Abstract
Aims
COVID‐19, a respiratory viral disease causing severe pneumonia, also affects the heart and other organs. Whether its cardiac involvement is a specific feature consisting of myocarditis, or simply due to microvascular injury and systemic inflammation, is yet unclear and presently debated. Because myocardial injury is also common in other kinds of pneumonias, we investigated and compared such occurrence in severe pneumonias due to COVID‐19 and other causes.
Methods and results
We analysed data from 156 critically ill patients requiring mechanical ventilation in four European tertiary hospitals, including all n = 76 COVID‐19 patients with severe disease course requiring at least ventilatory support, matched to n = 76 from a retrospective consecutive patient cohort of severe pneumonias of other origin (matched for age, gender, and type of ventilator therapy). When compared to the non‐COVID‐19, mortality (COVID‐19 = 38.2% vs. non‐COVID‐19 = 51.3%, P = 0.142) and impairment of systolic function were not significantly different. Surprisingly, myocardial injury was even more frequent in non‐COVID‐19 (96.4% vs. 78.1% P = 0.004). Although inflammatory activity [C‐reactive protein (CRP) and interleukin‐6] was indifferent, d‐dimer and thromboembolic incidence (COVID‐19 = 23.7% vs. non‐COVID‐19 = 5.3%, P = 0.002) driven by pulmonary embolism rates (COVID‐19 = 17.1% vs. non‐COVID‐19 = 2.6%, P = 0.005) were higher.
Conclusions
Myocardial injury was frequent in severe COVID‐19 requiring mechanical ventilation, but still less frequent than in similarly severe pneumonias of other origin, indicating that cardiac involvement may not be a specific feature of COVID‐19. While mortality was also similar, COVID‐19 is characterized with increased thrombogenicity and high pulmonary embolism rates.
Keywords: COVID‐19, Myocarditis, Thrombosis, Acute respiratory distress syndrome, Pneumonia
Introduction
The novel coronavirus disease COVID‐19, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has been spreading rapidly among the human population since December 2019 and was officially declared a pandemic on 11 March 2020 by the World Health Organization. COVID‐19 led to a worldwide healthcare crisis with over 20 million confirmed cases and over 700 000 fatalities (as of 11 August 2020). 1 While SARS‐CoV‐2 primarily involves the respiratory system, leading to severe pneumonia with consequent acute respiratory distress syndrome in up to 10% of all cases, the disease has also a considerable impact on the cardiovascular system according to latest studies. 2 Especially patients with pre‐existing cardiovascular conditions were shown to be at higher risk for a severe disease course and death. 2 Furthermore, COVID‐19 was associated with multiple direct and indirect cardiovascular complications including acute myocardial injury. Myocardial injury (CI) has thus been reported in 20–28% of hospitalized COVID‐19 patients, more common in patients in intensive care units (ICU), and was associated with increased mortality. 3 , 4 First case reports have also reported COVID‐19 associated myocarditis. 5 , 6 , 7 In line with these observations, histological changes of cardiac tissue are suggestive of COVID‐19‐specific cardiac involvement. 6 , 8 , 9 This theory is further supported by the high affinity of SARS‐CoV‐2 to angiotensin converting enzyme 2, 10 as well as from previous observations in SARS disease, which was also associated to specific cardiac involvement. 11 Furthermore, COVID‐19 seems to typically cause thrombotic events, which potentially further aggravate cardiovascular outcomes. 12
Another important point concerning SARS‐CoV‐2 and the heart is the deterioration and delay in medical treatment in areas severely affected by the pandemic, due to considerable shortages in ICU capacity, medical staff, and available tests. 13 , 14 , 15 Cardiac involvement, on the other hand, is also observed in patients suffering from pneumonias of other (non‐COVID‐19) origin, 16 associated to disease severity and outcome in critically ill patients. 17 , 18 Also thrombotic events, which seem to be very common in COVID‐19, are frequently observed in such ICU population. 19 It could be thus speculated, that in severe COVID‐19 disease, CI and cardiovascular outcome are primarily driven by severity of pulmonary disease with consequent end‐organ ischemia and generation of toxic radicals, rather than disease‐specific cardiac involvement.
This important pathophysiological issue is yet to be properly investigated. Comparison studies matching COVID‐19 to severe pneumonias of other origin are still scarce. Therefore, in this multicentre study, in order to characterize CI in COVID‐19, we examined cardiovascular outcomes in critically ill patients requiring ventilator therapy due to SARS‐CoV‐2‐induced pneumonia. To uncover COVID‐19‐specific characteristics of CI, we matched our study population to a historical cohort requiring respiratory support due to severe pneumonia of non‐COVID‐19 origin (non‐COVID‐19). To avoid unspecific effects caused by collapsing healthcare provision, patients were only recruited in two European countries (Germany and Austria), where medical systems were not overstrained due to COVID‐19 outbreak and enough ICU capacities were available. We hypothesized that compared with other pneumonias, COVID‐19 would promote higher CI rates with specific characteristics.
Key questions
What is already known about this subject?
Myocardial injury is a common occurrence in critical COVID‐19; hence, a disease‐specific involvement of the myocardium has been suggested. Yet myocardial injury also occurs in pneumonias of other origin, usually secondary to the severe pulmonary disease.
What does this study add?
In this multicentre study, myocardial injury and mortality were high but similar in patients requiring ventilatory support due to COVID‐19 or pneumonias of other origin. While thrombotic events were more frequent, as they seem to be a typical feature of COVID‐19, myocardial injury on the other hand, might be merely secondary to severe pulmonary disease and systemic inflammation.
How might this impact on clinical practice?
The herein presented clinical data shape our understanding on how COVID‐19 affects the cardiovascular system. The findings should have an impact on developing treatment strategies and the effective allocation of resources aimed at reducing the high mortality of the pandemic.
Methods
The study was conducted in four European tertiary centres (see further) in accordance with standards of good clinical practice and the principles of the Declaration of Helsinki and was approved by the respective local ethic committees.
Study cohorts
We included all critically ill 76 COVID‐19 patients, who required ventilator therapy between March and May 2020 [in Germany: University Hospital Münster (n = 18) and Maria Hilf Hospital Mönchengladbach (n = 18); in Austria: University Hospital Salzburg (n = 28) and Kepler University Hospital Linz (n = 12)]. Critical COVID‐19 disease was defined as the need for ventilator therapy, which was specified as either non‐invasive or invasive mechanical ventilation. Treatment of critical COVID‐19 pneumonia was performed according to current recommendations. 20 Diagnosis of COVID‐19 was established according to positive results of oropharyngeal or/and nasopharyngeal swabs test shown by real‐time reverse transcription–polymerase chain reaction assay for COVID‐19 (performed according to the manufacturer) and chest radiography and/or computer tomography of the thorax indicative for COVID‐19‐related pneumonia according to current recommendations. 21 Treatment of all patients was concluded, meaning they were either discharged or had died at the time of data analysis.
The control cohort was recruited from a consecutive, retrospective cohort of 1029 patients treated between 2016 and March 2020, requiring ventilator therapy (non‐invasive or invasive ventilation) due to respiratory failure induced by severe pneumonia of non‐COVID‐19 origin according to current intensive care guidelines. 22 To account for potential comorbidities and severity of respiratory failure, 76 patients were matched to COVID‐19 patients according to gender and age, as well as to the type of required ventilator therapy (non‐invasive or invasive ventilation). If more than one candidate in the retrospective non‐COVID‐19 cohort fully fulfilled the matching criteria, the patient with the closest admission time point as compared with the time point of the beginning of the recruitment of the COVID‐19 cohort (March 2020) was chosen for matching.
Data collection and analyses
A detailed description of data collection and analyses including applied definitions is provided in the supporting information. Briefly, in all eligible patients, patient data including demographics, medical history, laboratory examinations, comorbidities, complications, specific treatment measures, and outcomes were collected and analysed. To further characterize CI, available transthoracic echocardiography and radiographic images were investigated. CI was defined as high‐sensitive troponin (hs‐Tn) above the 99th‐percentile upper reference limit, 3 , 4 regardless of new abnormalities in electrocardiography and echocardiography. To account for the usage of different hs‐Tn assays in the recruiting centres, levels of hs‐Tn are given as the relative value (%) of the troponin assay‐specific cut‐off value (14.0 ng/L for hs‐TnT and 51.4 ng/mL for hs‐TnI).
Statistical analysis
The statistical analysis was carried out blindly by our statistical analytic team using SPSS 20 software package. Descriptive statistics were obtained for all study variables. All categorical variables were compared for the study outcome by using the Fisher exact test. Ordinal data are presented as median (interquartile range) values, and variables were compared using the Mann–Whitney U test. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. According to results, continuous variables were compared using the independent Student's t‐test or the Mann–Whitney U test, as appropriate. Continuous data are expressed as mean and standard deviation or median (interquartile range) values. A P value < 0.05 was regarded statistically significant.
Results
Baseline characteristics of the two cohorts are presented in Table 1 . According to matching criteria, there were no differences in relevant variables (gender, age, and respiratory therapy). The majority of non‐COVID‐19 presented with a pneumonia of primarily bacterial origin (Table 1 ). However, both cohorts did not present any differences in the prevalence of relevant pre‐existing pathologies (Table 1 ), indicating a similar status of comorbidities in both groups.
Table 1.
Baseline characteristics of investigated cohorts
| COVID‐19 (n = 76) | Non‐COVID‐19 (n = 76) | ||||
|---|---|---|---|---|---|
| Characteristic | n | Mean ± SD, Median (Q3–Q1) or % | n | Mean ± SD, Median (Q3–Q1) or % | P value |
| Gender (female) | 23/76 | 30.3% | 23/76 | 30.3% | >0.999 |
| Age (years) | 76 | 66.8 ± 13.4 | 76 | 65.3 ± 13.4 | 0.480 |
| BMI (kg/m2) | 76 | 27.5 (6.0) | 72 | 26.0 (7.8) | 0.159 |
| Aetiology of pneumonia | |||||
| Bacterial | 0/76 | 0% | 51/76 | 67.1% | |
| Viral | 76/76 | 100% | 22/76 | 28.9% | |
| Toxic | 0/76 | 0% | 3/76 | 3.9% | |
| Bacterial superinfection if viral or toxic | 28/76 | 36.8% | 19/76 | 25.0% | |
| Required respiratory therapy | |||||
| Non‐invasive ventilationa | 13/76 | 17.1% | 13/76 | 17.1% | >0.999 |
| Invasive ventilation | 63/76 | 82.9% | 63/76 | 82.9% | >0.999 |
| Medical history | |||||
| Arterial hypertension | 43/76 | 56.6% | 41/76 | 53.9% | 0.870 |
| Coronary artery disease | 10/76 | 13.2% | 14/76 | 18.4% | 0.505 |
| Peripheral vascular disease | 4/76 | 5.3% | 4/76 | 5.3% | >0.999 |
| Diabetes mellitus | 20/76 | 26.3% | 17/76 | 22.4% | 0.706 |
| Current smoking | 13/76 | 17.1% | 22/76 | 28.9% | 0.123 |
| Heart failure | 7/76 | 9.2% | 14/76 | 18.4% | 0.157 |
| Valvular heart disease | 3/76 | 3.9% | 5/76 | 6.6% | 0.719 |
| Atrial fibrillation | 9/76 | 11.8% | 16/76 | 21.1% | 0.189 |
| Pulmonary arterial hypertension | 4/76 | 5.3% | 2/76 | 2.6% | 0.681 |
| Obstructive lung disease | 11/76 | 14.5% | 17/76 | 21.1% | 0.295 |
| Restrictive lung disease | 3/76 | 3.9% | 7/76 | 9.2% | 0.327 |
| Malignancy | 11/76 | 14.5% | 17/76 | 22.4% | 0.295 |
BMI, body mass index; ICU, intensive care unit; SD, standard deviation.
Baseline characteristics of the investigated cohorts.
The term non‐invasive ventilation (NIV) refers to mechanical ventilation involving end‐expiratory and inspiratory positive air pressure support via a tightly fitted face mask or helmet, as opposed to invasive ventilation necessitating endotracheal intubation. All patients included in the study had some form of mechanical ventilation (patients who merely needed oxygen insufflation were not included).
P < 0.05.
Inspired by previous reports indicating a high burden of CI in COVID‐19, 3 , 4 we evaluated cardiac markers, echocardiography, and radiology images in both populations. Indeed, CI as indicated by elevated hs‐Tn levels was a frequent finding in both COVID‐19 and non‐COVID‐19 (Figure 1 A and Table 2 ). Surprisingly, despite the described previous reports in COVID‐19, non‐COVID‐19 presented higher levels of cardiac biomarkers [hs‐Tn, creatine kinase myoglobin fraction, and N‐terminal pro‐brain natriuretic peptide; Figure 1 B–D and Table 2 ] suggesting a higher burden of CI in non‐COVID‐19. Simultaneously, a higher frequency of CI in laboratory results and pulmonary congestion on radiography imaging in non‐COVID‐19 was observed (Figure 1 A and Table 2 ). Therefore, we speculated that a high burden of CI is generally observed in critical pneumonic disease of various non‐COVID‐19 origin and not specifically a COVID‐19‐dependent finding. In order to further evaluate our hypothesis, we investigated echocardiography and cardiac radiographic images. They revealed a high frequency of novel functional cardiac impairment, which however was similar in both groups (Figure 1 F–H and Table 2 ). We then evaluated if CI might rather depend on the severity of disease progression with consequent end‐organ ischemia. Therefore, we investigated lactate and pH levels. Indeed, as indicated by lower pH and higher lactate values, they revealed a more severe course in non‐COVID‐19 (Table 3 ). While this observation probably could explain a higher incidence of CI in this population, general outcomes and required ICU therapies like usage of catecholamine, extracorporal membrane oxygenation therapy, and rate/reasons of/for cardiopulmonary resuscitation were not significantly different in both groups (Table 4 ). Importantly, mortality was high in both groups and tended to be even higher in non‐COVID‐19 but not significantly different (Figure 1 E and Table 4 ), thus reflecting a poor outcome in both COVID‐19 and non‐COVID‐19‐related pneumonia. To further evaluate potential associations with inflammatory processes, further laboratory parameters were studied. However, besides increased procalcitonin (PCT) and leucocytes (Table 3 ), which could be driven by the high proportion of bacterial pneumonia in non‐COVID‐19 (Table 1 ), we did not observe any differences in other relevant inflammatory markers including C‐reactive protein (CRP), interleukin 6 (IL6), and fibrinogen (Table 3 ). While these biomarkers were strongly elevated, they indicated pronounced inflammatory processes in both COVID‐19 and non‐COVID‐19. Interestingly, despite a similar inflammatory activity (or even potentially higher inflammatory activity as indicated by leucocytes and PCT levels in non‐COVID‐19) in both populations, COVID‐19 revealed an increased d‐dimer levels (Figure 2 A and Table 3 ) suggesting a potentially higher thrombogenicity in this population. Consequently, with respect to our results as well recent literature dealing with COVID‐19 disease, 12 we investigated the incidence of thrombotic/thromboembolic events in both populations. Indeed, the incidence of these events was significantly higher in COVID‐19 (Figure 2 B–F and Table 4 ). Of note, this observation was primarily influenced by the high rate of pulmonary embolism in this population (Figure 1 D and Table 4 ). Therefore, our results indicate that independently of severity of pneumonic disease as well as inflammatory activity, COVID‐19 specifically provokes thrombogenicity, which potentially might drive cardiovascular outcome in the COVID‐19 population.
Figure 1.
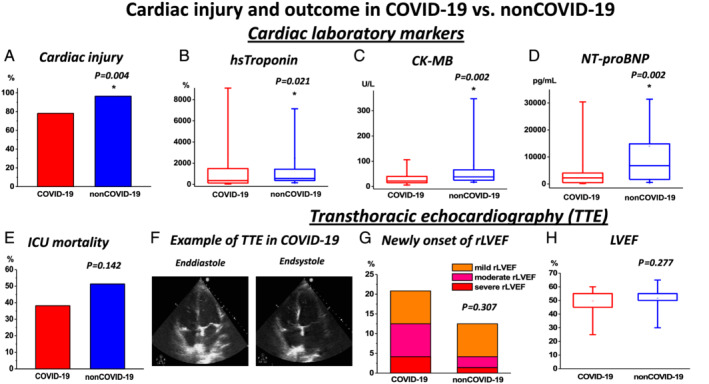
Myocardial injury and outcome in COVID‐19 vs. non‐COVID‐19: (A) Incidence of myocardial injury (for the definition, see supporting information) was high in both groups. However, higher rates were revealed in non‐COVID‐19. (B–D) Levels of relevant cardiac biomarkers: (B) high‐sensitive (hs) troponin, (C) CK‐MB, and (D) NT‐proBNP were increased in non‐COVID‐19. Highest values measured during the total period of intensive care unit (ICU) stay are presented as box plots (B–D). (E) ICU mortality rates were similar in both groups. (F–H) Transthoracic echocardiography (TTE) findings in COVID‐19 vs. non‐COVID‐19: (F) example of TTE obtained during ICU stay in a COVID‐19 patient; (G) incidence of newly onset of reduced left ventricular ejection fraction (rLVEF) with rates of severity of left ventricular ejection fraction (LVEF) impairment, rate of newly onset of rLVEF, and (H) LVEF values were not different in both groups. CK‐MB, creatine kinase myoglobin fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Table 2.
Parameters of cardiac outcome/function during ICU stay
| COVID‐19 (n = 76) | Non‐COVID‐19 (n = 76) | ||||
|---|---|---|---|---|---|
| Parameter | n | Median (Q3–Q1) or % | n | Median (Q3–Q1) or % | P value |
| Myocardial injury | 57/73 | 78.1% | 54/56 | 96.4% | 0.004* |
| Cardiac laboratory markers | |||||
| Initial hs‐Tn (%) | 73 | 178.6 (481.1) | 56 | 317.9 (398.2) | 0.003* |
| Max. hs‐Tn (%) | 73 | 354.3 (1409.6) | 56 | 550.0 (1108.9) | 0.021* |
| Initial CK (U/L) | 74 | 174.5 (320.8) | 76 | 103.0 (350.8) | 0.231 |
| Max. CK (U/L) | 74 | 518.0 (856.3) | 76 | 490.5 (949.0) | 0.864 |
| Initial CK‐MB (U/L) | 44 | 19.1 (16.6) | 51 | 27.3 (26.2) | 0.001* |
| Max. CK‐MB (U/L) | 44 | 22.0 (28.8) | 51 | 38.0 (42.0) | 0.002* |
| Initial NT‐proBNP (pg/mL) | 44 | 811.0 (2849.8) | 56 | 3890.0 (6926.3) | <0.001* |
| Max. NT‐proBNP (pg/mL) | 44 | 2217.1 (4481.3) | 56 | 6625.5 (13920.0) | 0.001* |
| Functional parameters on TTE | 48/76 | 63.2% | 72/76 | 94.7% | |
| Reduced LVEF | 14/48 | 29.2% | 18/72 | 25.0% | 0.676 |
| Newly onset of reduced LVEF | 10/48 | 20.8% | 9/72 | 12.5% | 0.307 |
| LVEF (%) | 48 | 55.0 (10.0) | 72 | 55.0(8.8) | 0.277 |
| LV dilatation | 1/44 | 2.3% | 2/66 | 3.0% | >0.999 |
| RV dilatation | 10/44 | 22.7% | 11/68 | 16.2% | 0.460 |
| Pericardial effusion | 3/47 | 6.4% | 8/71 | 11.3% | 0.522 |
| Radiology findings | |||||
| Cardiomegaly | 35/76 | 46.1% | 35/76 | 46.1% | >0.999 |
| Cardiomegaly during FU | 15/76 | 19.7% | 13/76 | 17.1% | 0.835 |
| Pulmonary venous congestion | 26/76 | 34.2% | 56/76 | 73.7% | <0.001* |
CK, creatine kinase; CK‐MB, creatine kinase myoglobin fraction; FU, follow‐up; hs‐Tn, high‐sensitive troponin; initial, first obtained value; LV, left ventricular; LVEF, left ventricular ejection fraction; Max., highest level of cardiac biomarker obtained during the total period of the ICU stay; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide, RV, right ventricular, TTE, transthoracic echocardiography.
Cardiac outcome of patients during intensive care unit (ICU) stay.
P < 0.05.
Table 3.
Relevant laboratory markers during ICU stay
| COVID‐19 (n = 76) | Non‐COVID‐19 (n = 76) | ||||
|---|---|---|---|---|---|
| Laboratory marker | n | Median (Q3–Q1) or Mean ± SD | n | Median (Q3–Q1) or Mean ± SD | P value |
| Lactate, U(L) | 76 | 2.62 (1.95) | 76 | 3.41 (4.49) | 0.005* |
| Min. pH | 76 | 7.21 (0.18) | 76 | 7.16 (0.15) | 0.007* |
| Creatinine (mg/dL) | 76 | 1.74 (2.12) | 75 | 2.20 (2.63) | 0.699 |
| Potassium (mmol/L) | 76 | 3.46 (0.48) | 76 | 3.37 (0.41) | 0.475 |
| Leucocytes (109/L) | 76 | 14.82 (11.28) | 76 | 20.20 (12.86) | <0.001* |
| Min. lymphocytes (109/L) | 75 | 3.00 (6.85) | 57 | 4.70 (6.55) | 0.095 |
| CRP (ng/mL) | 76 | 27.5 ± 12.2 | 76 | 27.0 ± 12.9 | 0.790 |
| PCT (ng/mL) | 76 | 1.59 (5.17) | 72 | 3.00 (22.70) | 0.003* |
| Interleukin 6 (pg/mL) | 68 | 518.9 (2079.6) | 32 | 391.9 (1086.4) | 0.897 |
| Fibrinogen (mg/dL) | 47 | 652.2 ± 203.6 | 65 | 598.8 ± 183.9 | 0.150 |
| d‐Dimer (mg/L) | 66 | 6.72 (15.04) | 37 | 3.21 (7.07) | 0.005* |
CRP, C‐reactive protein; Min., lowest level of laboratory biomarker obtained during the total period of ICU stay; PCT, procalcitonin.
Relevant laboratory findings obtained during intensive care unit (ICU) stay. If not other indicated, the highest obtained value during the whole period of ICU stay is presented.
P < 0.05.
Table 4.
Patients' outcome and relevant therapies during ICU stay
| COVID‐19 (n = 76) | Non‐COVID‐19 (n = 76) | ||||
|---|---|---|---|---|---|
| Outcome | n | Median (Q3–Q1) or % | n | Median (Q3–Q1) or % | P value |
| Death | 29/76 | 38.2% | 39/76 | 51.3% | 0.142 |
| Discharged from ICU | 47/76 | 61.8% | 37/76 | 48.7% | 0.142 |
| Duration of invasive ventilatory therapy (days) | 63 | 11 (16) | 63 | 10 (12) | 0.124 |
| Duration of ICU stay (days) | 76 | 15 (21) | 76 | 12 (17) | 0.033* |
| Required ICU therapy | |||||
| ECMO | 9/76 | 11.8% | 10/76 | 13.2% | >0.999 |
| Hemofiltration | 22/76 | 28.9% | 28/76 | 36.8% | 0.388 |
| Catecholamines | 59/76 | 77.6% | 68/76 | 89.5% | 0.079 |
| Electrical cardioversion/defibrillation | 6/76 | 7.9% | 14/76 | 18.4% | 0.091 |
| Relevant complications | |||||
| CPR | 6/76 | 7.9% | 10/76 | 13.2% | 0.429 |
| Sustained VT | 3/76 | 3.9% | 2/76 | 2.6% | >0.999 |
| Asystole | 2/76 | 2.6% | 7/76 | 9.2% | 0.167 |
| Pulseless electrical activity | 2/76 | 2.6% | 1/76 | 1.3% | >0.999 |
| Relevant bleeding | 4/76 | 5.3% | 5/76 | 6.6% | >0.999 |
| Thrombosis/thromboembolic event | 18/76 | 23.7% | 4/76 | 5.3% | 0.002* |
| Pulmonary embolism | 13/76 | 17.1% | 2/76 | 2.6% | 0.005* |
| Peripheral thrombosis/thromboembolism | 7/76 | 9.2% | 2/76 | 2.6% | 0.167 |
| Thromboembolic stroke/TIA | 4/76 | 5.3% | 0/76 | 0.0% | 0.120 |
CPR, cardiopulmonary resuscitation; ECMO, extracorporal membrane oxygenation; TIA, transient ischemic attack; VT, ventricular tachycardia defined as VT > 30 s.
Outcome of patients during intensive care unit (ICU) stay.
P < 0.05.
Figure 2.
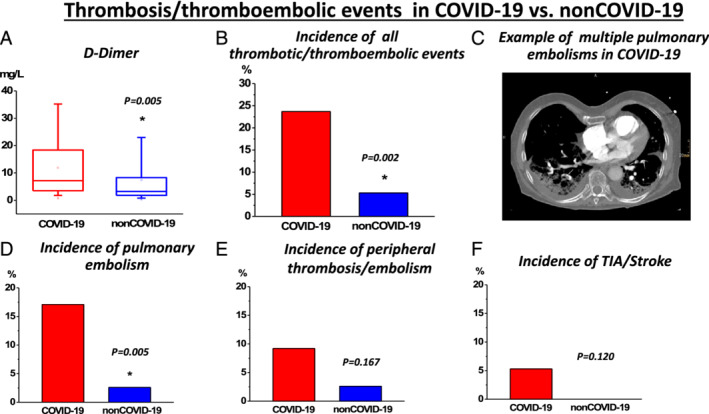
Thrombosis and/or thromboembolic events in COVID‐19 vs. non‐COVID‐19: (A) While d‐dimer levels were elevated (highest values measured during the total period of ICU stay are presented as box plots), (B) an increased incidence of thrombosis and/or thromboembolic events were revealed in COVID‐19. (C) Example of a computer tomography pulmonary angiogram of a COVID‐19 patient, who suffered from multiple, fatal periphery pulmonary embolism events. (D–F) Incidence of various thrombotic and/or thromboembolic events in COVID‐19: (D) pulmonary embolism, (E) peripheral thrombotic and/or thromboembolic events, and (F) thromboembolic stroke or transitory ischemic attack (TIA).
Discussion
Severe COVID‐19 disease is generally characterized by pneumonia with acute lung injury, yet a high incidence of CI has also been reported, 3 , 4 as well as cases with disease‐specific myocardial alterations. 5 , 6 , 7 , 8 , 9 On the other hand, CI in the absence of acute coronary syndrome has been well described in pneumonia of non‐COVID‐19 origin, 16 and is common in patients requiring ICU treatment. 3 , 4 , 17 , 18 , 23 In order to assess potential implications on the cardiovascular system, we retrospectively compared critically ill COVID‐19 patients with other similarly ill patients with pneumonia of other origin. Compared with other countries at initial outbreaks, 13 , 14 our fatality rate of 38.2% in mechanical‐ventilated COVID‐19 patients was low (Figure 1 E and Table 4 ), indicating adequate ICU support in less strained medical systems in Germany and Austria. Interestingly, such ICU mortality was not different in matched non‐COVID‐19 patients (Figure 1 E and Table 4 ). Our finding reassures the importance of available ICU capacities during the pandemic, as it seems that, given adequate ventilatory capacity, the mortality outcome of the severe cases of COVID‐19 is similar to that of non‐COVID‐19 pneumonias.
Our cohort presented a much higher rate of CI (78.1%; Figure 1 A and Table 4 ) than previous studies (20–28%), but those studies included both ICU and non‐ICU populations. 3 , 4 While we also included in‐hospital follow‐up investigations of cardiac biomarkers, our results might indicate a higher burden of CI in critical COVID‐19 patients than previously assumed. Surprisingly though, CI was even more frequent in non‐COVID‐19 (Figure 1 A–D and Table 4 ). Notably, our incidence rates in non‐COVID‐19 are in line with other reports investigating myocardial damage in critical pneumonias (55–81% in the first 24–48 h and 85% during a follow‐up of 7 days). 17 , 18 , 23
Several case series reported reduced left ventricular (LV) ejection fraction in COVID‐19 patients. 5 , 6 , 7 , 24 Consistently, we saw high rates of newly onset reduction of LV ejection fraction (20.8%; Table 2 and Figure 1 F–H ), with a consequent similar rate of cardiomegaly during follow‐up radiography (Table 2 ) in our cohort. However, consistent with previous reports indicating up to 30% incidence of transient cardiac functional impairment in pneumonias of non‐COVID‐19 origin, 16 functional impairment was not more severe in our COVID‐19 patients (Figure 1 F–H and Table 2 ). Our results suggest that in critical COVID‐19 pneumonia, myocardial impairment is a frequent observation in COVID‐19, but similar to other pneumonias (at least in terms of CI and systolic LV impairment). But although histologically confirmed cardiomyocyte injury has been described in non‐COVID‐19 pneumonias, 25 systolic LV impairment is usually a transient observation. 16 Whether a similar outcome is also true for COVID‐19 remains unclear.
A further aspect that reflects the severity of critical pneumonic disease is inflammation. Increased inflammatory burden was reported to drive CI in pneumonia of non‐COVID‐19 origin. Among others, in critically ill patients, specific inflammatory markers IL6 and PCT were linked to higher troponin levels. 17 , 26 In COVID‐19, an inflammatory cytokine storm with consequent acute respiratory distress syndrome is presumed to be the main drive of morbidity and fatality. 2 Similar to non‐COVID‐19, a high inflammatory activity indicated by CRP and PCT levels has been linked to CI. 2 , 3 , 4 In our study, inflammatory activity was high in both COVID‐19 and non‐COVID‐19, indicating the critical stage of disease in both populations. IL6 and CRP levels were not different between groups, but PCT and leucocytes were increased in non‐COVID‐19, possibly explained by the majority of bacterial infections in non‐COVID‐19 pneumonias (Table 1 ). Nevertheless, our data indicate a high inflammatory activity in both groups (Table 3 ), which probably drives CI. It can be speculated that in COVID‐19 (like in other pneumonias), CI and systolic impairment in severe disease is driven by pronounced inflammatory burden and reduced myocardial supply with consequent functional impairment of cardiac function. The likely speculation that the high inflammatory burden and reduced myocardial supply are responsible for CI and LV systolic impairment in severe COVID‐19 pneumonia is supported by a recent report of post‐mortem examination, which revealed cardiac inflammatory processes not meeting the criteria of true myocarditis in COVID‐19. 27
Despite the similar inflammatory activity, we saw a higher thrombogenicity in COVID‐19 (Figure 1 A and Tables 3 , 4 ). The observation in terms of thromboembolic events, including pulmonary embolism, is in line with reports from the Netherlands and the United Kingdom, which reported similar high rates (31% and 27%, respectively). 28 , 29 Although all our patients received anticoagulation at least at prophylactic dosages and there was no difference in rates of therapeutic anticoagulation between cohorts (Figure S1 ), thromboembolic events were much higher in COVID‐19. Our results are further supported by autopsy studies that found deep vein thrombosis and/or pulmonary embolism in most deceased COVID‐19 patients. 30 Indeed, the common occurrence of pulmonary embolism and other thromboembolic events seems one of the major challenges of present COVID‐19 therapy and research and could have potential impact on cardiovascular outcomes. The potential benefit of an effective anticoagulant therapy in COVD 19 needs to be further evaluated.
Limitations
The observational design presents the main study limitation, while the control cohort was not prospectively randomized. Assessment of CI was based on cardiac enzymes and echocardiography, not including more precise imaging techniques such as magnetic resonance imaging and/or myocardial biopsy. Our efforts to contain the hospital spread of the virus (from these ventilated, highly contagious patients) resulted in a restrictive approach to imaging and interventional procedures. Cardiac inflammatory burden and the question of COVID‐19‐associated myocarditis are thus not sufficiently covered. Advanced imaging and coronary/haemodynamic workups would have provided useful information on the ischaemic and inflammatory nature of CI. Not all patients had echocardiography (about 21%), and in some patients, cardiac enzymes were not measured (Table 2 ). Missing follow‐up analysis could also have revealed whether CI was reversible in survivors. Regarding the comparison group, PCR of infectious viruses and bacterial work‐up was not able to identify the infectious agents in all control patients. Therefore, while interpreting our results, we are not able to account for specific cardiac involvements, which are associated with some viral and bacterial agents. Additionally, the heterogeneity of our comparison group, which consists of patients suffering from bacterial, viral and/or toxic pneumonia might in part differ with regards to the pathogenetic mechanisms compared with COVID‐19 pneumonia ( Table S2 ). On the other hand, the high rates of superinfection in both groups might partly attenuate these differences. Nevertheless, the heterogenic aetiology of pneumonia in our control population remains one the main study limitations. The passionate use of untested treatments in a number of COVID‐19 patients (such as tocilizumab or hydroxychloroquine, Table S1 ) might have affected the results. Finally, instead of screening, diagnostic workups for thromboembolic events were only performed when clinically suspected and are therefore most probably underestimated.
In conclusion, we report a very high rate of CI in critical COVID‐19 disease, which in this study was even higher in severe pneumonias of other origin. While ICU mortality was similar, COVID‐19 differed significantly in terms of a high occurrence of thromboembolic events. Whether CI, including viral myocarditis, is a specific feature of the COVID‐19 disease is put in doubt by our findings and requires further investigation. Prevention and management of thrombotic complications, including pulmonary embolism, should be one of the main targets of the therapy of critically ill COVID‐19 patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
P.J., R.L., R.P., and L.J.M. substantially contributed to the conception, and design of the work. They also substantially took part in acquisition, analysis, and interpretation of data for the work and wrote the manuscript, prepared the figures, as well as revised the work critically for important intellectual content. Z.S., D.D., N.F., and H.J.F.S. contributed substantially to the acquisition and the interpretation of the data for the work as well as revised the work critically for important intellectual content. E.F., D.D., C.T., M.M., D.B., H.H., and T.T. contributed substantially to the acquisition of the data for the work and revised the work critically for important intellectual content. M.L., A.E., B.L., H.R., and U.C.H. contributed substantially to the conception and design of the work as well as the interpretation of data for the work. They also revised the work critically for important intellectual content. P.J., R.L., Z.S., E.F., D.D., C.T., N.F., M.M., D.B., H.H., H.J.F.S., T.T., M.L., A.E., B.L., H.R., U.C.H., R.P., and L.J.M. approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability statement
All presented data including deidentified study participant data are available upon reasonable request to the corresponding author: Rudin Pistulli at contact: rpistulli@yahoo.it. Reuse is only permitted after agreement of all coauthors of this study.
Patient consent and ethics approval
The study was approved by the respective local ethic committees of the participating medical centres: in Germany: University Hospital Münster: Nr. 2020‐306‐f‐S and Maria Hilf Hospital Mönchengladbach: Nr. 143/2020, and in Austria: University Hospital Salzburg: Nr. 1071/2020 and Kepler University Hospital Linz: Nr. 1085/2020. In accordance with the respective local ethic committees of the participating medical centres, no informed consent was necessary, due to the observational retrospective study design.
Supporting information
Table S1. Usage of specific therapies targeting COVID‐19 in COVID‐19 patients.
Usage of specific therapies potentially targeting COVID‐19 disease in COVID‐19 patients during their intensive care stay.
Table S2. Infectious workup for Covid‐19 and non‐Covid‐19 patients (sputum/tracheal secretion, throat swab and/or blood culture) – table depicts all pathogens detected during the course of ICU treatment including superinfections/secondary infections.
Figure S1. Rate of therapeutic anticoagulation was not significantly different in COVID‐19 vs. nonCOVID‐19.
Acknowledgement
Open access funding enabled and organized by Projekt DEAL.
Jirak, P. , Larbig, R. , Shomanova, Z. , Fröb, E. J. , Dankl, D. , Torgersen, C. , Frank, N. , Mahringer, M. , Butkiene, D. , Haake, H. , Salzer, H. J. F. , Tschoellitsch, T. , Lichtenauer, M. , Egle, A. , Lamprecht, B. , Reinecke, H. , Hoppe, U. C. , Pistulli, R. , and Motloch, L. J. (2021) Myocardial injury in severe COVID‐19 is similar to pneumonias of other origin: results from a multicentre study. ESC Heart Failure, 8: 37–46. 10.1002/ehf2.13136.
References
- 1. John Hopkins University n.d. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. (23 March 2020).
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 28: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 27: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 25: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J 2020; 41: 1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020; 22: 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 27: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang F‐S. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8: 420–422 Epub 2020 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. A pathological report of three COVID‐19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020; 49: 411–417. [DOI] [PubMed] [Google Scholar]
- 10. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med 2020; 382: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JWM, Yiu SF. Left ventricular performance in patients with severe acute respiratory syndrome: a 30‐day echocardiographic follow‐up study. Circulation 2003; 108: 1798–1803. [DOI] [PubMed] [Google Scholar]
- 12. Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, Merdji H, Clere‐Jehl R, Schenck M, Gandet FF, Fafi‐Kremer S. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Docherty AB, Harrison EM, Green CA, Hadwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen‐Van‐Tam JS, Ho A, Russel CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, Mcginn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. Jama 2020; 22: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenbaum L. Facing Covid‐19 in Italy—ethics, logistics, and therapeutics on the epidemic's front line. N Engl J Med 2020; 382: 1873–1875. [DOI] [PubMed] [Google Scholar]
- 16. Corrales‐Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet 2013; 381: 496–505. [DOI] [PubMed] [Google Scholar]
- 17. Ammann P, Maggiorini M, Bertel O, Haenseler E, Joller‐Jemelka HI, Oechslin E, Minder EI, Rickli H, Fehr T. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003; 41: 2004–2009. [DOI] [PubMed] [Google Scholar]
- 18. Bajwa EK, Boyce PD, Januzzi JL, Gong MN, Thompson BT, Christiani DC. Biomarker evidence of myocardial cell injury is associated with mortality in acute respiratory distress syndrome. Crit Care Med 2007; 35: 2484–2490. [DOI] [PubMed] [Google Scholar]
- 19. Minet C, Potton L, Bonadona A, Hamidfar‐Roy R, Somohano CA, Lugosi M, Cartier JC, Ferretti G, Schwebel C, Timsit JF. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care 2015; 19: 15–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley‐Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‐19). Intensive Care Med 2020; 46: 854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, Schluger NW, Volpi A, Yim JJ, Martin IB, Anderson DJ. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology 2020; 7: 2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, Forel JM, Guérin C, Jaber S, Mekontso‐Dessap A, Mercat A. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019; 9: 019–0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frencken JF, van Baal L, Kappen TH, Donker DW, Horn J, van der Poll T, van Klei WA, Bonten MJM, Cremer OL, de Beer FM, Bos LDJ, Glas GJ, van Hooijdonk RTM, Schouten LRA, Straat M, Witteveen E, Wieske L, van Vught LA, Wiewel M, Hoogendijk AJ, Huson MA, Scicluna B, Schultz MJ, Ong DSY, Klein Klouwenberg PMC, van de Groep K, Verboom D, Koster‐Brouwer ME. Myocardial injury in critically ill patients with community‐acquired pneumonia. A cohort study. Ann Am Thorac Soc 2019; 16: 606–612. [DOI] [PubMed] [Google Scholar]
- 24. Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID‐19: evidence from front‐line clinical observation in Wuhan, China. Int J Cardiol 2020; 8: 31115‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013; 187: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostermann M, Ayis S, Tuddenham E, Lo J, Lei K, Smith J, Sanderson B, Moran C, Collinson P, Peacock J, Rhodes A. Cardiac troponin release is associated with biomarkers of inflammation and ventricular dilatation during critical illness. Shock 2017; 47: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. Postmortem examination of patients with COVID‐19. Jama 2020; 21: 2518–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klok FA, Kruip MJ, Van der Meer NJ, Arbous MS, Gommers DA, Kant KM, Kaptein FH, van Paassen J, Stals MA, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; 10: 30120–30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, Lavinio A, Besser M. Thrombotic complications of patients admitted to intensive care with COVID‐19 at a teaching hospital in the United Kingdom. Thromb Res. 2020; 191: 76–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med 2020; 14: M20–M2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Usage of specific therapies targeting COVID‐19 in COVID‐19 patients.
Usage of specific therapies potentially targeting COVID‐19 disease in COVID‐19 patients during their intensive care stay.
Table S2. Infectious workup for Covid‐19 and non‐Covid‐19 patients (sputum/tracheal secretion, throat swab and/or blood culture) – table depicts all pathogens detected during the course of ICU treatment including superinfections/secondary infections.
Figure S1. Rate of therapeutic anticoagulation was not significantly different in COVID‐19 vs. nonCOVID‐19.
Data Availability Statement
All presented data including deidentified study participant data are available upon reasonable request to the corresponding author: Rudin Pistulli at contact: rpistulli@yahoo.it. Reuse is only permitted after agreement of all coauthors of this study.


