Abstract
It remains unclear if intubation and ventilation earlier in the disease course confers a survival advantage in acute respiratory distress syndrome. Our objective was to determine whether patients with COVID-19 who died following mechanical ventilation were more advanced in their disease compared to survivors. Forty-seven patients admitted directly to our centre received ventilation, of who 26 (57%) patients died. The rate of fall in SpO2:FiO2 ratio (p = 0.478) and increasing respiratory rate (p = 0.948) prior to IMV were similar between survivors and non-survivors. Our data support a trial of continuous positive airway pressure prior to IMV in patients with moderate-to-severe COVID-19 ARDS.
Keywords: Acute respiratory distress syndrome, Lung injury, Mechanical ventilation, Continuous positive airway pressure
Abbreviations: ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; CRP, C-reactive protein; ICU, Intensive Care Unit; IMV, Invasive mechanical ventilation; p-SILI, patient self-inflicted lung injury; SpO2:FiO2, Oxygen saturation: fraction of inspired oxygen ratio; VILI, ventilation-induced lung injury
Dear Editor:
The relative harm of lung injury induced by prolonged trials of spontaneous breathing with assisted non-invasive support (patient self-inflicted lung injury; p-SILI) versus early mechanical ventilation-induced lung injury (VILI) has engendered vigorous debate [1,2]. It remains unclear if intubation and IMV earlier in the disease course confers a survival advantage in acute respiratory distress syndrome (ARDS). Our objective was to determine whether patients with COVID-19 pneumonia who died following invasive mechanical ventilation (IMV) were more advanced in their disease compared to survivors.
We conducted a single-centre retrospective cohort study of mechanically ventilated COVID-19 patients treated at University College London Hospital (UCLH) between 1 March and 30 June 2020. Our strategy, adopted from the outset, was to utilize continuous positive airways pressure (CPAP) in patients deteriorating with facemask oxygen alone in either the intensive care unit (ICU) or a newly-established respiratory high dependency unit (HDU). Patients proceeded to IMV if they showed a trajectory of worsening hypoxaemia and/or increasing respiratory effort/fatigue.
Data were extracted from electronic healthcare records on patient demographics, clinical features (duration of symptoms, timing of intubation, daily peak temperature, and daily median SpO2, FiO2 and respiratory rate), and routine biochemistry (C-reactive protein (CRP), neutrophil count). Respiratory and inflammatory variables were collected three days preceding IMV requirement. Continuous and categorical variables are reported as median (interquartile range) and n (%), respectively. For comparison of continuous variables, Mann Whitney U test for comparison between 2 groups. The change in continuous variables over time between survivors and non-survivors was assessed using two-way ANOVA. Categorical data were compared using the chi-squared test. Statistical analysis was performed, and graphs constructed using Prism (GraphPad Software, Version 5.0d, San Diego, California, US).
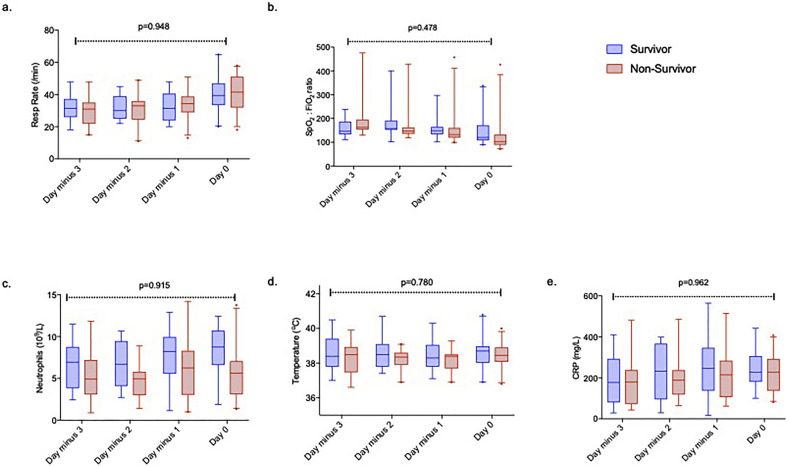
Ninety-nine patients received IMV. Fifty-two (52%) patients transferred from other hospitals were excluded as complete clinical data prior to IMV were unavailable. Forty-seven patients admitted directly to UCLH received IMV, of who 26 (57%) patients died in hospital. Six patients were intubated shortly after hospital admission. Of the remaining 40 patients, 39 (98%) received CPAP. Duration from symptom onset to hospitalisation (p = 0.436), days from hospitalisation to IMV (p = 0.398), respiratory rate (p = 0.704) and SpO2:FiO2 ratio (p = 0.066) immediately prior to IMV and use of CPAP (p = 0.119) were similar between survivors and non-survivors. A trajectory of falling SpO2:FiO2 ratio (p = 0.056) and increasing tachypnoea (p < 0.001) prior to intubation was noted in all patients. However, the rate of change of SpO2:FiO2 ratio (p = 0.478) and increasing respiratory rate (p = 0.948) prior to IMV were similar between survivors and non-survivors. The change in inflammatory parameters preceding IMV were similar between survivors and non-survivors (Fig. 1 ).
Fig. 1.
Trajectory of respiratory rate and SpO2: FiO2 ratio and inflammatory variables over the three days preceding invasive mechanical ventilation. (a-b). An increase in respiratory rate (p < 0.001) and fall in SpO2: FiO2 ratio (p = 0.056) occur over time but with no difference in the rate in change over time between patients who survive or die. No difference is seen in inflammatory parameters; c). neutrophils, d). peak daily temperature, or e). Box and whisker plots represent median (horizontal line), interquartile range (box) and 95% confidence intervals (whiskers). Two- way ANOVA comparing change over time between survivors and non-survivors.
Consistent with international expert consensus recommendations on airway management in COVID-19, we intubated patients in their individual pathology and pathophysiology, the acute trajectory of their illness, in addition to their responsiveness to trials of noninvasive airway management [3]. On IMV initiation, 61% of patients fulfilled the Berlin criteria for severe ARDS. This contrasts with 39% in the first 24 h of ICU admission in the UK national database [4]. Despite this higher proportion, the mortality rate was comparable for patients mechanically ventilated within the first 24 h [4]. While cognisant of the potential detrimental effects of p-SILI, this does not appear to have had any attributable impact on mortality. Furthermore, a third of our patients initiated on CPAP improved without needing IMV [5].
The mortality among COVID-19 ARDS patients not initiated on IMV within the first 24 h of admission to UK ICUs was lower than later initiation of IMV, supporting our observation [4]. Furthermore, a 21% improvement in UK ICU mortality rates over the course of the COVID pandemic mirrors an equivalent reduction in use of IMV, including a 42% reduction in IMV within the first 24 h, despite unchanged PaO2:FiO2 ratios [6]. Recent data from the second surge show this trend is continuing [7].
Advancing age was associated with mortality consistent with UK data [4]. Inflammatory parameters including CRP and neutrophil: lymphocyte ratio predict survival among hospitalised patients with COVID-19 [8,9]. We therefore explored the trajectory of inflammatory variables among COVID-19 patients receiving invasive mechanical ventilation but found no difference in neutrophil count, temperature, nor CRP change prior to IMV between survivors and non-survivors.
As with all retrospective analyses, we acknowledge residual confounding, and that results are associative. Furthermore, the small sample size limits generalisability. Nonetheless, these data support a trial of CPAP prior to IMV in patients with moderate-to-severe COVID-19 ARDS, especially in a resource- limited setting. Randomised controlled trials evaluating timing of IMV in ARDS are imperative.
Ethics approval
Ethics to report observational data on critical care patients at UCLH is covered by the National Research Ethics Service (14/LO/103).
Consent for publication
N/A.
Availability of data and materials
Upon reasonable request.
Funding
None.
Authors' contributions
Study design (NA), data collection (AL, TB, AK, JG), Statistics (NA), Drafting manuscript (NA). All authors read and approved the final manuscript.
Declaration of Competing Interest
None.
Acknowledgements
UCLH Intensive Care.
References
- 1.Gattinoni L., Marini J.J., Camporota L. The respiratory drive: an overlooked tile of COVID-19 pathophysiology. Am J Respir Crit Care Med. 2020;202(8):1079–1080. doi: 10.1164/rccm.202008-3142ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin M.J., Jubran A., Laghi F. Hypoxaemia does not necessitate tracheal intubation in COVID-19 patients. Br J Anaes. 2020 Nov 16 doi: 10.1016/j.bja.2020.11.007. [S0007-0912(20)30922-3; In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei H., Jiang B., Behringer E.C., Hofmeyr R., Myatra S.M., Wong D.T., et al. Controversies in airway management of COVID-19 patients: updated information and international expert consensus recommendations. Br J Anaes. 2020 Nov 6 doi: 10.1016/j.bja.2020.10.029. S0007-0912(20)30896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards-Belle A., Orzechowska I., Gould D.W., Thomas K., Doidge J.C., Mouncey P.R., et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern, Ireland. Am J Respir Crit Care Med. 2020 Dec 11 doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arina P., Baso B., Moro V., Patel H., Ambler G., Group, U. C. L, UCL Critical Care COVID-19 Research Group Critical Care COVID-19 Research. Discriminating between CPAP success and failure in COVID-19 patients with severe respiratory failure. Intensive Care Med. 2020 Nov 16:1–3. doi: 10.1007/s00134-020-06304-y. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doidge, JC, Mouncey, PR, Thomas, K, Gould DW, Ferrando- Vivas, P, Shankar-Hari, M, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. [DOI] [PMC free article] [PubMed]
- 7.ICNARC report on COVID-19 in critcal care: England, Wales and Northern Ireland. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. (Accessed 6th Nov 2020).
- 8.Manson J.J., Crooks C., Naja M., Ledlie A., Goulden B., Liddle T., et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2(10):e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request.