Abstract
Aims
The clinical reliability of echocardiographic surrogate markers of left ventricular filling pressures (LVFPs) across different cardiovascular pathologies remains unanswered. The main objective was to evaluate the evidence of how effectively different echocardiographic indices estimate true LVFP.
Methods and results
Design: this is a systematic review and meta‐analysis. Data source: Scopus, PubMed and Embase. Eligibility criteria for selecting studies were those that used echocardiography to predict or estimate pulmonary capillary wedge pressure or left ventricular end‐diastolic pressures. Twenty‐seven studies met criteria. Only eight studies (30%) reported both correlation coefficient and bias between non‐invasive and invasively measured LVFPs. The majority of studies (74%) recorded invasive pulmonary capillary wedge pressure as a surrogate for left ventricular end‐diastolic pressures. The pooled correlation coefficient overall was r = 0.69 [95% confidence interval (CI) 0.63–0.75, P < 0.01]. Evaluation by cohort demonstrated varying association: heart failure with preserved ejection fraction (11 studies, n = 575, r = 0.59, 95% CI 0.53–0.64) and heart failure with reduced ejection fraction (8 studies, n = 381, r = 0.67, 95% CI 0.61–0.72).
Conclusions
Echocardiographic indices show moderate pooled association to invasively measured LVFP; however, this varies widely with disease state. In heart failure with preserved ejection fraction, no single echocardiography‐based metric offers a reliable estimate. In heart failure with reduced ejection fraction, mitral inflow‐derived indices (E/e′, E/A, E/Vp, and EDcT) have reasonable clinical applicability. While an integrated approach of several echocardiographic metrics provides the most promise for estimating LVFP reliably, such strategies need further validation in larger, patient‐specific studies.
Keywords: Left ventricular end‐diastolic pressure, Echocardiography, Invasive heart catheterization
Introduction
The primary pathophysiological process in heart failure (HF) is raised intracardiac pressure. 1 For most patients with HF, this is associated with increased left ventricular filling pressure (LVFP) or left ventricular end‐diastolic pressure (LVEDP), caused by either systolic or diastolic impairment of the left ventricle (LV). 2 LVEDP, a strong predictor of morbidity and mortality, has long been regarded as the key measurement for raised LVFP. 2 , 3 , 4 Early diagnosis of raised LVFP is critical to inform the diagnosis of HF and also guide treatment optimization. 5
The current standard approach to LVFP or LVEDP estimation is by transthoracic echocardiography (TTE). TTE allows for a detailed assessment of both LV diastolic and systolic function (Figure 1 ).
Figure 1.
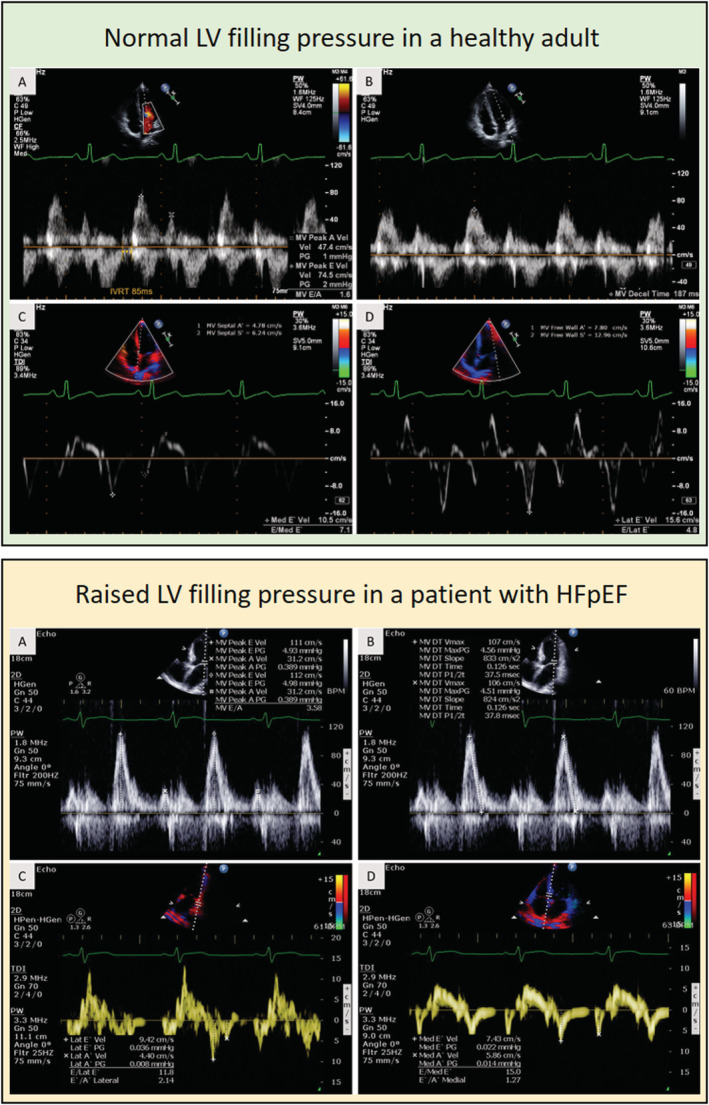
Case examples of Doppler‐based methods for left ventricular filling pressure (LVFP) assessment. The first case (green panel) is a healthy 20‐year‐old male; the second case (yellow panel) is a 60‐year‐old male patient admitted with heart failure (HF). In the healthy adult case, the E/e′ is <8 and suggests normal LVFP vs. in the patient, it is >12 and suggests raised LVFP.
Transthoracic echocardiography methods including mitral inflow, tissue Doppler annular velocities, tricuspid regurgitation velocity (TRV), and left atrial volume (LAV) have been widely studied to estimate LVFP. In particular, E/e′ has been the main focus of the majority of these studies. The current recommendations advocate sub‐categorization of patients depending on LV ejection fraction (LVEF). For patients with reduced LVEF (<50%), they recommend a focus on E/A ratio to estimate LVFP. And for those with preserved LVEF (>50%), a combination of E/e′, e′ velocity, TRV, and left atrial volume indexed for body surface area (LAVi) is advised to predict LVFP. 6
The combined diagnostic accuracy of these TTE methods, to predict LVEDP in different HF states, including heart failure with preserved ejection fraction (HFpEF), heart failure with reduced ejection fraction (HFrEF), and even in valvular heart disease remains unclear. Systematic evidence synthesis is required to develop further understanding of which TTE methods are clinically applicable to predict LVFP in a specific HF state.
The aim of this systematic review and meta‐analysis was, therefore, to summarize current literature, systematically consolidate studies that have estimated LVEDP using TTE, and ascertain their predictive association with invasive measurements in different HF states.
Methods
Systematic review and meta‐analysis registration
This project was registered (CRD42020164642) with, and the ethics approved by, the prospective register of systematic review (PROSPERO).
Eligibility criteria
Eligible studies were those that used TTE to predict or estimate pulmonary capillary wedge pressure (PCWP) or LVEDP. The primary endpoint was the agreement between invasive and non‐invasive methods. This was evaluated by both the correlation coefficient and statistical bias in the respective studies. This study mainly focused on the correlation coefficient (r), and all subsequent meta‐analyses were planned using r values. We limited our search to peer‐reviewed journals, medicine, and human adult (age ≥18 years) participants. Studies with fewer than 12 patients were excluded.
Search strategy
The Scopus database, which incorporates all MEDLINE and Embase results, formed the basis of the literature search, undertaken on 21 November 2019. Search terms included ‘invasive’, ‘noninvasive/echocardiography’, and ‘LVEDP/Pulmonary Capillary Wedge Pressure/PCWP’. The full search strategy is included in the supporting information. An identical search of the Cochrane Central database returned no additional results. An extensive review of the referenced literature identified 11 additional studies. Duplicate results (n = 1) were removed, and the remaining results were combined (n = 207).
Study selection
Preliminary vetting of the results, according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, was undertaken by R. J. The shortlist was subsequently reviewed by an independent assessor, P. G. A third independent expert, A. J. S., was involved to resolve disagreements. The PRISMA flow chart is detailed in Figure 2 .
Figure 2.
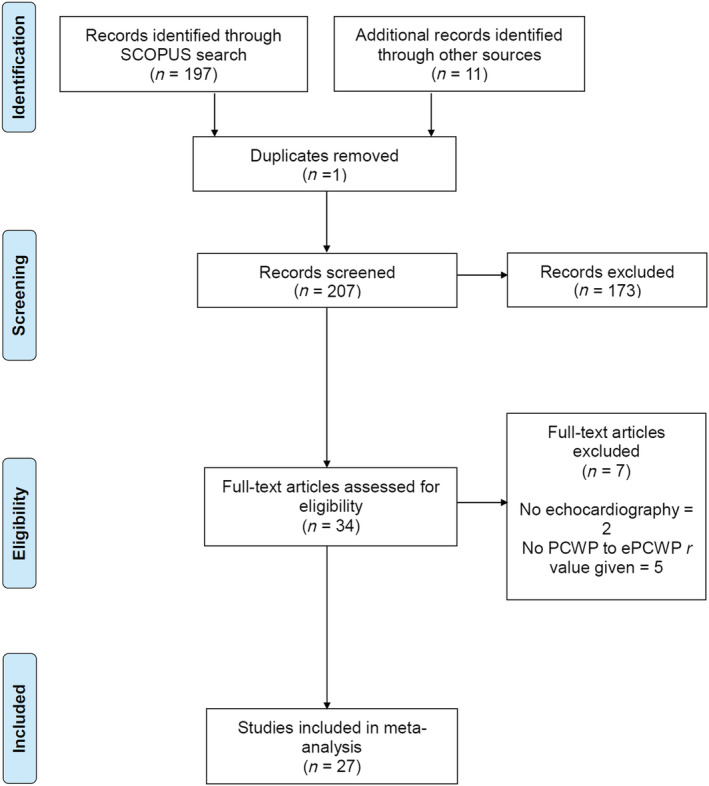
Search strategy flow chart as per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses 2009 guidance.
Assessment of clinical applicability
We adapted the Critical Appraisal Skills Programme (CASP) tool to ensure clinical applicability to the evidence synthesis. This is detailed in the Supporting Information, Tables S1 and S2 .
Statistical analysis and meta‐analysis
Statistical analysis was performed using MedCalc (MedCalc Software, Belgium, Version 19.1.5). We pooled the values of correlation coefficients and numbers recruited in each study for agreement between invasive and non‐invasive LVFPs. Detailed statistical methods are described in the supporting information.
Results
The initial search identified 197 studies. A further 11 studies were identified through a thorough review of the referenced literature. After an initial screening of title and abstract, 34 research outcome papers were evaluated, and from these, 27 articles met the inclusion criteria 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 (Figure 2 ). The total number of patients studied across all the 27 studies was 2058.
Study characteristics
All studies were diagnostic cohort studies, using the invasive haemodynamic study as the reference test for LVFP. Consecutive patient recruitment was reported in 17 studies. Except for Nagueh et al. 27 and Andersenet al., 32 all studies were single tertiary centre studies. All studies were published between 1997 and 2019. While some studies focused on specific disease states such as HFrEF (n = 8) and HFpEF (n = 11), some recruited heterogeneous patient populations (n = 11). From the 27 studies, only 8 studies (30%) reported both correlation coefficient and bias (Supporting Information, Table S3 ). The majority of studies (74%, 20/27) recorded invasive PCWP as a surrogate for LVEDP. Seven studies recorded LVEDP directly. 10 , 14 , 18 , 22 , 24 , 30 , 31
Meta‐analysis results for all studies
The pooled analysis across all studies showed a statistically significant association of invasive haemodynamic assessment to echocardiographic methods—the weighted average (random effects) correlation coefficient for all the 27 studies was 0.69 [95% confidence interval (CI) 0.63–0.75, P < 0.01] (Supporting Information, Table S4 ). Figure 3 shows the forest plot of the pooled correlation coefficients, CIs, and percentage weighting for all studies included. Visual scrutiny of the forest plots suggests that a degree of between‐study variation exists in terms of the effect sizes. I 2 values of 81.6% confirmed significant levels of heterogeneity.
Figure 3.
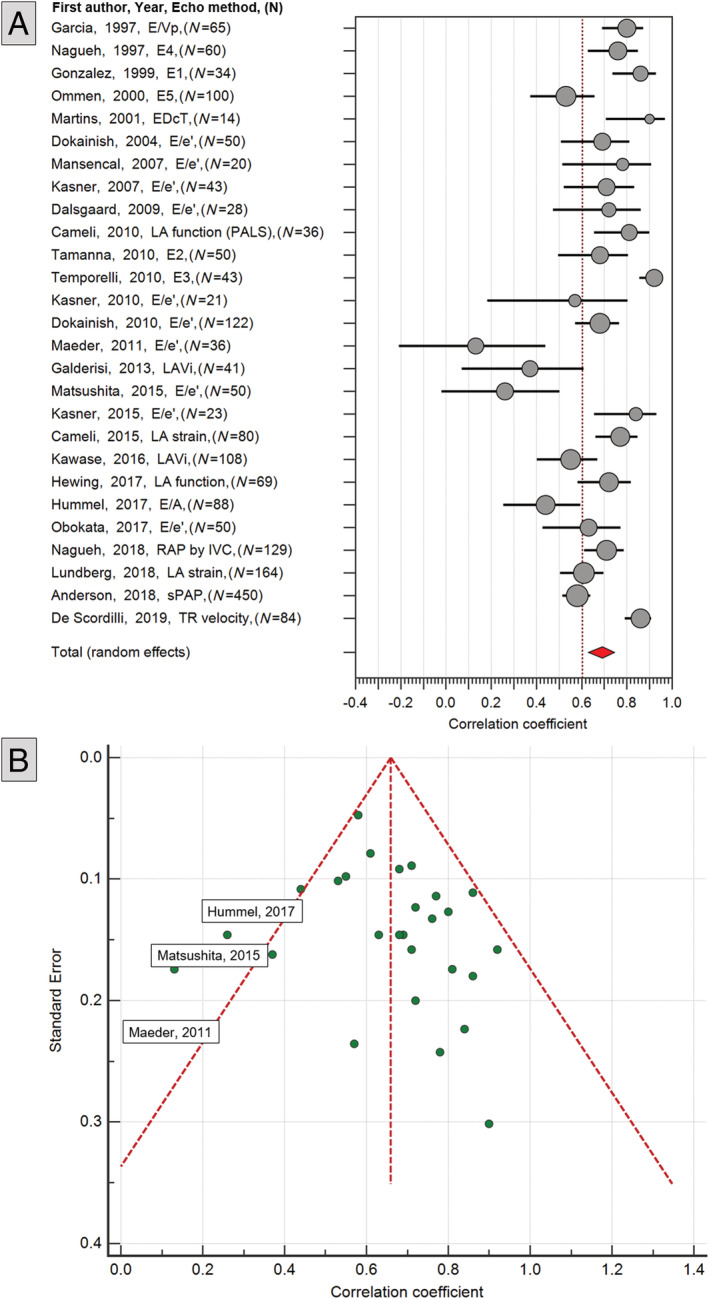
(A) Forest plot of all the 23 studies evaluated in meta‐analysis for non‐invasive assessment of left ventricular filling pressure. (B) Funnel plot of all the 23 studies.
Temporelli et al. demonstrated the highest correlation coefficient with invasive measurement (r = 0.92, 95% CI 0.86–0.96), using an integrated mitral inflow velocity (MIV) equation (Figure 3 ). 29 However, they recruited only 43 patients, reducing the overall weight of the study. The study by Anderson et al. had the highest weight, closely followed by the Lundberg et al. work into left atrial (LA) strain. 32 , 33
Eight studies reported bias in the agreement between echocardiographic modelled LVFP vs. invasively measured LVFP. The maximum bias (bias = 9 mmHg) was reported by Maeder et al. in an HFpEF cohort (Supporting Information, Table S3 ). 19 In HFrEF, the bias was comparatively less for the two studies by Cameli et al. and Temporelli et al. 16 , 29
Meta‐analysis results for echocardiography metric
The first group, MIVs, had a pooled (random effects) correlation coefficient of 0.58 (95% CI 0.46–0.68, P < 0.01) (Figure 4 , Supporting Information, Table S5 ). Four MIV studies comprised a heterogeneous patient population: two studies investigated HFrEF (n = 91), and two investigated coronary artery disease (CAD) (n = 56). The study with the highest correlation was that by Martins et al. (r = 0.90, 95% CI 0.71–0.97). 11 However, this study had the lowest weighting within this category, recruiting only 14 patients. The most highly weighted study was that by Anderson et al. (n = 450), showing moderate association between E/A and invasive LVFP (r = 0.53). 32 The most widely studied MIV parameter was E/A (n = 665). Other echocardiography metrics studied included E/Vp (n = 85) and EDcT (n = 64).
Figure 4.
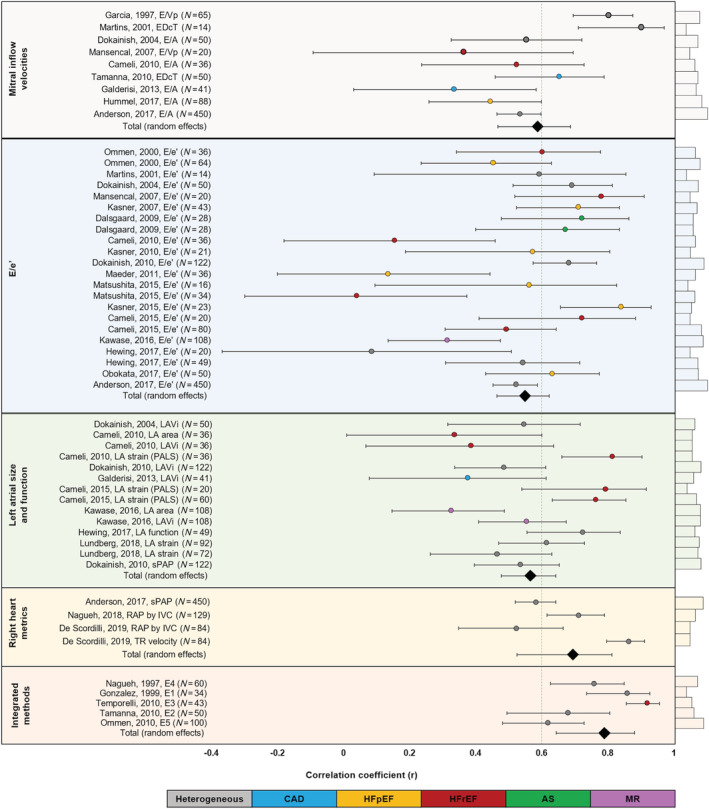
Forest plots of all the methods applied by echocardiography for non‐invasive assessment of left ventricular filling pressure.
The second category, E/e′, was the largest, incorporating 17 studies and 1348 patients [HFpEF, n = 253; HFrEF, n = 126; heterogeneous, n = 785; aortic stenosis (AS), n = 28; mitral regurgitation (MR), n = 108]. E/e′ had a pooled random effects correlation coefficient of 0.55 (95% CI 0.46–0.62, P < 0.01, z = 11). Correlation coefficients varied greatly between studies, ranging from 0.08 (95% CI −0.38 to 0.5) (Hewing et al.) to 0.84 (95% CI 0.65–0.93) (Kasner et al.). 22 , 24 The Kawase et al. study investigating E/e′ in MR patients (r = 0.33) recruited 108 patients, contributing significant weight in reducing the pooled correlation coefficient. 23
The third major parameter studied was LA size and function. Overall, these studies had a pooled random effects correlation of 0.56 (95% CI 0.47–0.64, P < 0.01, z = 10, n = 952). Of these, LAVi was investigated in five studies, including 357 patients; LA area was also investigated in 144 patients, and LA strain assessments were undertaken in 280 patients. One study evaluated LAVi in MR cases (n = 108) and found a correlation coefficient of 0.55 (95% CI 0.40–0.67) (Kawase et al.), much stronger than that found for LA area alone (r = 0.33, 95% CI 0.00–0.59). 23 Of the LA parameters, LA strain assessment had the strong correlations, ranging from 0.46 (Lundberg et al.) to 0.81 (Cameli et al.).
Assessment of the right heart comprised three studies and is the most novel category, with all studies published since 2017. These studies combined had a strong correlation coefficient of 0.69 (95% CI 0.52–0.811 P < 0.01, z = 6.1, n = 747). The largest study was by Anderson et al., investigating systolic pulmonary artery pressure (r = 0.58, 95% CI 0.52–0.64). 32 Nagueh et al. 27 and de Scordilli et al. investigated right atrial pressure, with the latter also studying TRV. 28 TRV had a stronger correlation (r = 0.86, 95% CI 0.79–0.91), compared with right atrial pressure; however, this is weighted lower due to fewer patient numbers. All studies recruited heterogeneous populations. The five integrated approach studies (n = 287) combined several echocardiographic parameters into equations (Table 1 ) to estimate LVFP. This category had the highest weighted average (random effects) correlation coefficient at 0.79 (95% CI 0.64–0.88, P < 0.001, z = 6.84). Ommen et al. had the highest weighting at 28.6% (n = 100) but also had the lowest correlation of all the equations (r = 0.62, 95% CI 0.48–0.73) and the lowest clinical applicability of all studies. 10 In comparison, Temporelli et al. found a correlation of 0.92 (95% CI 0.86–0.96) using an equation focusing on MIV and had excellent clinical applicability. 29 The integrated approach studies incorporated a range of patient cohorts; Nagueh et al. and Gonzalez‐Vilchez et al. studied heterogeneous populations (n = 95), Ommen et al. and Temporelli et al. recruited HFrEF patients (n = 63), Ommen et al. further studied 64 patients with HFpEF, and Tamanna et al. investigated CAD (n = 50). 8 , 9 , 10 , 17 , 29
Table 1.
Integrated equations identified in the literature for estimating left ventricular filling pressure
| Abbreviation | First author | Year | n | Approach | Disease state |
|---|---|---|---|---|---|
| E1 | Gonzalez‐Vilchez | 1999 | 54 | 103/([2·IVRT] + FPV) | Heterogeneous |
| E2 | Tamanna | 2010 | 50 | 1.43 × DR + 1.32 × E/A − 0.024 × DT + 0.02 × MLAV + 9.2 | CAD |
| E3 | Temporelli | 2010 | 43 | 32.16 + (−0.1045E) + (0.1345A) + (−0.17 DT) + (4.95 E/A) | HFrEF |
| E4 | Nagueh | 1997 | 125 | 1.91 + (1.24*E/e′) | Heterogeneous |
| E5 | Ommen | 2000 | 100 | 11.96 + (0.596*E/e′) | HFpEF + HFrEF |
CAD, coronary artery disease; DR, deceleration rate; DT, deceleration time; FPV, flow propagation velocity; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IVRT, isovolumetric relaxation time; MLAV, maximal left atrial volume.
Meta‐analysis results for disease
From the 27 eligible studies, five primary patient cohorts were recruited: HFpEF, HFrEF, CAD, AS, and MR. Furthermore, 11 studies did not specify diagnoses, grouped into ‘heterogeneous’ for the purpose of this meta‐analysis.
The pooled analysis across all cohorts revealed a modest association between echocardiographic and invasive haemodynamic assessment (r = 0.64, 95% CI 0.56–0.71, P < 0.01, z = 11.9) (Figure 5 , Supporting Information, Table S6 ).
Figure 5.
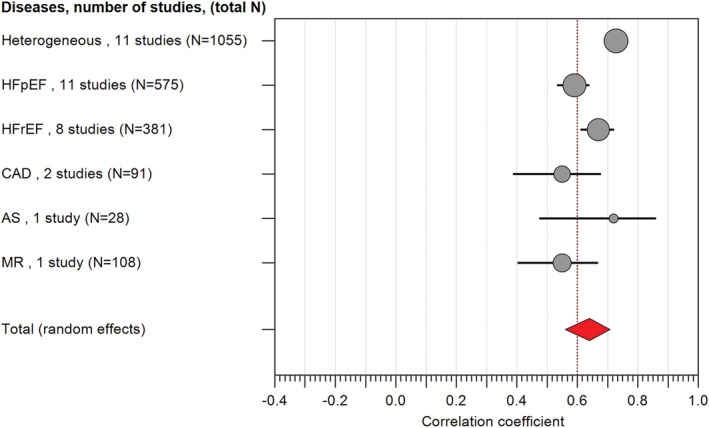
Forest plots of all the heart disease states in which echocardiography has been tested for non‐invasive estimation of left ventricular filling pressure. It is worth noting that the heterogeneous group represented the most patients in the total meta‐analysis (40.6%).
In total, 1055 patients were categorized as ‘heterogeneous’, and this group demonstrated the best association with the least effect size (r = 0.73, 95% CI 0.70–0.76) and highest weighting (22.16%). A small proportion of these patients were intubated in intensive care (n = 160). HFpEF (r = 0.59, 95% CI 0.53–0.64, n = 575) patients were found to have a lower correlation coefficient than HFrEF patients (r = 0.67, 95% CI 0.61–0.72, n = 381). Patients recruited with a diagnosis of CAD and MR demonstrated the lowest correlation coefficient (r = 0.55, 95% CI 0.38–0.68, n = 91 and r = 0.55, 95% CI 0.40–0.67, n = 108, respectively).
Clinical applicability (Critical Appraisal Skills Programme) results
The modified CASP tool showed that 19% (n = 5) of studies were ‘highly clinically applicable’, 56% (n = 15) ‘moderately clinically applicable’, and 26% (n = 7) ‘less clinically applicable’. Of those ‘less clinically applicable’, most failed to report bias or undertake suitable reproducibility assessments (Supporting Information, Table S2 ). Three studies scored 100%; all evaluated a heterogeneous cohort.
Discussion
This systematic review and meta‐analysis found that non‐invasive echocardiographic estimates of LVFP demonstrate moderate association to invasive right heart catheterization. Five echocardiographic methods were evaluated, highlighting the integrated approach as best correlated. However, evidence for the integrated approach lacked in all HF phenotypes, particularly HFpEF. Of the single approaches, LA strain showed promising results.
Principal findings
This meta‐analysis demonstrates that echocardiographic approaches to estimating LVFP are feasible and can be applied clinically. However, there was significant heterogeneity in both the quality of studies and their findings. Many studies recruited a heterogeneous cohort of patients, which makes it challenging to apply their methods to predict LVFP in groups of HF patients.
In patients with MR, Kawase et al. demonstrated that LA volume and function (minimum LAVi and KT index, a marker of LV function) appears to have a better correlation to invasive PCWP than E/e′. 23 The main limitation was the poor reproducibility for the novel KT index. In patients with AS, a single small study demonstrated promising results for E/e′ (r = 0.72, 95% CI 0.47–0.86), but more evidence is needed to predict LVFP in this cohort. We did not identify any study that has developed personalized non‐invasive models in either mitral stenosis or aortic regurgitation.
Patients with HFpEF represent the second largest group where LVFP has been studied non‐invasively. The pooled correlation with invasive LVFP was modest. Maeder et al. demonstrated that E/e′ has a weak association to LVFP in patients with HFpEF. 19 Two other studies by Hummel et al. and by Ommen et al. again confirmed a poor correlation of LVEDP to E/e′. 10 , 25 The only study showing promising correlation and high clinical applicability within HFpEF was Obokata et al. (r = 0.63, 95% CI 0.43–0.77, n = 50). 26 They also identified the incremental role of non‐invasive stress E/e′ to improve diagnostic sensitivity.
In HFrEF, the pooled correlation coefficient was much stronger for clinical translation across five studies. However, only one study demonstrated excellent clinical applicability for estimating LVFP by an integrated equation focusing on MIV profile (E3) (Temporelli et al.). 29 The main limitation of this proposed equation is that no validation via a multicentre trial has been undertaken to support widespread clinical adoption. Cameli et al. demonstrated a strong correlation using LA strain parameters in HFrEF in a small study of 36 patients. In patients with CAD, the pooled association was only modest, and both studies that specifically recruited CAD patients demonstrated only medium to limited clinical applicability.
Further, our study is unique due to its prominent inclusion of heterogeneous patient populations, consisting of a number of cardiac pathologies as well as patients with normal LVFP. While the algorithm laid out in ASE/EACVI 2016 recommendations excludes patients without cardiac pathologies, 6 we decided a heterogeneous cohort better matches that seen in clinical practice, where diagnoses are often unclear at the time the algorithm is applied. 34 This decision is supported by our finding that the most clinically applicable methods comprised the heterogeneous cohorts. 7 , 8 , 9 , 28 These studies demonstrated good association and scored high on the modified CASP. However, one of these studies was on only intubated patients. 7 Nonetheless, Nagueh et al. recruited a combination of intubated and non‐intubated patients to derive their non‐invasive equation and overall, demonstrated a high correlation coefficient of 0.87. 8 However, overall, there is a lack of clarity of evidence in HFpEF patients supporting the routine clinical use of E/e′ as an accurate estimate of LVFP.
Comparisons with other recent meta‐analyses
Sharifov et al. undertook a systematic review and meta‐analysis of 24 studies investigating the correlation between invasive LVFP using non‐invasive E/e′ in patients with HFpEF. 35 However, there are differences between ours and the Sharifov et al. work. Firstly, the majority of their included studies did not perform simultaneous echocardiography and right heart catheterization. Our study has addressed this issue, with 26/27 of our included studies having simultaneous investigation. Sharifov et al. concluded a lack of evidence for a substantive correlation with E/e′, requiring further validation, and this statement is supported by our findings. Furthermore, Nauta et al. also performed a systematic review studying the correlation between echocardiographically estimated and invasively measured LVFP in patients with HFpEF and again found a modest correlation using the E/e′ method. 36 This study further considered the prognostic relevance of this, demonstrating improvements when using multiple parameters simultaneously. These conclusions strengthen our findings on the utility of integrated approaches. These studies, however, are limited to HFpEF cohorts. Our meta‐analysis has divided patients by a variety of diagnoses, providing a broader view of the usefulness of echocardiography in estimating LVFP in HF.
Context, implications for health policy, and future research
It is clear that one single non‐invasive echocardiographic parameter cannot reliably estimate LVFP across all cardiovascular pathologies. Importantly, in HFpEF, where it is critically important to have such a non‐invasive diagnostic tool, we have very limited evidence to support any current single or even integrated method by echocardiography. This meta‐analysis supports previous publications calling for further research to validate non‐invasive LVFP estimates in HFpEF. Future studies should consider robust clinical validation including reproducibility tests, which was identified as one of the reasons why some studies were less clinically applicable. When reporting correlation coefficients, the studies should also report either bias or standard error, between non‐invasive and invasive methods. In patients with HFrEF, the evidence is much stronger, and a cautious approach to inform LVFP can be applied.
Limitations
In this systematic review and meta‐analysis, we did not include studies specifically looking at the diagnostic accuracy of any echocardiographic metric or integrated equation on a categorical scale. Hence, we did not report studies that have performed sensitivity/specificity analysis. We did this intentionally to investigate the true continuum of any non‐invasive method vs. the reference invasive method. In addition, this work did not evaluate the prognostic role of non‐invasive echocardiographic parameters.
Conclusions
Pooled echocardiographic indices show moderate association to invasively measured LVFP; however, this varies with cardiovascular pathology. In HFpEF or valvular heart disease, no single echocardiography measure offers a reliable estimate of LVFP. In HFrEF patients, mitral inflow‐derived indices demonstrate promise for routine clinical use. Most studies included heterogeneous patient groups and thus cannot be applied to a precision medicine approach. Integrated methods incorporating several echocardiographic metrics appear most promising for estimating LVFP reliably but require further validation in larger, patient‐specific studies.
Conflict of interest
None declared.
Funding
This work was supported by Wellcome Trust grants (A.R.—206632/Z/17/Z), (P.G.‐220703/Z/20/Z) and (A.J.S.—205188/Z/16/Z). L.Z. was supported by the National Medical Research Council (NMRC/OFIRG/0018/2016). P.G. was supported by the Academy of Sciences Starter Grant (SGL018\1100).
Supporting information
Table S1. The modified CASP questions used in the systematic review process.
Table S2. Full results of the Modified Critical Appraisal Skills Programme (CASP) tool results.
Table S3. Studies reporting bias between invasively measured versus non‐invasively predicted LVEDP/PCWP.
Table S4. All the 27 studies which were pooled together in systematic review for further meta‐analysis with respective random effects weighting in percentage (%).
Table S5. Data of all echocardiographic methods studied for their association to LV filling pressure.
Table S6. Supplementary data to Figure 3a showing correlation between echocardiographically estimated LVEDP and invasive measurements in different disease states.
Jones, R. , Varian, F. , Alabed, S. , Morris, P. , Rothman, A. , Swift, A. J. , Lewis, N. , Kyriacou, A. , Wild, J. M. , Al‐Mohammad, A. , Zhong, L. , Dastidar, A. , Storey, R. F. , Swoboda, P. P. , Bax, J. J. , and Garg, P. (2021) Meta‐analysis of echocardiographic quantification of left ventricular filling pressure. ESC Heart Failure, 8: 566–576. 10.1002/ehf2.13119.
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as supporting information.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Brienesse SC, Davies AJ, Khan A, Boyle AJ. Prognostic value of LVEDP in acute myocardial infarction: a systematic review and meta‐analysis. J Cardiovasc Transl Res 2018; 11: 33–35. [DOI] [PubMed] [Google Scholar]
- 3. Fruhwald FM, Dusleag J, Eber B, Fruhwald S, Zweiker R, Klein W. Long‐term outcome and prognostic factors in dilated cardiomyopathy. Preliminary results. Angiology 1994; 45: 763–770. [DOI] [PubMed] [Google Scholar]
- 4. Nagendran J, Norris CM, Appoo JJ, Ross DB, Nagendran J. Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Left ventricular end‐diastolic pressure predicts survival in coronary artery bypass graft surgery patients. Ann Thorac Surg 2014; 97: 1343–1347. [DOI] [PubMed] [Google Scholar]
- 5. Satpathy C, Mishra TK, Satpathy R, Satpathy HK, Barone EJ. Diagnosis and management of diastolic dysfunction and heart failure. American family physician 2006; 73: 841–846. [PubMed] [Google Scholar]
- 6. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 7. Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD. An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol 1997; 29: 448–454. [DOI] [PubMed] [Google Scholar]
- 8. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997; 30: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez‐Vilchez F, Ares M, Ayuela J, Alonso L. Combined use of pulsed and color M‐mode Doppler echocardiography for the estimation of pulmonary capillary wedge pressure: an empirical approach based on an analytical relation. J Am Coll Cardiol 1999; 34: 515–523. [DOI] [PubMed] [Google Scholar]
- 10. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures. Circulation 2000; 102: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 11. Martins S, Soares RM, Branco L, Salomão S, Antunes AM. Non‐invasive monitoring of pulmonary capillary wedge pressure in heart failure. Eur J Heart Fail 2001; 3: 41–46. [DOI] [PubMed] [Google Scholar]
- 12. Dokainish H, Zoghbi WA, Lakkis NM, Al‐Bakshy F, Dhir M, Quinones MA, Nagueh SF. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B‐type natriuretic peptide in patients with pulmonary artery catheters. Circulation 2004; 109: 2432–2439. [DOI] [PubMed] [Google Scholar]
- 13. Mansencal N, d'Allonnes LR, Beauchet A, Fabre S, Digne F, Farcot J‐C, Joseph T, Dubourg O. Reliability of echocardiography for hemodynamic assessment of end‐stage heart failure. Am J Cardiol 2007; 100: 998–1001. [DOI] [PubMed] [Google Scholar]
- 14. Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss H‐P, Pauschinger M, Tschöpe C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction. Circulation 2007; 116: 637–647. [DOI] [PubMed] [Google Scholar]
- 15. Dalsgaard M, Kjaergaard J, Pecini R, Iversen KK, Køber L, Moller JE, Grande P, Clemmensen P, Hassager C. Left ventricular filling pressure estimation at rest and during exercise in patients with severe aortic valve stenosis: comparison of echocardiographic and invasive measurements. J Am Soc Echocardiogr 2009; 22: 343–349. [DOI] [PubMed] [Google Scholar]
- 16. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 2010; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamanna RJ, Jahan R, Zaher A, Akhanda AK. Correlation of Doppler echocardiography with cardiac catheterization in estimating pulmonary capillary wedge pressure: a tertiary level hospital finding in Bangladesh. Univ Heart J 2008; 4: 1–8. [Google Scholar]
- 18. Kasner M, Gaub R, Sinning D, Westermann D, Steendijk P, Hoffmann W, Schultheiss HP, Tschope C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure‐volume loop analysis. Eur J Echocardiogr 2010; 11: 743–751. [DOI] [PubMed] [Google Scholar]
- 19. Maeder MT, Karapanagiotidis S, Dewar EM, Gamboni SE, Htun N, Kaye DM. Accuracy of Doppler echocardiography to estimate key hemodynamic variables in subjects with normal left ventricular ejection fraction. J Card Fail 2011; 17: 405–412. [DOI] [PubMed] [Google Scholar]
- 20. Galderisi M, Rapacciuolo A, Esposito R, Versiero M, Schiano‐Lomoriello V, Santoro C, Piscione F, De Simone G. Site‐dependency of the E/e′ ratio in predicting invasive left ventricular filling pressure in patients with suspected or ascertained coronary artery disease. Eur Heart J ‐ Cardiovasc Imag 2013; 14: 555–561. [DOI] [PubMed] [Google Scholar]
- 21. Matsushita K, Minamishima T, Goda A, Ishiguro H, Kosho H, Sakata K, Satoh T, Yoshino H. Comparison of the reliability of E/E′ to estimate pulmonary capillary wedge pressure in heart failure patients with preserved ejection fraction versus those with reduced ejection fraction. Int J Cardiovasc Imaging 2015; 31: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 22. Kasner M, Sinning D, Burkhoff D, Tschöpe C. Diastolic pressure‐volume quotient (DPVQ) as a novel echocardiographic index for estimation of LV stiffness in HFpEF. Clin Res Cardiol Off J Ger Card Soc 2015; 104: 955–963. [DOI] [PubMed] [Google Scholar]
- 23. Kawase Y, Kawasaki M, Tanaka R, Nomura N, Fujii Y, Ogawa K, Sato H, Miyake T, Kato T, Tsunekawa T, Okubo M, Tsuchiya K, Tomita S, Matsuo H, Minatoguchi S. Noninvasive estimation of pulmonary capillary wedge pressure in patients with mitral regurgitation: a speckle tracking echocardiography study. J Cardiol 2016; 67: 192–198. [DOI] [PubMed] [Google Scholar]
- 24. Hewing B, Theres L, Spethmann S, Stangl K, Dreger H, Knebel F. Left atrial strain predicts hemodynamic parameters in cardiovascular patients. Echocardiography 2017; 34: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 25. Hummel YM, Liu LCY, Lam CSP, Fonseca‐Munoz DF, Damman K, Rienstra M, Van der Meer P, Rosenkranz S, Van Veldhuisen DJ, Voors AA, Hoendermis ES. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail 2017; 19: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 26. Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction. Circulation 2017; 135: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagueh SF, Smiseth OA, Dokainish H, Andersen OS, Abudiab MM, Schutt RC, Kumar A, Gude E, Sato K, Harb SC, Klein AL. Mean right atrial pressure for estimation of left ventricular filling pressure in patients with normal left ventricular ejection fraction: invasive and noninvasive validation. J Am Soc Echocardiogr 2018; 31: 799–806. [DOI] [PubMed] [Google Scholar]
- 28. de Scordilli M, Pinamonti B, Albani S, Gregorio C, Barbati G, Daneluzzi C, Korcova R, Perkan A, Fabris E, Geri P, Biolo M, Lo Giudice F, Confalonieri M, Sinagra G. Reliability of noninvasive hemodynamic assessment with Doppler echocardiography: comparison with the invasive evaluation. J Cardiovasc Med 2019; 20: 682–690. [DOI] [PubMed] [Google Scholar]
- 29. Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P. Doppler echocardiography in advanced systolic heart failure. Circ Heart Fail 2010; 3: 387–394. [DOI] [PubMed] [Google Scholar]
- 30. Dokainish H, Nguyen J, Sengupta R, Pillai M, Alam M, Bobek J, Lakkis N. New, simple echocardiographic indexes for the estimation of filling pressure in patients with cardiac disease and preserved left ventricular ejection fraction: New diastolic indices. Echocardiography 2010; 27: 946–953. [DOI] [PubMed] [Google Scholar]
- 31. Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D'Ascenzi F, Focardi M, Favilli R, Pierli C, Fineschi M, Mondillo S. Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end‐diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography 2016; 33: 398–405. [DOI] [PubMed] [Google Scholar]
- 32. Andersen OS, Smiseth OA, van der Dokainish H, Abudiab MM, Schutt RC, van Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha J‐W, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017; 69: 1937–1948. [DOI] [PubMed] [Google Scholar]
- 33. Lundberg A, Johnson J, Hage C, Bäck M, Merkely B, Venkateshvaran A, Lund LH, Nagy AI, Manouras A. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin Res Cardiol. 2019;108: 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355: 251–259. [DOI] [PubMed] [Google Scholar]
- 35. Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic accuracy of tissue doppler index E/è for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: A systematic review and meta‐analysis. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2016;25: e002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20: 1303–1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The modified CASP questions used in the systematic review process.
Table S2. Full results of the Modified Critical Appraisal Skills Programme (CASP) tool results.
Table S3. Studies reporting bias between invasively measured versus non‐invasively predicted LVEDP/PCWP.
Table S4. All the 27 studies which were pooled together in systematic review for further meta‐analysis with respective random effects weighting in percentage (%).
Table S5. Data of all echocardiographic methods studied for their association to LV filling pressure.
Table S6. Supplementary data to Figure 3a showing correlation between echocardiographically estimated LVEDP and invasive measurements in different disease states.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supporting information.


