Abstract
Aims
There is no quality of life tool specifically developed for patients with severe aortic stenosis (AS) to assess how this chronic condition and its treatment affect patients. The Toronto Aortic Stenosis Quality of Life Questionnaire (TASQ) has been developed to overcome this gap. The results of the validation of the TASQ in patients undergoing treatment for severe AS are presented.
Methods and results
Prospective study at 10 centres in Europe and Canada, which enrolled 274 patients with severe symptomatic AS undergoing surgical or transcatheter aortic valve replacement. Mean TASQ score at baseline was 71.2 points and increased to 88.9 three months after aortic valve implantation (P < 0.001). Increases were seen for the emotional impact (32.0 to 39.0; P < 0.001), physical limitations (14.8 to 22.0; P < 0.001), and physical symptoms (8.5 vs. 11.0; P < 0.001) domains. Internal consistency was good/excellent for overall TASQ score (α = 0.891) and for the physical limitation, emotional impact, and social limitation domains (α = 0.815–0.950). Test–retest reliability was excellent or strong for the overall TASQ (intraclass correlation coefficient of 0.883) and for the physical symptoms, physical limitation, emotional impact, and social limitation domains (intraclass correlation coefficient of 0.791–0.895). Responsiveness was medium overall (Cohen's d = 0.637) and medium/large for physical symptoms, emotional impact, and physical limitations (0.661–0.812). Sensitivity to change was significant for physical symptoms, physical limitations (both P < 0.001), emotional impact (P = 0.003), and social limitations (P = 0.038).
Conclusions
The TASQ is a new, brief, self‐administered, and clinically relevant health‐specific tool to measure changes in quality of life in patients with AS undergoing an intervention.
Keywords: Aortic stenosis, Aortic valve replacement, Quality of life, Questionnaire
Introduction
Chronic heart failure (HF) is characterized by congestion of the lungs, shortness of breath, and a decline in physical capacity. Most causes of HF such as myocardial infarction are irreversible, and treatment options include pharmacotherapy and implantable supporting devices. HF may also be due to aortic valve stenosis (AS), which is a common condition in the elderly 1 but tends to be asymptomatic for a very long time. As soon as symptoms (e.g. angina, shortness of breath, and dizziness/syncope) develop, the prognosis is significantly impaired, as is quality of life (QoL). The symptoms resemble the clinical presentation of HF; however, AS is curable in principle, and replacement of the stenosed valve reinstates the non‐diseased state.
A gain in quality‐adjusted life years is the ultimate goal for the treatment of both common HF and AS‐related HF. Specific questionnaires, such as the Kansas City Cardiomyopathy Questionnaire (KCCQ), were developed as a health status measure for patients with common forms of HF. Because of the similarities with symptoms seen in AS‐related HF, the KCCQ has also been used extensively in aortic valve (AV) replacement trials. 2 , 3 , 4 However, the KCCQ is focused on HF‐specific symptoms and may not capture symptoms that are specific to severe AS as well 5 where the left ventricular function is often normal. It also fails to take into account features relevant prior to and after undergoing an AV intervention, such as surgical AV replacement (SAVR) or transcatheter AV implantation (TAVI), and is unlikely to be able to differentiate between outcomes after the various procedures.
It is for these reasons that the Toronto Aortic Stenosis Quality of Life Questionnaire (TASQ) was developed. 5 , 6 This questionnaire reflects AS‐specific symptoms and how they affect a patient's physical and mental well‐being, as well as evaluating the patient's assessment of their general health. It is short, convenient to use and dedicated for patients with AS. It has been shown to provide an accurate picture of QoL in patients with severe AS before and after treatment. 5 , 6 Here, we present the results of the validation of the TASQ in patients undergoing treatment for severe AS, based on a prospective, multinational registry.
Methods
The TASQ registry was a prospective observational registry with a follow‐up period of 3 months. 5 , 6 Patients with severe symptomatic AS were recruited from 10 centres in nine countries in Europe (Austria/Germany, 2 France, 2 Italy, 2 Spain, 2 and the UK 1 ) and one centre in Canada with the intention to have at least two sites for each language. Patients either underwent transfemoral (TF) TAVI using the balloon expandable SAPIEN 3 valve (Edwards Lifesciences) or SAVR using any commercially available surgical valve. The treatment decision was made by the heart team at each centre, based on standard in‐house protocols, and was independent of the study. Patients were excluded from the study if they were unable to complete the questionnaire due to cognitive impairment. The study protocol was approved by the independent ethics committee or institutional review board at each centre. The registry was conducted in accordance with the Declaration of Helsinki and its amendments, as well as country‐specific laws and regulations. Patients were required to provide written informed consent.
Toronto Aortic Stenosis Quality of Life Questionnaire, Kansas City Cardiomyopathy Questionnaire, and Short Form‐12 version 2 questionnaires
For the purpose of this registry, the TASQ was produced in English (available open access 5 , 6 ) and translated into French, German, Italian, and Spanish. Validated translations were performed in the target countries and supervised by an experience Clinical Outcomes specialist. Questionnaires were forward translated twice and back translated once by qualified translators, followed by a cognitive interview on five patients with a heart condition. Questionnaires were then proofread and released. For comparative purposes, patients were required to complete the TASQ, the KCCQ, and the Short Form‐12 version 2 (SF‐12v2) at baseline prior to the intervention, pre‐discharge, and at 30 days and 3 months of follow‐up. The three questionnaires were given to the patient sequentially, but in a random order.
The scoring of the TASQ 6 is based on a consistent 7‐point scale for each of the 16 questions, covering response options from ‘not very much’ to ‘very much’. The TASQ consists of five domains: physical symptoms (Questions 1 and 14), physical limitations (Questions 3, 6, 7, and 15), emotional impact (Questions 2 and 8 to 13), social limitations (Questions 4 and 5), and health expectations (Question 16). Each question has a maximum score of 7, giving the complete questionnaire a maximum total score of 112 with a higher score indicating improved QoL. The full questionnaire is available at www.tasq-q.com.
The KCCQ 7 is a 23‐item self‐administered questionnaire that addresses specific health domains: physical limitation, symptoms, QoL, social limitation, symptom stability, and self‐efficacy—the first four of which are combined into an overall summary scale. Values for the domains range from 0 to 100, with higher scores indicating lower symptom burden and better QoL. The self‐efficacy domain is designed to assess whether or not patients feel they have the knowledge and skills to manage their HF as an outpatient. The KCCQ has been used in several AS‐related analyses. 3 , 8 , 9
Generic health status was assessed with the SF‐12v2. The SF‐12 is a reliable and valid measure of generic health status that provides overall physical and mental component summary scores. 10 Scores are standardized using norm‐based methods to generate a mean of 50 and an SD of 10, with higher scores indicating better health status. 11 The maximum score for both physical and mental component summary scores is 100.
Study objectives
The principal objective of the registry was to validate the TASQ in patients with severe symptomatic AS undergoing valve replacement.
Statistical analysis
The overall analytic approach is outlined in Supporting Information, Table S1 . The internal consistency of items in the TASQ was measured using Cronbach's alpha. Construct validity was correlated with the KCCQ, the SF‐12v2 Physical and Mental Component Scores, and the New York Heart Association (NYHA) class as applicable. Floor effects were calculated by comparing the number of patients scoring the worst possible score on the TASQ to the number of the patients scoring the worst possible score on the KCCQ. Ceiling effects were calculated in the same manner. Responsiveness and sensitivity to change before and after the AV procedures and at the follow‐up time points were analysed with paired t‐tests. Furthermore, because of normally distributed values, t‐tests were used to compare baseline to pre‐discharge, 30 days and 3 months of outcomes, and to compare 30 days to 3 months in terms of both QoL and patient expectations. Changes between pre‐discharge, 30 days and 3 months of test results measured responsiveness and sensitivity to change. Lastly, comparison analyses of QoL among the three QoL tools (TASQ, KCCQ, and SF‐12v2) were performed to identify interactions between procedural group and measurement tool. The overall summary score was correlated to the NYHA class, the KCCQ, and the SF‐12v2.
Results
Patient population
A total of 274 patients (137 undergoing TAVI and 137 undergoing SAVR) were enrolled. Questionnaires were applied in English (n = 64), French (n = 49), German (n = 49), Italian (n = 64), and Spanish (n = 48). The average age of patients was 77.6 years, most were male (62.8%), and the mean Society of Thoracic Surgeons risk score was 3.83 (Supporting Information, Table S2 ). Most patients were independent, with a mean Katz Index of 5.80 (max. score of 6.0) and an Instrumental Activities of Daily Living score of 6.88 (max. score of 8.0). All patients had normal cognitive capabilities (mean Mini Mental State Examination‐2 score of 26.2; max. score of 30).
Toronto Aortic Stenosis Quality of Life Questionnaire, Kansas City Cardiomyopathy Questionnaire, and Short Form‐12 version 2 over 3 months
There was a steady increase in the mean TASQ total score from baseline (71.2) to 3 months of post‐treatment follow‐up (88.9), with a mean difference of 17.7 points (Figure 1 ). This increase was steady and statistically significant for the emotional impact (39.0 vs. 32.0 points), physical limitations (22.0 vs. 14.8 points), and physical symptoms (11.0 vs. 8.5 points) domains (each P < 0.001). On the other hand, the score for social limitations dropped to 9.6 at discharge from a baseline value of 10.2; it increased to 12.0 at 3 months. Health expectations were essentially flat with a slight decline from 5.8 to 4.8 at 3 months.
Figure 1.
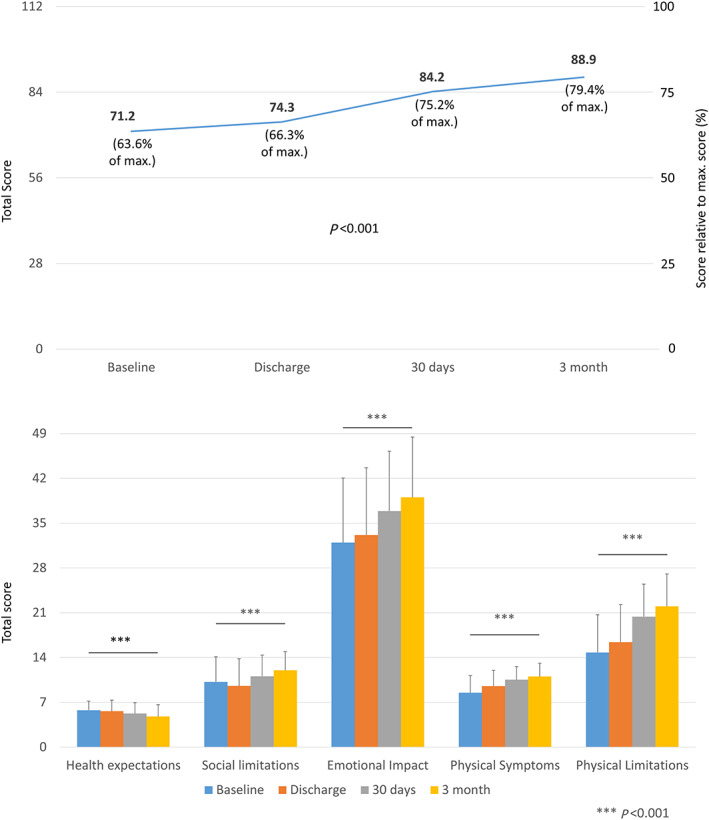
Toronto Aortic Stenosis Quality of Life Questionnaire score before and at 30 days and 3 months after aortic valve replacement (surgical aortic valve replacement or transcatheter aortic valve replacement)—total score (upper panel) and by domain (lower panel).
For the KCCQ, a similar increase in the total score was observed (mean increase of 19.0 points for the overall summary score between baseline and Month 3, Figure 2 ), with each domain being higher at 3 months than baseline. Physical limitation and social limitation were the only domains for which an intermediate drop at the time of hospital discharge was seen (from 65.0 to 56.7 and from 59.3 to 54.8, respectively). The KCCQ does not have a health expectations domain, so comparison of this domain was not possible. Compared with the TASQ and the KCCQ, the non‐specific SF‐12v2 was largely unchanged although significant between baseline and Month 3 (absolute difference of 2.6 for the mental summary score and 5.8 for the physical summary score; Figure 3 ).
Figure 2.
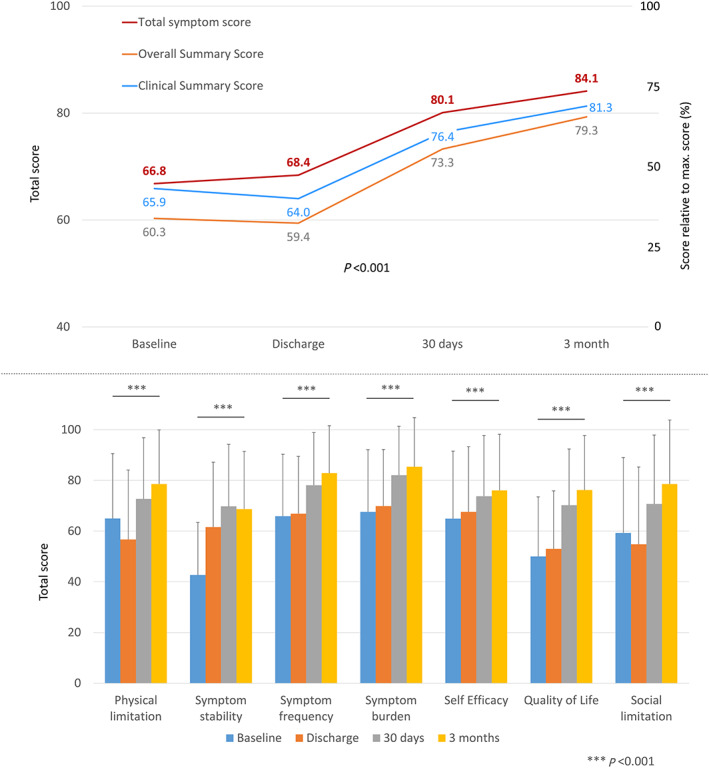
Kansas City Cardiomyopathy Questionnaire score before and at 30 days and 3 months after aortic valve replacement (surgical aortic valve replacement or transcatheter aortic valve replacement)—total score (upper panel) and by domain (lower panel).
Figure 3.
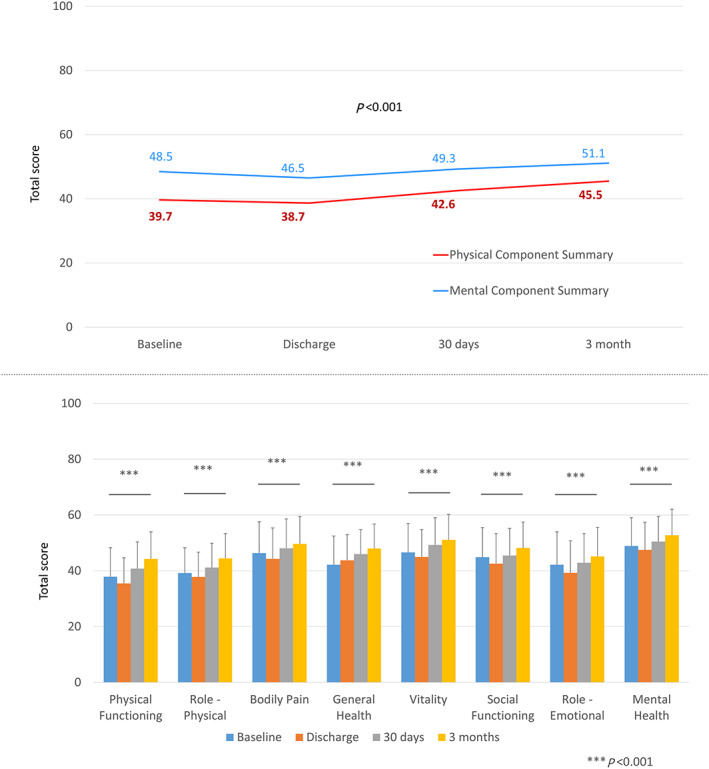
Short Form‐12 version 2 score before and at 30 days and 3 months after aortic valve replacement (surgical aortic valve replacement or transcatheter aortic valve replacement)—total score (upper panel) and by domain (lower panel).
Reliability
Internal consistency for the TASQ was tested using Cronbach's alpha (Table 1 ). The overall TASQ score had good consistency with an alpha of 0.891. This was also the case for the physical limitations (0.815) and emotional impact (0.815) domains, while social limitations had excellent consistency (0.950). Consistency was poor for physical symptoms, with an alpha of 0.579 (vs. threshold of 0.6).
Table 1.
Internal consistency of the TASQ
| Domain | Cronbach's α a | Degree of consistency |
|---|---|---|
| Physical symptoms (1, 14) | 0.579 | Poor consistency α < 0.6 |
| Physical limitations (3, 6, 7, 15) | 0.815 | Good consistency 0.9 > α ≥ 0.8 |
| Emotional impact (2, 8–13) | 0.815 | Good consistency 0.9 > α ≥ 0.8 |
| Social limitations (4, 5) | 0.950 | Excellent consistency α ≥ 0.9 |
| Health expectations (16) | n.a. b | n.a. b |
| TASQ total score | 0.891 c | Good consistency 0.9 > α ≥ 0.8 |
n.a., not applicable; TASQ, Toronto Aortic Stenosis Quality of Life Questionnaire.
Cronbach's α ≥ 0.9 indicates excellent consistency, 0.9 > α ≥ 0.8 is quite good, 0.8 > α ≥ 0.7 is acceptable, 0.7 > α ≥ 0.6 is questionable, and α < 0.6 is poor. 12
With only one item, no Cronbach's α can be determined.
Cronbach's α increases to 0.901 if Question 16 is not considered.
Test–retest reliability vs. 1 and 3 months (Table 2 ) was excellent for the overall TASQ score (intraclass correlation coefficient of 0.883) as well as for the physical symptoms (0.852), physical limitations (0.826), and emotional impact (0.895) domains. It was strong for social limitations (0.791) and moderate for health expectations (0.524).
Table 2.
TASQ test–retest reliability
| Domain | 1 month mean | 3 months mean | Mean difference | P‐value | ICC a | Agreement |
|---|---|---|---|---|---|---|
| Physical symptoms (1, 14) | 11.0 | 11.3 | 0.31 | 0.039 | 0.852 | Excellent |
| Physical limitations (3, 6, 7, 15) | 21.4 | 22.5 | 1.14 | 0.003 | 0.826 | Excellent |
| Emotional impact (2, 8–13) | 38.3 | 39.9 | 1.59 | 0.009 | 0.895 | Excellent |
| Social limitations (4, 5) | 11.4 | 12.2 | 0.82 | 0.001 | 0.791 | Strong |
| Health expectations (16) | 4.9 | 4.5 | −0.43 | 0.054 | 0.524 | Moderate |
| TASQ total score | 87.0 | 90.4 | 3.43 | 0.002 | 0.883 | Excellent |
ICC, intraclass correlation coefficient; TASQ, Toronto Aortic Stenosis Quality of Life Questionnaire.
No patients with surgical aortic valve replacement were considered for this analysis as recovery is prolonged vs. transcatheter aortic valve implantation. No patients were hospitalized between Months 1 and 3, and no patients had a change in New York Heart Association class.
The ICC (ratio of between‐groups variance:total variance) ranges from 0 to 1, with higher scores indicating increased test–retest reliability. In general, an intraclass correlation coefficient of 0 to 0.2 indicates poor agreement, 0.3 to 0.4 fair agreement, 0.5 to 0.6 moderate agreement, 0.7 to 0.8 strong agreement, and >0.8 excellent agreement.
Responsiveness
Toronto Aortic Stenosis Quality of Life Questionnaire responsiveness was determined among patients who underwent AV intervention and were alive at 1 month after the procedure (Table 3 ). Overall responsiveness was rated as medium (Cohen's d = 0.637). Among the domains, the effect size was only rated as large for physical limitations (0.812), while it was medium for physical symptoms (0.661) and emotional impact (0.456), and small for both social limitations (0.208) and health expectations (−0.307).
Table 3.
Determining TASQ responsiveness to clinical change
| Domain | Baseline mean a | 1 month mean a | Mean difference | P‐value | Effect size (Cohen's d) | |
|---|---|---|---|---|---|---|
| Physical symptoms (1, 14) | 8.53 ± 2.64 | 10.53 ± 2.06 | 2.00 | <0.001 | 0.661 | Medium |
| Physical limitations (3, 6, 7, 15) | 14.82 ± 5.93 | 20.37 ± 5.07 | 5.55 | <0.001 | 0.812 | Large |
| Emotional impact (2, 8–13) | 32.03 ± 10.30 | 36.86 ± 9.37 | 4.83 | <0.001 | 0.456 | Medium |
| Social limitations (4, 5) | 10.17 ± 3.98 | 11.08 ± 3.32 | 0.91 | 0.001 | 0.208 | Small |
| Health expectations (16) | 5.78 ± 1.42 | 5.26 ± 1.71 | −0.52 | <0.001 | −0.307 | Small |
| TASQ total score | 71.09 ± 19.43 | 84.15 ± 17.33 | 13.06 | <0.001 | 0.637 | Medium |
TASQ, Toronto Aortic Stenosis Quality of Life Questionnaire.
The responsiveness of the TASQ domains to a clinical change was first assessed among patients who underwent aortic valve replacement and were alive at 1 month after the procedure (n = 243). Scores at baseline and 1 month were compared using paired t‐tests. Cohen's d effect size, which quantifies the magnitude of change relative to baseline variation, was also used to assess the responsiveness of the questionnaire to clinical change. In general, an effect size of 0.2 to 0.3 indicates a small effect, around 0.5 is a medium effect, and ≥0.8 is a large effect.
Mean ± standard deviation.
The sensitivity to change after valve replacement (Table 4 ) was significant for physical symptoms, physical limitations, emotional impact, and social limitations but did not reach statistical significance for health expectations.
Table 4.
Sensitivity to change: mean 1 month change in TASQ stratified by change in NYHA class
| Domain vs. NYHA | Total (N = 243) | ↓ by 3 (N = 3) | ↓ by 2 (N = 39) | ↓ by 1 (N = 106) | No change (N = 77) | Worsening (N = 11) | P‐value for trend a |
|---|---|---|---|---|---|---|---|
| Physical symptoms (1, 14) | 2.00 | 3.33 | 2.49 | 2.71 | 0.89 | −0.00 | <0.001 |
| Physical limitations (3, 6, 7, 15) | 5.55 | 7.33 | 7.01 | 7.00 | 3.10 | 1.09 | <0.001 |
| Emotional impact (2, 8–13) | 4.83 | 9.67 | 8.18 | 5.79 | 2.27 | −1.73 | 0.003 |
| Social limitations (4, 5) | 0.91 | 0.33 | 2.00 | 1.23 | −0.07 | −0.82 | 0.038 |
| Health expectations (16) | −0.52 | 0.0 | −0.21 | −0.67 | −0.35 | −1.18 | 0.374 |
| TASQ total score | 13.06 | 20.67 | 19.42 | 16.31 | 6.24 | −2.64 | <0.001 |
NYHA, New York Heart Association; TASQ, Toronto Aortic Stenosis Quality of Life Questionnaire.
To examine the sensitivity of the TASQ to clinically relevant changes and its ability to discriminate between different levels of change, we calculated the change in domain scores for all patients from baseline to 1 month stratified by their change in NYHA class during the same period and examined the relation between the change in domain scores vs. the change in NYHA class using a linear trend test.
ANOVA.
Validity
Construct validity was tested by correlating the TASQ and its components to the NYHA class, the KCCQ, and the SF‐12v2 (Table 5 ). There was an adequate correlation (Pearson >0.5) between the overall TASQ score and the KCCQ and the SF‐12v2, and a negative correlation with the NYHA class (−0.367; P < 0.001). Correlations with the KCCQ and the SF‐12v2 were generally strong for the TASQ domains of physical symptoms, physical limitations, emotional impact, and social limitations, with the weakest correlations seen for TASQ emotional impact (0.433) and social limitations (0.452) with the SF‐12v2 physical component score. Correlations were weak for the TASQ health expectations domain.
Table 5.
Construct validity: testing correlations (Pearson) of TASQ domains with other measures at 3 months
| TASQ | ||||||
|---|---|---|---|---|---|---|
| Domain | Physical symptoms | Physical limitations | Emotional impact | Social limitations | Health expectations | Total TASQ score |
| NYHA class | −0.399 | −0.412 | −0.192 | −0.225 | 0.042 | −0.367 |
| KCCQ | ||||||
| Total symptom score | 0.708 | 0.730 | 0.524 | 0.597 | 0.090 | 0.686 |
| Overall summary score | 0.741 | 0.792 | 0.669 | 0.736 | 0.035 | 0.803 |
| Clinical summary score | 0.707 | 0.732 | 0.574 | 0.627 | 0.046 | 0.713 |
| Social limitation | n.a. | n.a. | n.a. | 0.603 | n.a. | n.a. |
| QoL | n.a. | n.a. | 0.714 | 0.708 | 0.046 | 0.801 |
| SF‐12v2 | ||||||
| Physical component score | 0.587 | 0.611 | 0.433 | 0.452 | 0.007 | 0.555 |
| Mental component score | 0.574 | 0.561 | 0.628 | 0.597 | 0.113 | 0.680 |
| Social functioning | n.a. | n.a. | n.a. | 0.660 | n.a. | n.a. |
| Role ‐ emotional | n.a. | n.a. | 0.582 | n.a. | 0.161 | n.a. |
KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; QoL, quality of life; SF‐12v2, Short Form‐12 version 2; TASQ, Toronto Aortic Stenosis Quality of Life Questionnaire.
Discussion
The results of this study confirm that the TASQ is a reliable, responsive, and valid measure of QoL in patients with severe AS and is sensitive to change in patients undergoing AV interventions.
Although various scales are available to evaluate QoL in patients with cardiovascular disease, until now, there has not been one that specifically assesses QoL in patients with AS. In the past, the KCCQ has often been used in AS studies because there are some similarities between some of the symptoms of severe AS and those of HF due to other causes. A level of validity for the KCCQ has been established in trials of AV intervention. 2 , 3 , 4 However, the KCCQ focuses on HF‐specific symptoms and does not capture parameters that are specific to AS. 5 In addition, the treatment of severe AS, and the likely outcome of such treatment, differs from that for other types of HF.
Patients with severe AS develop symptoms of HF in the presence of a preserved ejection fraction (HFpEF). Long‐standing outflow obstruction leads to maladaptive concentric myocardial hypertrophy, which causes diastolic dysfunction independently of ejection fraction. 13 Importantly, this secondary form of HFpEF responds—at least in part—to treatment of the underlying valvular disease. 14 This contrasts with primary HFpEF, which results from the interaction of metabolic and haemodynamic risk factors (such as hypertension, obesity, diabetes mellitus, chronic kidney disease, and atrial fibrillation) and does not have a specific underlying cause. 13 The cardinal symptoms of severe AS include angina and syncope as well as symptoms of HF such as dyspnoea. 15 A recent European registry study of patients with newly diagnosed severe AS found that 91% of symptomatic patients had dyspnoea, 30% had dizziness on exertion/syncope, and 29% had chest pain. 1 In comparison, among patients with primary HFpEF with structural heart disease (left ventricular hypertrophy or left atrial enlargement) enrolled in the large PARAGON‐HF trial, the most common symptoms were dyspnoea on exertion (92%), oedema (38%), and angina (29%). 16 In the AS registry, the most common co‐morbidities in patients with severe AS were severe renal impairment (27%), atrial fibrillation (17%), and chronic lung disease (13%). 1 Among patients with primary HFpEF in PARAGON‐HF, the most common co‐morbidities were hypertension (95%), diabetes mellitus (43%), and atrial fibrillation (32%). 16 Treatment of symptomatic severe AS with AV intervention reduces morbidity and mortality. 15
Thus, although there are some similarities between HF associated with AS and HF due to some other causes, there are also differences. The KCCQ was not designed to evaluate symptoms specific to AS, or the changes in QoL resulting from AV intervention. It would therefore be helpful to have a measurement tool that can assess the effects of AS‐specific symptoms/factors, and of AV intervention, on QoL. The TASQ has been designed to address that need, by taking into account the physical, emotional, and social factors associated with severe AS. 5 It also includes a health expectations domain, which reflects how hopeful the patient is that their health will improve, a factor that is not assessed by the KCCQ. Some research identifies that patient expectations and perceptions may impact post‐procedural outcomes including QoL. 17 Preliminary evaluation of the TASQ produced promising results. 5 The current study has validated the TASQ in a larger cohort of patients undergoing AV intervention.
The mean TASQ score increased (improved) steadily from baseline to 3 months of post‐treatment follow‐up. The increase was most pronounced for the emotional impact, physical limitations, and physical symptoms domains. A similar increase in the KCCQ total score was seen between baseline and 3 months. In contrast, the SF‐12v2, a non‐specific QoL questionnaire, did not change substantially between baseline and 3 months.
Internal consistency was good or excellent (Cronbach's α > 0.8) for the overall TASQ score and for the physical limitations, emotional impact, and social limitations domains. This is generally consistent with the findings of a study assessing the validity of the KCCQ in patients with severe AS, in which good/excellent internal consistency was seen for the KCCQ overall summary score and the physical limitation, symptoms, and social limitation domains, with acceptable consistency (α = 0.72) for the QoL domain. 3 In the current study, test–retest reliability was excellent or strong for the overall TASQ score and for the physical symptoms, physical limitations, emotional impact, and social limitations domains and was moderate for health expectations, further supporting the reliability of the TASQ.
In terms of the responsiveness of the TASQ to clinical change (1 month post‐treatment vs. baseline), a medium effect size was seen for the overall TASQ score and for the physical symptoms and emotional impact domains, with a large effect size for the physical limitations domain. Only small effects were seen for the social limitations and health expectations domains. In the KCCQ validation study in AS, a large responsiveness effect size was seen for overall summary, QoL, and symptoms domains and a moderate effect size for physical limitations and social limitations. 3 In the evaluation of sensitivity to change (as indicated by the relationship between change in NYHA class and change in domain score), the TASQ overall score and physical symptoms, physical limitations, emotional impact, and social limitations domains showed excellent discriminatory ability. This is consistent with the results reported previously for KCCQ domains in patients with severe AS. 3
In the evaluation of construct validity, a correlation (Pearson >0.5) was seen between the overall TASQ score and both the KCCQ and the SF‐12v2. Strong correlations with the KCCQ were seen for the TASQ domains of physical symptoms, physical limitations, emotional impact, and social limitations. Correlations with the SF‐12v2 were strong for the TASQ domains of physical symptoms, physical limitations, emotional impact, and social limitations (except for the latter two domains and the SF‐12v2 physical component score). A negative correlation was seen between the TASQ overall score and NYHA class, with similar correlations seen for the physical symptoms, physical limitations, emotional impact, and social limitations domains. Only weak correlations were seen between the TASQ health expectations domain and the KCCQ, SF‐12v2, or NYHA class.
Quality of life is an important consideration for patients with severe AS, who are often elderly and have multiple co‐morbid conditions. 18 , 19 , 20 , 21 The KCCQ has been shown to be valid and relevant in patients with severe AS, 2 , 3 , 4 but it focuses specifically on symptoms of HF and does not take into account factors that are specific to AS and its treatment. The SF‐12v2 evaluates general health‐related QoL 11 and is not able to differentiate between the effects of AS and the effects of other conditions, which is important, given that patients with AS tend to have multiple co‐morbidities. The TASQ incorporates parameters based on insight provided by patients with severe AS, including symptoms, emotional and social factors, and the implications of AS and its treatment on the patient's future. 5 It also includes questions about changes in symptoms over the previous 2 months, facilitating the assessment of how valve replacement has affected QoL. This instrument will enable more accurate evaluation of the QoL of patients with severe AS, including the response to treatment, and may facilitate appropriate treatment decisions.
Limitations
The sample size used non‐probability sampling; recruitment was aimed at facilitating equal distribution of patient numbers across languages. Patients were asked to complete three different questionnaires at each time point, which may have added to patient burden and may have been inconvenient for some patients, potentially leading to poor compliance and missing responses. In order to spread the risk of this across all three forms, the questionnaires were administered to patients in a random order. Patients were also evaluated to ensure that they had adequate capability for completing the forms and complying with study procedures. As such, patients with cognitive impairment were excluded from the study. Finally, the TAVI subgroup included only those undergoing TF‐TAVI. This is because TF access was the standard procedure used at participating centres.
Conclusions
The TASQ is a new, brief, self‐administered, and appropriate health‐specific tool to measure changes in QoL in patients with AS. Use of the TASQ may help to adequately assess health outcomes in patients undergoing AV intervention.
Conflict of interest
P.B. is the representative of the Institute for Pharmacology and Preventive Medicine, Cloppenburg, Germany. L.S., J.K., and M.T. are employees of the funder. All centres were paid by IPPMed for the enrolment and documentation of patients.
Funding
This work was supported by a research grant provided by Edwards Lifesciences (Nyon, Switzerland) to the Institute for Pharmacology and Preventive Medicine (Cloppenburg, Germany).
Author contributions
D.F., S.K., L.S., C.M.L., L.Sy., J.K., M.T., P.B., and R.S. were involved in the conception and design of the registry. The remaining authors gave feedback on the final protocol and included patients. D.F., C.M.L., and P.B. drafted the manuscript, and all other authors revised the article for important intellectual content. All authors gave final approval of the version to be published. All authors are fully accountable for the content of the manuscript.
Study coordinators
Michelle Dimas, Mamta Kapoor, and Jermel Pierre (Toronto, Canada); Mervyn Andiapen, Rita Adrego, and Adedolapo Adeleke (London, UK); Mathilde Le Marchand, Genevieve Mottin, and Sharanya Logeswaran (Massy, France); Reza Farnoud (Bichat, France); Florian Fulisch, Nathalie Guessefeld, and Renate Kahl (Kiel, Germany); Mattia Lunardi and Ilaria Franzese (Verona, Italy); Mira Stolcova (Florence, Italy); Maria Angeles Carmona and Teresa Hemodinamica (Barcelona, Spain); and Elena Paz and Maria Rita Soler Martin (Coruna, Spain).
Supporting information
Table S1. Summary of analytics approaches.
Table S2. Baseline demographic and clinical characteristics by cohort.
Acknowledgement
Data were captured using the s4trials Software provided by Software for Trials Europe GmbH, Berlin, Germany. Open access funding enabled and organized by Projekt DEAL.
Frank, D. , Kennon, S. , Bonaros, N. , Stastny, L. , Romano, M. , Lefèvre, T. , Di Mario, C. , Stefàno, P. , Ribichini, F. , Himbert, D. , Urena‐Alcazar, M. , Salgado‐Fernandez, J. , Castillo, J. J. C. , Garcia del Blanco, B. , Deutsch, C. , Sykorova, L. , Kurucova, J. , Thoenes, M. , Lüske, C. M. , Bramlage, P. , and Styra, R. (2021) Aortic valve replacement: validation of the Toronto Aortic Stenosis Quality of Life Questionnaire. ESC Heart Failure, 8: 270–279. 10.1002/ehf2.12961.
[Correction added on 5 December 2020, after first online publication: Projekt Deal funding statement has been added.]
References
- 1. Frey N, Steeds RP, Rudolph TK, Thambyrajah J, Serra A, Schulz E, Maly J, Aiello M, Lloyd G, Bortone AS, Hauptmann KE, Clerici A, Delle Karth G, Rieber J, Indorfi C, Mancone M, Belle L, Lauten A, Arnold M, Bouma BJ, Lutz M, Pohlmann C, Kurucova J, Thoenes M, Bramlage P, Messika‐Zeitoun D, group Ir . Symptoms, disease severity and treatment of adults with a new diagnosis of severe aortic stenosis. Heart 2019; 105: 1709–1716. [DOI] [PubMed] [Google Scholar]
- 2. Arnold SV, Reynolds MR, Wang K, Magnuson EA, Baron SJ, Chinnakondepalli KM, Reardon MJ, Tadros PN, Zorn GL, Maini B, Mumtaz MA, Brown JM, Kipperman RM, Adams DH, Popma JJ, Cohen DJ, CoreValve USPTI. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal Trial. JACC Cardiovasc Interv 2015; 8: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail 2013; 6: 61–67. [DOI] [PubMed] [Google Scholar]
- 4. Arnold SV, Spertus JA, Vemulapalli S, Li Z, Matsouaka RA, Baron SJ, Vora AN, Mack MJ, Reynolds MR, Rumsfeld JS, Cohen DJ. Quality‐of‐life outcomes after transcatheter aortic valve replacement in an unselected population: A report from the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol 2017; 2: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frank D, Kennon S, Bonaros N, Romano M, Lefevre T, Di Mario C, Stefano P, Ribichini FL, Himbert D, Urena‐Alcazar M, Salgado‐Fernandez J, Cuenca Castillo JJ, Garcia B, Kurucova J, Thoenes M, Luske C, Bramlage P, Styra R. Trial protocol for the validation of the ‘Toronto Aortic Stenosis Quality of Life (TASQ) Questionnaire’ in patients undergoing surgical aortic valve replacement (SAVR) or transfemoral (TF) transcatheter aortic valve implantation (TAVI): the TASQ registry. Open Heart 2019; 6: e001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Styra R, Dimas M, Svitak K, Kapoor M, Osten M, Ouzounian M, Devins G, Deckert A, Horlick E. Toronto aortic stenosis quality of life questionnaire (TASQ): validation in TAVI patients. BMC Cardiovasc Disord 2020; 20: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 8. Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ. Placement of aortic transcatheter valves I. Health‐related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 2011; 124: 1964–1972. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ, Investigators PT. Health‐related quality of life after transcatheter or surgical aortic valve replacement in high‐risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol 2012; 60: 548–558. [DOI] [PubMed] [Google Scholar]
- 10. Muller‐Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)‐12 health status instrument with the SF‐36 in patients with coronary heart disease. Heart 2004; 90: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 12. Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess 2003; 80: 99–103. [DOI] [PubMed] [Google Scholar]
- 13. Del Buono MG, Buckley L, Abbate A. Primary and secondary diastolic dysfunction in heart failure with preserved ejection fraction. Am J Cardiol 2018; 122: 1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato K, Kumar A, Jones BM, Mick SL, Krishnaswamy A, Grimm RA, Desai MY, Griffin BP, Rodriguez LL, Kapadia SR, Obuchowski NA, Popovic ZB. Reversibility of cardiac function predicts outcome after transcatheter aortic valve replacement in patients with severe aortic stenosis. J Am Heart Assoc 2017; 6: e005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimard BH, Safford RE, Burns EL. Aortic stenosis: diagnosis and treatment. Am Fam Physician 2016; 93: 371–378. [PubMed] [Google Scholar]
- 16. Chandra A, Vaduganathan M, Lewis EF, Claggett BL, Rizkala AR, Wang W, Lefkowitz MP, Shi VC, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV, Solomon SD. Health‐related quality of life in heart failure with preserved ejection fraction: the PARAGON‐HF trial. JACC Heart Fail 2019; 7: 862–874. [DOI] [PubMed] [Google Scholar]
- 17. Waljee J, McGlinn EP, Sears ED, Chung KC. Patient expectations and patient‐reported outcomes in surgery: a systematic review. Surgery 2014; 155: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001; 20: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 19. Kraai IH, Vermeulen KM, Luttik ML, Hoekstra T, Jaarsma T, Hillege HL. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail 2013; 15: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 20. Horrocks J, Closs J, Astin F. Quality of life in older adults with aortic stenosis: a narrative review. Int J Older People Nurs 2014; 9: 227–246. [DOI] [PubMed] [Google Scholar]
- 21. Arnold SV, Spertus JA, Lei Y, Green P, Kirtane AJ, Kapadia S, Thourani VH, Herrmann HC, Beohar N, Zajarias A, Mack MJ, Leon MB, Cohen DJ. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes 2013; 6: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of analytics approaches.
Table S2. Baseline demographic and clinical characteristics by cohort.


