Abstract
Aims
The role of dynamic changes in lactate concentrations on prognosis in acute heart failure has been poorly investigated. The aim of this study was to explore the predictive value of 24 h time‐weighted lactate (LACTW) in patients with acute heart failure.
Methods and results
Ninety‐six consecutive acute heart failure patients presenting to the Emergency Department of San Paolo Hospital, Naples, Italy, were prospectively enrolled. Arterial blood lactate was measured at admission and during the following 24 h at random time intervals. LACTW was obtained by the sum of the average lactate values among consecutive time points multiplied by the intervals between consecutive time points and dividing the sum by the total time (24 h). The outcome was a composite of need of admission to the intensive care unit, hospitalization duration >7 days, or intra‐hospital death. Admission lactate, maximum measured lactate, and LACTW were collected. Univariate and multivariate Cox regression analysis was applied to determine the hazard ratio (HR) of developing the outcome. Forty‐three patients experienced the pre‐specified outcome. In sex‐adjusted and age‐adjusted multivariable analysis, LACTW predicted the outcome occurrence (HR: 1.51, 95% confidence interval: 1.24, 1.84, P < 0.001). Risk stratification analysis based on LACTW tertiles demonstrated a gradual increase in risk of developing the outcome (HR: 17.32, 95% confidence interval: 2.30, 130.23, P = 0.006) for the highest LACTW tertile.
Conclusions
In acute heart failure patients, 24 h LACTW had a significant independent predictive value for adverse intra‐hospital outcome. LACTW could be a useful index at identifying high‐risk patients who may require a more aggressive treatment during hospitalization.
Keywords: Time‐weighted lactate, Acute heart failure, Prognosis, Hospitalization, Intensive care
Introduction
Acute heart failure (AHF) is a life‐threatening medical condition that represents one of the most frequent causes of admission to the emergency department. 1 Beside its specific aetiology, AHF refers to a rapid onset or worsening of symptoms and/or signs of heart failure (HF), characterized by a mismatch between energy supply, oxygen demand, and consumption. 2 The detection of a high lactate concentration associated with a low blood pH (lactic acidaemia) is useful in describing the severity of such an imbalance. 3 In this regard, lactate concentration does not reflect the severity of heart dysfunction per se, but rather, it features the epiphenomenon of the metabolic consequences of the multiorgan insult driven by the acute circulatory decompensation. 4 Several studies have shown the usefulness of lactate concentration as a prognostic marker in acute myocardial infarction and cardiogenic shock. 5 , 6 Furthermore, it has been recently demonstrated that, in patients with AHF, elevated values of blood lactate on admission are associated with a higher risk of death. 7
Beside single static lactate measurement upon admission, there is general agreement that in the acute setting, prolonged high values of blood lactate concentration are associated with a worse prognosis. 8 The persistent increase in blood lactate concentration could be related to increase in its production, decrease in its clearance, or both conditions simultaneously. 9 Furthermore, even minimal fluctuation in lactate concentration above the normal value could reflect subtle phases of hypoperfusion due to a more severe underlying morbid condition, or insufficient response to therapeutic interventions, which could be related to a worse prognosis. In the absence of severe renal and/or hepatic dysfunction, dynamic changes in lactate concentration in the critically ill patient may predict prognosis more accurately than static indices. 10 Several dynamic parameters of lactate homeostasis are potentially measurable, but to date, no comparison has been performed among them. In the setting of cardiorespiratory failure, an earlier lactate clearance could help in the assessment of the metabolic response to therapy and in the identification of patients able to restore the oxygen debt rapidly. 11 Moreover, persistent hyperlactataemia within the first 24 h of hospitalization is a strong predictor of a worse outcome in AHF and is related to higher rates of in‐hospital adverse events and 1 year mortality. 12 Recently, time‐weighted (TW) average lactate (LACTW), an index of lactate homeostasis that takes into account the amount of time spent at each lactate concentration in relation to the total period of time observed, demonstrated to be superior to static indices of lactate concentration in outcome prediction in a large heterogeneous population of critically ill patients. 10 Until now, no prospective study has investigated the role of dynamic changes in lactate in predicting outcome in AHF. Here we aim at exploring the predictive value on adverse intra‐hospital outcome of the TW average lactate during the first 24 h of hospitalization (LACTW) in comparison with static and other dynamic parameters of lactate homeostasis in patients admitted to the emergency department for AHF.
Some of the results of this study have been previously reported in abstract form. 13
Methods
Patients and experimental design
Consecutive patients with AHF referred to the Emergency Department of San Paolo Hospital in Naples, Italy, were prospectively enrolled. All eligible patients were diagnosed with AHF according the following criteria 1 : signs and symptoms of congestion and/or hypoperfusion further confirmed by appropriate additional investigations: brain natriuretic peptide (BNP) values higher than 100 pg/mL when available, bilateral B‐lines or comets on lung ultrasound, or typical findings on chest radiography. Exclusion criteria included clinical and laboratory signs of infection, BNP values lower than 100 pg/mL, malignant ventricular arrhythmias, cardiogenic shock, the need for intra‐aortic balloon pump implantation, and electrocardiographic diagnosis of acute coronary syndrome upon admission. Moreover, to avoid clinical conditions potentially affecting lactate clearance regardless of HF itself, patients with end‐stage renal disease or advanced liver failure were excluded. Patients were treated in accordance with the European Society of Cardiology guidelines. 1 Non‐invasive ventilation, including both continuous positive airway pressure and bi‐level positive pressure ventilation, was used when needed. 14 Step‐down care from the emergency department (hospitalization on an ordinary inpatient ward or direct discharge to home with advice to be followed clinically in an outpatient clinic) was dictated by clinical stabilization. Patients with persistent, significant dyspnoea or haemodynamic instability were transferred to a more intensive ward (intensive care unit or coronary intensive care unit).
The study was performed in accordance with the Declaration of Helsinki on human research and the Good Clinical Practice standards. All participants gave their written informed consent, and the protocol was approved by the local ethics committee ‘Campania Centro’.
Study procedures
Upon enrolment, all patients underwent the following study procedures: clinical history collection; physical examination; electrocardiogram; venous blood sample tests including troponin, urea, creatinine, electrolytes, glucose, complete blood count, and liver function tests; and arterial blood gas analyses. Bedside lung ultrasound for detection of bilateral anterior comets and transthoracic echocardiography for the assessment of left ventricular function through the measurement of ejection fraction (Simpson's method) were also performed.
Arterial blood samples were processed and instantly analysed trough a mobile point of care system (Cobas b 123, Roche, Basel, Switzerland). Lactate concentrations were measured at hospital arrival and during the following 24 h at random time intervals. Static [admission lactate (LACSTART) and maximum measured lactate (LACMAX)] and dynamic [2 h clearance (Cl2hLAC) and TW lactate (LACTW)] indices of lactate homeostasis during hospitalization were recorded and included in the analyses. Cl2hLAC was calculated as the difference between lactate after 2 h and lactate on admission, divided by the lactate on admission and expressed as a percentage. LACTW was determined by the sum of the mean lactate values among consecutive time points multiplied by the period of time between consecutive time points and then dividing by the total time (24 h). 10
Outcome definition
Outcome occurrence was defined by a composite of need of admission to a more intensive ward (intensive care unit or coronary unit), hospitalization duration of more than 7 days, and hospital mortality.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. Student's t‐test for unpaired values was used to compare the means of groups for quantitative variables. Bonferroni correction was applied when indicated. Categorical variables were presented as frequencies and percentages and compared using χ 2 test with Yates correction. Correlations between variables were examined by determining Pearson's coefficient. Univariate and multivariate Cox regression analyses were used to determine the hazard ratio (HR) of experiencing the primary endpoint: composite endpoint was the dependent variable, whereas LACSTART, LACMAX, Cl2hLAC, LACTW, and troponin were included as independent variables. The ability of TW lactate to predict the outcome was assessed by measurement of the area under the receiver operating characteristic (ROC) curve (or c‐index). The best threshold of the ROC curve was chosen using bootstrap analysis and maximization of the Youden index. A P‐value <0.05 was considered statistically significant.
All data were collected and entered in an Excel database (Microsoft Office 2016, Redmond, WA, USA), and statistical analyses were performed using SPSS (IBM SPSS Statistics Version 25, SPSS Inc., Chicago, IL).
Results
Characteristics of the study population
Among 121 screened patients, five patients were excluded because of the presence of acute coronary syndrome, one for a diagnosis of cardiogenic shock who required intra‐aortic balloon pump, and two for ventricular fibrillation. In addition, 10 patients were excluded for a concomitant diagnosis of bacterial pneumonia, six for end‐stage renal disease, and one for advanced liver failure.
Ninety‐six patients were included in the final analysis. The characteristics of the study population including co‐morbidities and arterial blood gas parameters are presented in Table 1 .
Table 1.
Anthropometric characteristics and laboratory and clinical parameters of the whole study population divided according to outcome occurrence
| Characteristics | Study population (N = 96) | No outcome (n = 53) | Yes outcome (n = 43) | P‐value a |
|---|---|---|---|---|
| Sex (female), n (%) | 54 (56) | 27 (50.9) | 27 (62.8) | ns |
| Age (years) | 76.3 ± 10.4 | 74.1 ± 8.9 | 78.8 ± 11.5 | 0.02 |
| Systolic blood pressure (mmHg) | 171.8 ± 37.3 | 179.2 ± 35.6 | 162.4 ± 37.4 | 0.02 |
| Diastolic blood pressure (mmHg) | 96.4 ± 18.7 | 101.8 ± 16.4 | 90.1 ± 20.1 | 0.002 |
| BNP (pg/mL) | 777.6 ± 184.9 | 800 ± 188.3 | 749.4 ± 178.8 | ns |
| Creatinine (mg/dL) | 1.32 ± 0.68 | 1.36 ± 0.79 | 1.26 ± 0.52 | ns |
| LVEF (%) | 33.3 ± 9.6 | 34.3 ± 9.0 | 32.2 ± 10.2 | ns |
| Troponin (mg/L) | 0.14 ± 0.3 | 0.08 ± 0.1 | 0.22 ± 0.4 | 0.01 |
| Respiratory rate (b.p.m.) | 27.5 ± 5.5 | 26.7 ± 5.5 | 28.8 ± 5.3 | ns |
| Clinical presentation | ||||
| Congestion, n (%) | 21 (21.9) | 16 (30.2) | 5 (11.6) | 0.016 |
| Hypoperfusion, n (%) | 26 (27.1) | 9 (17) | 17 (39.6) | |
| Congestion and hypoperfusion, n (%) | 49 (51) | 28 (52.8) | 21 (48.8) | |
| Chest X‐ray findings | ||||
| Cardiomegaly, n (%) | 62 (64.6) | 33 (62.3) | 29 (67.4) | ns |
| Pulmonary congestion, n (%) | 78 (81.2) | 43 (81.1) | 35 (81.4) | ns |
| Pleural effusion, n (%) | 51 (53.1) | 32 (60.4) | 19 (44.2) | ns |
| Number of chest X‐ray findings: 0/1/2/3, n (%) | 2 (2.1)/22 (22.9)/47 (49)/25 (26) | 2 (3.8)/10 (18.9)/25 (47.1)/16 (30.2) | 0 (0)/12 (27.8)/22 (51.1)/9 (20.1) | ns |
| Co‐morbidities | ||||
| Ischaemic heart disease, n (%) | 74 (77.1) | 40 (75.5) | 34 (79.1) | ns |
| Atrial fibrillation, n (%) | 22 (22.9) | 12 (22.6) | 10 (23.2) | ns |
| Valvulopathy, n (%) | 11 (11.4) | 7 (13.2) | 4 (9.3) | ns |
| Hypertension, n (%) | 85 (88.5) | 47 (88.7) | 38 (88.4) | ns |
| Type 2 diabetes, n (%) | 45 (48.4) | 26 (49.1) | 20 (46.5) | ns |
| Chronic kidney disease, n (%) | 40 (41.7) | 23 (43.4) | 17 (39.5) | ns |
| COPD, n (%) | 16 (16.7) | 10 (18.9) | 6 (13.9) | ns |
| HFrEF, n (%) | 65 (67.7) | 35 (66.1) | 30 (69.8) | ns |
| HFmrEF, n (%) | 23 (24) | 13 (24.5) | 10 (23.2) | ns |
| HFpEF, n (%) | 8 (8.3) | 5 (9.4) | 3 (7.0) | ns |
| ICD, n (%) | 18 (18.7) | 13 (24.5) | 5 (11.6) | ns |
| Suspected cause of AHF | ||||
| Acute coronary syndrome, n (%) | 18 (18.8) | 0 (0) | 18 (41.9) | <0.001 |
| Cardiogenic shock, n (%) | 8 (8.3) | 1 (1.9) | 7 (16.3) | |
| Worsening HF, n (%) | 17 (17.7) | 9 (17) | 8 (18.5) | |
| Hypertensive HFpEF, n (%) | 18 (18.8) | 15 (28.3) | 3 (7) | |
| Hypertensive HFrEF, n (%) | 35 (36.4) | 28 (52.8) | 7 (16.3) | |
| Arterial blood gas parameters | ||||
| pH | 7.2 ± 0.1 | 7.23 ± 0.15 | 7.19 ± 0.15 | ns |
| pCO2 (mmHg) | 55.1 ± 20.8 | 57.3 ± 22.1 | 54.2 ± 22.5 | ns |
| pO2 (mmHg) | 52.3 ± 11.1 | 52.9 ± 11.3 | 51.4 ± 10.9 | ns |
| LACSTART (mmol/L) | 5.3 ± 3.0 | 4.3 ± 2.6 | 6.5 ± 2.9 | <0.001 |
| LACMAX (mmol/L) | 5.4 ± 2.9 | 4.4 ± 2.6 | 6.6 ± 2.9 | <0.001 |
| Cl2hLAC (%) | 28.1 ± 30.8 | 37.2 ± 31.7 | 14.4 ± 25.0 | <0.0001 |
| LACTW | 2.5 ± 1.6 | 1.59 ± 0.7 | 3.7 ± 1.6 | <0.00001 |
AHF, acute heart failure; BNP, brain natriuretic peptide; Cl2hLAC, 2 h clearance of lactate; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implanted cardioverter defibrillator; LACMAX, maximum lactate; LACSTART, lactate at admission; LACTW, time‐weighted lactate; LVEF, left ventricular ejection fraction; ns, not significant.
Data expressed as mean ± standard deviation or frequencies and percentage as appropriate.
P‐value refers to the comparison between patients who experienced the outcome and patients who did not.
The study population had a mean age of 76.3 ± 10.4 years, with a slightly higher prevalence of female sex (56%). Concerning the cardiological history, 67.7% of the patients had HF with reduced ejection fraction, 24% of them had HF with mid‐range ejection fraction between 40% and 49%, and 8.3% had HF with preserved ejection fraction. The mean left ventricular ejection fraction value measured at bedside echocardiography performed upon admission was 33.3 ± 9.6%. From a clinical standpoint, 21 (21.9%) patients presented with signs of pulmonary congestion, 26 (27.1%) patients with signs of systemic hypoperfusion, and 49 (51%) patients with signs of both congestion and hypoperfusion. Details on chest X‐ray findings are described in Table 1 .
The most frequent suspected cause of AHF was hypertensive HF with reduced ejection fraction (36.4% of cases), followed by hypertensive HF with preserved ejection fraction (18.8%), acute coronary syndrome (18.8%), worsening HF (17.7%), and cardiogenic shock (8.3%).
The mean number of blood gas analyses performed during the first 24 h from admission for each patient was 3.7 ± 0.9. As shown in Table 1 , blood gas analysis on admission showed low pH (7.2 ± 0.1) and pO2 (52.3 ± 11.1 mmHg) with high level of pCO2 (55.1 ± 20.8 mmHg) and lactate (5.3 ± 3 mmol/L). Troponin's mean value on admission was 0.14 ± 0.3 mg/L. BNP values were 777.6 ± 184.9 pg/mL.
Time‐weighted lactate and intra‐hospital outcome
Forty‐three patients experienced the pre‐specified primary composite endpoint. In particular, 18 patients were admitted to the intensive care unit for haemodynamic instability or need of tracheal intubation, 14 patients were admitted to the coronary intensive care unit for the development of acute coronary syndrome or end‐stage HF, and finally, 11 patients died during the hospitalization.
Table 1 shows a comparison between patients that experienced the outcome and those who did not. Patients who experienced the outcome were significantly older with higher levels of troponin on admission and slightly lower values of both systolic and diastolic blood pressure. No differences were found in terms of self‐reported co‐morbidities. For indices of lactate homeostasis, we observed a statistically significant difference between the two groups for both static measures (LACSTART and LACMAX) and dynamic changes in lactate concentration (Cl2hLAC and LACTW) (Table 1 ).
As shown in Table 2 , on multivariate Cox regression analysis adjusted for age and sex, LACTW was significant for developing the primary outcome (HR: 1.51, 95% confidence interval: 1.24, 1.84, P < 0.001). This was also observed for the other indices of lactate homeostasis (Table 2 ).
Table 2.
Cox regression analysis of primary composite outcome for lactate on admission, maximum measured lactate, and time‐weighted lactate
| Parameter | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| LACSTART | 1.12 (1.01, 1.25) | 0.033 | 1.16 (1.02, 1.31) | 0.024 |
| LACMAX | 1.13 (1.01, 1.26) | 0.031 | 1.16 (1.02, 1.32) | 0.022 |
| Cl2hLAC | 0.99 (0.98, 1.01) | ns | 0.99 (0.98, 1.00) | ns |
| LACTW | 1.40 (1.17, 1.68) | <0.001 | 1.51 (1.24, 1.84) | <0.001 |
| Abnormal chest X‐ray finding | ||||
| Cardiomegaly | 1.06 (0.53, 2.11) | ns | 1.04 (0.51, 2.10) | ns |
| Pulmonary congestion | 1.31 (0.51, 3.41) | ns | 1.31 (0.50, 3.43) | ns |
| Pleural effusion | 0.78 (0.40, 1.53) | ns | 0.72 (0.36, 1.45) | ns |
| BNP | 1.00 (0.99, 1.00) | ns | 1.00 (0.99, 1.00) | ns |
| Clinical presentation (congestion and/or hypoperfusion) | ||||
| Only congestion | Reference | — | Reference | — |
| Only hypoperfusion | 1.78 (0.63, 4.97) | ns | 1.78 (0.63, 5.07) | ns |
| Congestion and hypoperfusion | 1.71 (0.63, 4.67) | ns | 1.70 (0.62, 4.66) | ns |
| Troponin | 5.52 (1.85, 16.42) | 0.002 | 5.78 (1.91, 17.47) | 0.002 |
| LVEF | 0.97 (0.93, 1.01) | ns | 0.96 (0.93, 1.00) | ns |
| Systolic blood pressure | 0.99 (0.98, 1.00) | ns | 0.99 (0.98, 1.00) | ns |
| Diastolic blood pressure | 0.99 (0.97, 1.01) | ns | 0.99 (0.97, 1.01) | ns |
| Creatinine | 0.59 (0.32, 1.07) | ns | 0.58 (0.31, 1.08) | ns |
| Age | 1.00 (0.97, 1.04) | ns | 1.01 (0.97, 1.05) | ns |
| pH | 0.98 (0.17, 5.53) | ns | 0.99 (0.18, 5.58) | ns |
| pCO2 | 1.00 (0.98, 1.01) | ns | 0.98 (0.98, 1.01) | ns |
| pO2 | 0.99 (0.95, 1.03) | ns | 0.99 (0.95, 1.03) | ns |
BNP, brain natriuretic peptide; CI, confidence interval; Cl2hLAC, 2 h clearance of lactate; HR, hazard ratio; LACMAX, maximum lactate; LACSTART, lactate at admission; LACTW, time‐weighted lactate; LVEF, left ventricular ejection fraction; ns, not significant.
Adjusted for age and sex.
Risk stratification analysis based on LACTW tertiles demonstrated a gradual increase in risk of developing the primary endpoint across LACTW tertiles, with an HR of 17.32 (2.30, 130.23, P = 0.006) for the highest LACTW tertile (Table 3 and Figure 1 ). In particular, no death was observed among the patients belonging to the first TW lactate tertile, two deaths occurred among the patients belonging to the second TW lactate tertile, and nine deaths among the ones in the third TW lactate tertile.
Table 3.
Cox regression analysis of primary composite outcome according to time‐weighted lactate LACTW tertile
| Tertile (range) | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| T1 (0.53–1.59) | Reference | — | Reference | — |
| T2 (1.60–2.78) | 9.39 (1.21, 72.54) | 0.032 | 9.20 (1.19, 71.28) | 0.034 |
| T3 (2.79–8.73) | 17.32 (2.30, 130.23) | 0.006 | 21.05 (2.73, 162.19) | 0.003 |
CI, confidence interval; HR, hazard ratio.
Adjusted for age and sex.
Figure 1.
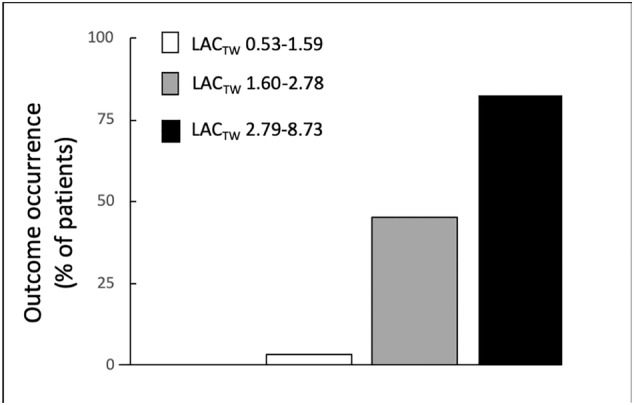
Relationship between adverse outcome occurrence and each tertile of time‐weighted lactate (LACTW).
Receiver operating characteristic curve analysis demonstrated an excellent ability of LACTW in predicting the outcome, with an area under the ROC curve of 0.928 (standard error 0.024, P < 0.0001, 95% confidence interval: 0.880, 0.976) (Figure 2 ). Using bootstrap analysis and maximization of the Youden index, the best cut‐off was 2.2 (with a sensibility of 88% and a specificity of 83%).
Figure 2.
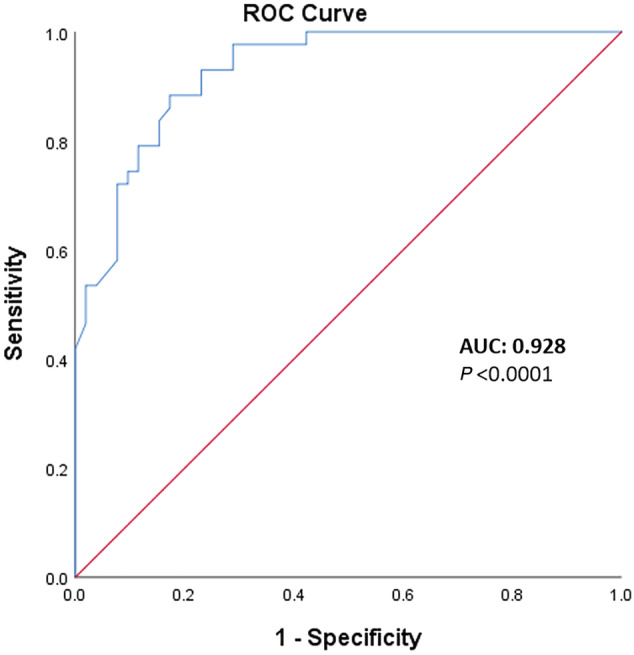
Receiving operating characteristic (ROC) curves of time‐weighted lactate.P‐values refer to the comparison of the area under the ROC curve (AUC) vs. 0.50 (i.e. no discrimination, red line).
Discussion
To our knowledge, this is the first prospective study that demonstrates that high overall levels of lactate during the first 24 h of hospitalization strongly predict the prognosis in patients presenting with AHF. We also show that LACTW has a greater predictive value than the other static and dynamic indices of lactate homeostasis. Moreover, we demonstrated that each tertile of LACTW identifies a different risk category in AHF patients.
Several studies reported the prognostic role of lactate concentrations in critically ill patients, including those with septic shock 15 and trauma, 16 after cardiac arrest 17 or major surgery. 18 While in chronic HF the prevalence of increased levels of lactataemia is low and does not correlate with arterio‐venous oxygen difference or cardiac index, 19 elevated blood lactate levels upon admission are associated with high mortality in AHF patients. 7 Lactate does not reflect the severity of heart dysfunction per se but rather reveals the metabolic consequences and the severity of the insult driven by the sudden HF. 4 Indeed, there are several different pathways that could potentially contribute to increase lactate levels during AHF: the insufficient supply of blood and oxygen to the peripheral tissues, 4 the neurohormonal activation, 20 and diaphragm fatigue. 21 Among these mechanisms, only the proportion of hyperlactataemia due to the activation of anaerobic metabolism following tissue hypoperfusion is directly related to the severity of the disease and to mortality rates. 22
Some studies 23 have already underlined that serial evaluation of lactate concentrations may be more helpful than a single value, and dynamic changes of lactataemia may represent a useful monitoring tool of response to treatment. In this regard, a Cl2hLAC demonstrated to be a good marker to guide the therapeutic approach in acute cardiorespiratory insufficiency. 11 Nevertheless, some concerns exist regarding the significance of lactate clearance and the best time to repeat lactate measurement. 23 In fact, during the first phases of hospitalization, blood lactate concentrations could have complex fluctuations above the normal value and persistent hyperlactataemia could identify further period of hypoperfusion due to a more severe underlying morbid condition or insufficient response to therapeutic interventions. 24
Among the dynamic indices, we demonstrate that LACTW is more useful in predicting outcome than Cl2hLAC in patients admitted with AHF. In this study, LACTW was significantly associated with mortality outcome as well as prolonged length of stay, therefore identifying patients with significant potential morbidity.
There are many advantages in the use of LACTW; this index describes not only the magnitude but also the duration and trend over time of lactate homeostasis. As demonstrated in this study, each tertile of LACTW had an increased risk of developing the primary endpoint in comparison with the lowest LACTW tertile. Furthermore, in this present study, a LACTW of more than 1.6 identified patients who had the worst outcome, as compared with subjects with a LACTW less than 1.6. Patients with LACTW more than 2.79 had an especially poor outcome. The prognostic significance of LACTW persisted after adjustment for age and sex. Thus, our results suggest that LACTW is a powerful predictor of patient adverse outcomes in AHF. Interestingly, according to Nichol et al., relatively mild hyperlactataemia in AHF patients is independently associated with the negative outcome, such as in the middle tertile in our study. 24 Finally, to our knowledge, this is the first study in which LACTW was determined prospectively in a pure cohort of AHF patients.
Some limitations need to be accounted for in this study. First, the actual cause of increase in circulating lactate levels is still a matter of debate and it does not necessarily reflect organ hypoperfusion. Lactate is a complex, and highly volatile marker, with large fluctuations that are not only related to perfusion and anaerobic metabolism, but rather a complex and dynamic interplay between the sum of production and elimination in important organs such as muscle, heart, kidneys, and liver. 25 Furthermore, this was a single‐centre experience with a relatively small sample size. The present study did not compare the different treatments and different aetiologies of AHF, and thus, optimal treatment of these patients remains elusive and requires further investigation. We chose a composite endpoint of intra‐hospital morbidity and mortality; therefore, data on intermediate‐term or long‐term mortality are lacking. Finally, the clinical staff was not blinded to individual lactate measurements; nevertheless, lactate measurements did not influence per se the clinical management: all the clinical decisions were taken according to the current recommendations, taking into account the whole clinical picture and the results of the diagnostic work‐up.
In conclusion, in patients presenting with AHF, LACTW concentrations collected during the first 24 h after emergency department admission had a significant independent predictive value of adverse outcomes. LACTW could be a useful index in identifying patients at higher risk of adverse outcomes, who may require more aggressive therapy during hospitalization. Further studies are needed in order to validate this index in the assessment of patients hospitalized for AHF.
Conflict of interest
None declared.
Funding
This study was not funded.
Author contributions
G.B., V.M., G.G., F.G.N., C.G.T., and F.S. contributed in the conception and design of the study; G.B., A.P., G.P., E.A., C.S., and G.G. in the collection of data; G.B., V.M., and N.D. in the analysis and interpretation of data; G.B., V.M., and N.D. in drafting of the article; and G.B., V.M., N.D., A.P., G.P., E.A., C.S., G.G., F.G.N., C.G.T., and F.S. in the revision and final approval of the manuscript.
Bosso, G. , Mercurio, V. , Diab, N. , Pagano, A. , Porta, G. , Allegorico, E. , Serra, C. , Guiotto, G. , Numis, F. G. , Tocchetti, C. G. , and Schiraldi, F. (2021) Time‐weighted lactate as a predictor of adverse outcome in acute heart failure. ESC Heart Failure, 8: 539–545. 10.1002/ehf2.13112.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Wagner S, Cohn K. Heart failure: a proposed definition and classification. Arch Intern Med 1977; 137: 675–678. [DOI] [PubMed] [Google Scholar]
- 3. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 2015; 372: 1078–1079. [DOI] [PubMed] [Google Scholar]
- 4. Biegus J, Zymliński R, Sokolski M, Gajewski P, Banasiak W, Ponikowski P. Clinical, respiratory, haemodynamic, and metabolic determinants of lactate in heart failure. Kardiol Pol 2019; 77: 47–52. [DOI] [PubMed] [Google Scholar]
- 5. Gjesdal G, Braun OO, Smith JG, Scherstén F, Tydén P. Blood lactate is a predictor of short‐term mortality in patients with myocardial infarction complicated by heart failure but without cardiogenic shock. BMC Cardiovasc Disord 2018; 18: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawase T, Toyofuku M, Higashihara T, Okubo Y, Takahashi L, Kagawa Y, Yamane K, Mito S, Tamekiyo H, Otsuka M, Okimoto T, Muraoka Y, Masaoka Y, Shiode N, Hayashi Y. Validation of lactate level as a predictor of early mortality in acute decompensated heart failure patients who entered intensive care unit. J Cardiol 2015; 65: 164–170. [DOI] [PubMed] [Google Scholar]
- 7. Zymliński R, Biegus J, Sokolski M, Siwołowski P, Nawrocka‐Millward S, Todd J, Jankowska EA, Banasiak W, Cotter G, Cleland JG, Ponikowski P. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur J Heart Fail 2018; 20: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 8. Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, Laggner AN. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore) 2004; 83: 274–279. [DOI] [PubMed] [Google Scholar]
- 9. Vernon C, Letourneau JL. Lactic acidosis: recognition, kinetics, and associated prognosis. Crit Care Clin 2010; 26: 255–283. [DOI] [PubMed] [Google Scholar]
- 10. Nichol A, Bailey M, Egi M, Pettila V, French C, Stachowski E, Reade MC, Cooper DJ, Bellomo R. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care 2011; 15: R242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott S, Antonaglia V, Guiotto G, Paladino F, Schiraldi F. Two‐hour lactate clearance predicts negative outcome in patients with cardiorespiratory insufficiency. Crit Care Res Prac 2010; 2010: 917053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biegus J, Zymliński R, Gajewski P, Sokolski M, Siwołowski P, Sokolska J, Swoboda K, Banasiak M, Banasiak W, Ponikowski P. Persistent hyperlactataemia is related to high rates of in‐hospital adverse events and poor outcome in acute heart failure. Kardiol Pol 2019; 77: 355–362. [DOI] [PubMed] [Google Scholar]
- 13. Bosso G, Mercurio V, Diab N, Numis F, Pagano A, Porta G, Paladino F, Guiotto G, Schiraldi F. Time weighted lactate as a predictor of adverse outcomes in acute heart failure. Am J Respir Crit Care Med 2018; B44: A3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pagano A, Numis FG, Rosato V, Russo T, Porta G, Bosso G, Serra C, Masarone M, Visone G, Paladino F. Pressure support ventilation vs continuous positive airway pressure for treating of acute cardiogenic pulmonary edema: a pilot study. Respir Physiol Neurobiol 2018; 255: 7–10. [DOI] [PubMed] [Google Scholar]
- 15. Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009; 37: 1670–1677. [DOI] [PubMed] [Google Scholar]
- 16. Raux M, Le Manach Y, Gauss T, Baumgarten R, Hamada S, Harrois A, Riou B, Duranteau J, Langeron O, Mantz J, Paugam‐Burtz C, Vigue B, TRAUMABASE Group . Comparison of the prognostic significance of initial blood lactate and base deficit in trauma patients. Anesthesiology 2017; 126: 522–533. [DOI] [PubMed] [Google Scholar]
- 17. Riveiro DF, Oliveira VM, Braunner JS, Vieira SR. Evaluation of serum lactate, central venous saturation, and venous‐arterial carbon dioxide difference in the Prediction of mortality in postcardiac arrest syndrome. J Intensive Care Med 2016; 31: 544–552. [DOI] [PubMed] [Google Scholar]
- 18. Meregalli A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high‐risk, surgical patients. Crit Care 2004; 8: R60–R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adamo L, Nassif ME, Novak E, LaRue SJ, Mann DL. Prevalence of lactic acidemia in patients with advanced heart failure and a depressed cardiac output. Eur J Heart Fail 2017; 19: 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qvisth V, Hagstrom‐Toft E, Enoksson S, Bolinder J. Catecholamine regulation of local lactate production in vivo in skeletal muscle and adipose tissue: role of β‐adrenoreceptor subtypes. J Clin Endocrinol Metab 2008; 93: 240–246. [DOI] [PubMed] [Google Scholar]
- 21. Kisaka T, Stringer WW, Koike A, Agostoni P, Wasserman K. Mechanisms that modulate peripheral oxygen delivery during exercise in heart failure. Ann Am Thorac Soc 2017; 14: S40–S47. [DOI] [PubMed] [Google Scholar]
- 22. Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, Kluge S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 2016; 42: 202–210. [DOI] [PubMed] [Google Scholar]
- 23. Vincent JL, Quintairos E, Silva A, Couto L Jr, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016; 20: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, Cooper DJ. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multicentre study. Crit Care 2010; 14: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 2018; 118: 691–728. [DOI] [PubMed] [Google Scholar]


