Abstract
Aims
The aim of the present paper was to provide an up‐to‐date view on epidemiology and risk factors of heart failure (HF) development after myocardial infarction.
Methods and results
Based on literature review, several clinical risk factors and biochemical, genetic, and imaging biomarkers were identified to predict the risk of HF development after myocardial infarction.
Conclusions
Heart failure is still a frequent complication of myocardial infarction. Timely identification of subjects at risk for HF development using a multimodality approach, and early initiation of guideline‐directed HF therapy in these patients, can decrease the HF burden.
Keywords: Heart failure, Clinical risk factors, Biomarkers, Genetics, Adverse remodelling
Introduction
Despite the remarkable advances in the treatment of coronary artery disease and acute myocardial infarction (MI) over the past two decades, MI remains the most common cause of heart failure (HF). 1 According to the time sequence of MI occurrence and HF development, three clinical presentations differing in pathophysiology, clinical characteristics, and outcomes can be identified: (i) HF onset at the time of MI presentation, (ii) HF developing during hospitalization for MI, and (iii) HF onset after discharge from the index hospitalization.
Heart failure developing at the time of myocardial infarction hospitalization
The factors that contribute to the pathogenesis of HF development at the time of the MI hospitalization include myocardial compromise due to myocardial necrosis, myocardial stunning, and mechanical complications such as papillary muscle rupture, ventricular septal defect, and ventricular free wall rupture. Within 30 min of ischaemia, cardiomyocyte structural changes and oedema develop, leading to progressive myocyte death after 3 h of ischaemia. Reperfusion itself causes a second wave of injury through the production of reactive oxygen species. Despite successful epicardial reperfusion, the embolization of thrombotic debris leads to ongoing microvascular dysfunction and myocardial ischaemia. 2 The inflammatory response to myocyte death also contributes to HF development. Furthermore, HF at this stage can be also triggered by exacerbation of pre‐existing HF and co‐morbidities, for example, anaemia, chronic kidney disease (CKD), or chronic obstructive pulmonary disease.
Opposing trajectories of HF incidence presenting at MI admission and during a hospital stay have been observed in the last decades. While the proportion of HF cases at MI admission has increased (from 4% in 1992–1996 3 to 12–13% in 2001–2011 4 , 5 ), the proportion of HF cases developing during the hospital stay has decreased (from 39% 3 to 4–28% 4 , 5 , 6 ). The increase in HF at MI presentation may be explained by recent improvements in pre‐hospital care, which led to a decrease in out of hospital mortality. 7 , 8 On the other hand, the decrease in in‐hospital HF may be caused by the introduction of percutaneous coronary intervention (PCI), which leads to more substantial myocardial salvage as compared with thrombolysis. In the nationwide SWEDEHEART registry, 6 the incidence of in‐hospital HF complicating MI has fallen from 46% in the thrombolytic era (the year 1996) to 28% in PCI era (the year 2008) (Figure 1 ). Higher myocardial salvage by PCI can also explain the increase in the proportion of patients with HF with preserved ejection fraction, which increased from 18% in 1998 to 30% in 2008. The second explanation for the in‐hospital HF decrease may be the change in MI diagnosis, which is currently based on troponin level and allows the detection of less severe MI cases with a lower risk of HF development.
Figure 1.
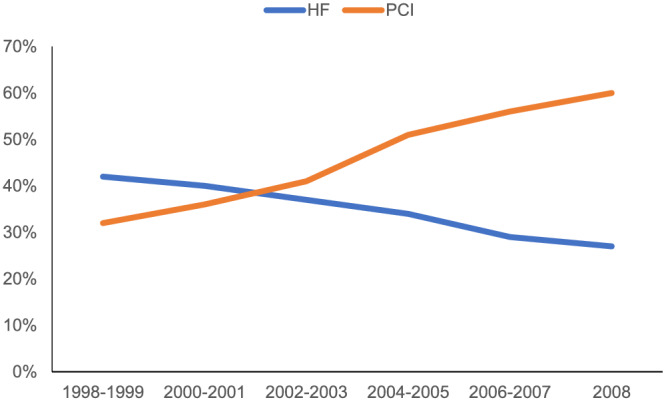
Percentage of patients with index myocardial infarction undergoing percutaneous coronary intervention (PCI) (orange line) and with in‐hospital heart failure (HF) (blue line)—adapted from SWEDEHEART study. 6
Heart failure developing after myocardial infarction hospitalization
Heart failure developing after MI hospitalization is a consequence of cardiomyocyte death and scar formation, which triggers chronic neurohumoral activation (renin–angiotensin–aldosterone and sympathetic nervous system up‐regulation) and ventricular remodelling. Left ventricular (LV) remodelling is more pronounced in men, patients with larger infarct size, and late or unsuccessful reperfusion of epicardial or microvascular bed. 9 Ventricular remodelling changes ventricular geometry and leads to wall thinning, ischaemic mitral regurgitation, and further cardiomyocyte loss.
Heart failure development after hospital discharge is very prevalent. It is diagnosed in approximately 13% of patients at 30 days and 20–30% at 1 year after discharge for MI. 10 , 11 The incidence of HF after MI discharge is highest in the first months, and then it drops and remains stable at a rate of 1.3–2.2% per year afterwards. 11
Clinical impact of heart failure after myocardial infarction
The development of HF after MI has a significant impact on outcomes, regardless of the HF type. 12 Among patients with a history of MI, HF development increases total mortality risk three‐fold and cardiovascular mortality four‐fold. The timing of HF development also has an impact on adverse events. HF developing more than 3 days after MI is associated with a 43% higher mortality risk as compared with patients with HF developing in the first 3 days after MI. 12 This may be explained by different risk factors and mechanisms leading to HF at different time points.
The need for heart failure screening and prevention
As recognized by both the European Society of Cardiology 13 and the American Heart Association, 14 HF prevention is an urgent public health need. The population of patients after MI represent a high‐risk group for HF development, in which HF screening and prevention is of particular importance. Missed or delayed diagnosis of HF compromises patient prognosis and increases therapy costs. This underscores the need for close follow‐up of MI patients at risk for HF development, which has been shown to result in improved patient adherence, higher prescription of recommended therapy, and lower cardiovascular hospitalizations. 15 , 16 , 17
The present paper reviews risk factors and biomarkers associated with HF development after MI and suggests a multimodality approach for screening (Figure 2 ). This information is intended to assist clinicians in identifying patients at particular risk of HF development after MI and early initiation of guideline‐directed HF therapy in these patients at high risk of adverse clinical events.
Figure 2.
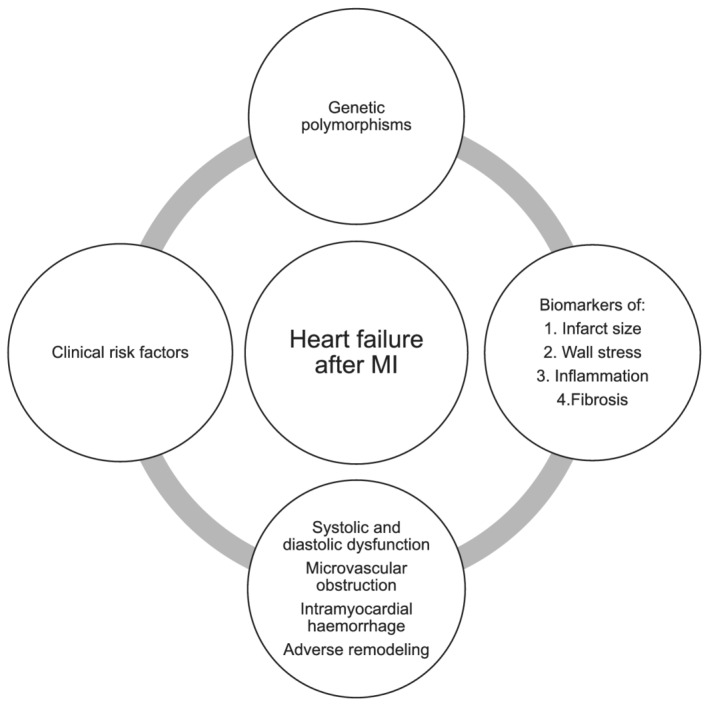
Multimodal approach for prediction of heart failure after myocardial infarction (MI).
Clinical risk factors
The impact of different clinical risk factors on the HF risk after MI is shown in Table 1 .
Table 1.
Clinical risk factors for HF
| Clinical risk factors | Increase in risk of post‐MI HF |
|---|---|
| Age, increase by 10 years | 20–50% |
| Female sex | 15–34% |
| History of previous MI | 21–89% |
| Hypertension | 7–70% |
| Diabetes | 30–42% |
| Glomerular filtration, decrease by 10 mL/min/1.73 m2 | 10% |
| Heart rate, increase by 10 b.p.m. | 7–23% |
| Atrial fibrillation | 20–51% |
HF, heart failure; MI, myocardial infarction.
Age
The incidence of in‐hospital HF is three times higher in patients 75–85 years old as compared with those 25–54 years old. After hospital discharge, HF incidence is six times higher in the older age group. 11 After multivariate adjustment, the in‐hospital HF risk increases by approximately 50% 5 and post‐discharge HF by 20–50% 18 , 19 , 20 for every 10 years of age.
Gender
Female sex was found to be independently associated with increased HF risk after MI in some studies, but not in others. 18 , 19 , 21 , 22 , 23 In the studies that reported higher risk associated with female sex, the excess HF risk ranged from 15% to 34%. 6 , 20 , 24
Several reasons may explain higher HF risk in women. Compared with men, female patients presenting with MI are older and have a higher prevalence of co‐morbidities and worse functional status. 25 The impact of co‐morbidities such as diabetes, hypertriglyceridemia, and metabolic syndrome on cardiovascular risk appears to be higher in women than men. Furthermore, gender disparities in MI presentation 26 , 27 and less aggressive hospital care of female patients, 27 including the underuse of revascularization, may further contribute to the higher HF risk in women. 28
Number and location of infarct‐related artery
Multi‐vessel disease (MVD) reflects the high atherosclerotic burden with more prominent endothelial dysfunction and systemic inflammation. 29 Patients with MVD are generally older and have diabetes and renal impairment as common co‐morbidities. MVD is associated with lower ejection fraction 22 , 29 and increased risk of major adverse cardiovascular events (MACE), including HF, by 80%. 29 Anterior MI is associated with a higher risk of adverse remodelling 30 and HF. 31 The higher risk of HF associated with anterior MI is caused by the greater magnitude of irreversible LV damage, as compared with other MI locations. 32
Prior myocardial infarction
A history of MI increases the risk of HF by 21–89%. 5 , 6 , 18 , 21 , 24 , 33 , 34 Excess risk may be explained by pre‐existing systolic and/or diastolic dysfunction.
Arterial hypertension
Many studies reported that arterial hypertension increases the risk of HF. The excess risk associated with arterial hypertension ranged from 7% to 70%. 6 , 18 , 20 , 21 More common microvascular injury and myocardial haemorrhage contribute to the excess HF risk in patients with arterial hypertension. 35 Furthermore, higher neurohormonal activation and more common LV remodelling was described in hypertensive patients after MI. 36
Higher heart rate
A higher heart rate at admission was a risk factor for HF after acute MI in several studies. 5 , 18 , 21 , 22 , 33 The risk rises by 7–23% for every 10 beats. 5 , 21 , 22 Tachycardia may reflect MI severity and imminent cardiac dysfunction.
Atrial fibrillation
New‐onset atrial fibrillation complicates 2–21% cases of MI and may reflect left atrial pressure increase and atrial fluid overload during MI. 37 Atrial fibrillation increases the risk of HF after MI by 20–51%. 18 , 33
Diabetes
After MI, the incidence of HF among diabetic patients is 60–70% higher than in patients without diabetes. 38 After accounting for other co‐morbidities associated with diabetes, its presence still results in 30–42% higher risk of HF after MI. 5 , 6 , 39 , 40 Compared with non‐diabetic patients with similar infarct size, 41 similar systolic function, and infarct‐related coronary artery patency rate, 42 diabetic patients develop more often adverse LV remodelling and HF. 43 This may be explained by a more common microvascular obstruction 42 and diastolic dysfunction in those with diabetes. 44 The excess risk seems to be similar in patients with pre‐existing diabetes and diabetes diagnosed at the time of MI. 41 , 45
Chronic kidney disease
Chronic kidney disease increases the risk of HF development after MI approximately two‐fold. 20 Excess HF risk in CKD can be explained by accelerated atherosclerosis, more common MVD, atypical MI presentation, and lower odds of revascularization, which results in larger infarct size and more severe ventricular dysfunction. Moreover, CKD leads to fluid overload, secondary hypertension, anaemia, chronic inflammation, and alterations of the renin–angiotensin–aldosterone system. 46 , 47 Lower prescription of evidence‐based medications in CKD patients has also been reported. 48
Ischaemic preconditioning and heart failure after myocardial infarction
Preconditioning is the process by which brief, repetitive episodes of ischaemia reduce the size of a subsequent MI. Compared with those without antecedent angina, patients with angina have a decreased risk of HF development during MI, lower risk of mortality or adverse LV remodelling after MI, and enhanced recovery of cardiac contractile function after MI. 49 , 50 The protective effect of angina has been described up to 3 months prior to MI. 51 The difference in outcomes in patients with and without angina preceding MI may be explained by ischaemic preconditioning or a larger extent of collateral circulation in patients with antecedent angina.
Whether the ischaemic preconditioning data could be applied to clinical care to improve outcomes remains to be seen. Remote ischaemic conditioning with transient ischaemia and reperfusion of the arm or leg has been shown in several small randomized controlled trials to reduce myocardial infarct size and increase myocardial salvage in patients with ST‐segment elevation MI (STEMI). 52 , 53 However, in the recent large CONDI‐2 trail among 5401 STEMI patients, remote ischaemic conditioning by intermittent ischaemia and reperfusion applied to the arm did not decrease the risk of death or hospitalization for HF. 54
Coronavirus disease 2019
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 likely affects the risk of HF development after MI. However, hard clinical data are still lacking. There are several mechanisms by which COVID‐19 pandemic may influence the risk of HF development after MI.
First, the incidence of acute coronary syndrome (ACS) increases in the setting of viral infection, likely due to inflammation‐mediated plaque destabilization. The risk in the setting of COVID‐19 infection is unknown, but other viruses are associated with a 3‐fold to 10‐fold increased risk. 55 However, a decrease in MI hospitalizations was observed in several countries, 56 , 57 , 58 with a parallel increase in fatality and complication rates. Also, both patient‐related and system‐related delays were noted, with a 40% increase in symptom onset to coronary angiography time during the COVID‐19 pandemic. Thus, the absence or delay in coronary revascularization during MI may increase the proportion of patients with HF.
Second, there is growing evidence that COVID‐19 leads to direct myocardial injury. Within 24 h from admission for COVID‐19, troponin elevation is present in 36% of patients. 59 Among recovering patients evaluated a mean of 71 days after confirmed COVID‐19 diagnosis, 78% of patients have demonstrable cardiac involvement via cardiac magnetic resonance imaging (MRI), 76% have detectable high‐sensitivity troponin, and 60% have evidence of active myocardial inflammation. 60 The mechanisms responsible for myocardial injury during COVID‐19 infection may include inflammation, cytokine storm, hypercoagulable state with formation of microthrombi and macrothrombi, direct viral invasion of the myocardium, and myocardial supply/demand imbalance. 59 MI in the setting of COVID‐19 infection likely increases not only the mortality risk 56 but also the risk of subsequent HF development.
Biochemical markers
Biomarkers of infarct size
Cardiac troponin, a biomarker of choice in MI diagnostics, measured at plateau phase (48–72 h after MI symptom onset) is associated with MRI determined infarct size. 61 Similarly, peak levels of creatine kinase (CK) and CK‐MB are associated with infarct size on single‐photon emission computed tomography. 62 Several studies have shown the association of troponin or CK‐MB level with MACE, including HF. 19 , 63 , 64 Yet the association of peak troponin or CK‐MB with HF has not been seen in all investigations. 65 , 66
Natriuretic peptides
Alongside troponin, natriuretic peptides are associated with infarct size and cardiac dysfunction. 67 , 68 In addition to the magnitude of natriuretic peptides elevation, 66 , 69 its pattern is also associated with adverse events. While in some patients the brain natriuretic peptide increase after MI has a monophasic pattern with a peak at 16 h after admission, in others, the rise is biphasic with a second peak at 5 days. Patients demonstrating the biphasic pattern have a higher risk of LV remodelling and HF. 70 , 71 Additionally, premorbid N‐terminal prohormone brain natriuretic peptide levels, as well as high‐sensitivity troponin T levels measured at a median time of 6 years before MI, have also been associated with adverse events, including HF. 72
Inflammation markers
There is growing evidence that prolongation or expansion of the post‐infarction inflammatory response significantly contributes to LV remodelling and HF development. 73 Numerous methods of inflammatory response quantification have shown promise in HF prediction. C‐reactive protein level predicted the risk of adverse events after MI, including HF, in several studies. 74 , 75 , 76 , 77 The neutrophil‐to‐lymphocyte ratio, an indicator of systemic inflammation, predicted MACE and HF in a meta‐analysis of 14 studies. 78
Cytokines are strategic regulators of inflammation. In a study of 4939 patients with ACS, pro‐inflammatory cytokine interleukin 6 (IL‐6) was an independent predictor of MACE and HF. 79 IL‐32 is a relatively novel pro‐inflammatory cytokine that induces the release of other inflammatory cytokines such as tumour necrosis factor‐α, IL‐1β, IL‐6, IL‐8, and IL‐18. Xuan et al. showed that IL‐32 is an independent predictor of cardiac death and HF among patients after MI. 80
Renal biomarkers
The estimated glomerular filtration rate (eGFR) is independently associated with HF risk after MI. Fox et al. used a creatinine‐based MDRD equation for eGFR calculation to show that, after multivariate adjustments, the excess risk of HF attributable to renal dysfunction ranged from 30% to 90% depending on CKD stage. 81 Similar results were reported from the VALIANT study where the risk of HF rose by 10% for each 10 mL/min/1.73 m2 decrease in eGFR. 18
Cystatin C is a sensitive marker of renal impairment that, unlike creatinine, is not affected by age, sex, and muscle mass. In the SOLID‐TIMI 52 study among patients with ACS, cystatin C provided incremental information for risk stratification, including HF hospitalization, independent of other biomarkers including eGFR. 82
Biomarkers of fibrosis
Suppressor of tumourigenesis (ST2) is a member of the IL‐1 receptor family that is involved in the process of myocardial remodelling and fibrosis., 83 , 84 It has two isoforms: transmembrane ligand and soluble form. Binding of the soluble form (sST2) to IL‐33 prevents the beneficial effect of this IL on the reduction of cell death and fibrosis. While several studies demonstrated the prognostic utility of sST2 testing in HF, there is less evidence for the predictive value of sST2 after MI. Among consecutive MI patients from the Mayo Clinic, sST2 elevation was independently associated with excess risk of death and HF. Patients in the upper tertial of sST2 had three‐fold the risk of HF as compared with the lowest tertial. 85
Galectin‐3, a β‐galactoside‐binding lectin mainly secreted by activated macrophages, is also reflective of fibrosis and cardiac remodelling in response to myocardial injury. 86 The American College of Cardiology/American Heart Association guidelines recommend measurement of both sST2 and galectin‐3 for risk prediction in patients with HF. 87 A recent study suggested an independent predictive value of galectin‐3 also among unselected MI patients. In a prospectively enrolled incident MI cohort, galectin‐3 was associated with increased risk of death and HF even after adjustment for MI severity, co‐morbidities, and sST2. 88
Other biomarkers
Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade collagen and other proteins of the extracellular matrix. After MI, MMPs regulate the remodelling process by facilitating extracellular matrix turnover and inflammatory signalling. MMP‐8 and MMP‐9 were shown to predict LV remodelling 89 , 90 and adverse outcomes, including HF development. 91
Clusterin is a protein that regulates complement activity, apoptosis, and lipid transport. Proteomic analysis of plasma from patients after the first anterior MI identified increased plasma levels of clusterin to be associated with LV remodelling. 92 Whether clusterin is also associated with the risk HF after MI needs to be determined.
The prognostic utility of serial biomarker measurement and multi‐marker approach after MI was evaluated by Reinstadler et al., 93 who measured several biomarkers as aspartate and alanine aminotransferases, high‐sensitivity troponin T, N‐terminal prohormone brain natriuretic peptide, lactate dehydrogenase, and high‐sensitivity C‐reactive protein daily for 4 days after admission for an MI. They reported that the peak level of the biomarkers studied was associated with LV remodelling, while the admission value was not. Furthermore, combined biomarker analysis was superior to any of the individual biomarkers. 93 Thus, not only the selection of a biochemical biomarker but also timing of the measurement after MI may be of importance in HF risk prediction.
Genetic aspects
There is a paucity of data on genetic predictors of HF development after MI.
A recent study using weighted gene co‐expression network analysis identified genes BCL3, HCK, PPIF, S100A9, SERPINA1, and TBC1D9B to be involved in the inflammatory response, apoptosis, and HF development after MI. 94
MicroRNAs are products of non‐coding DNA transcription consisting of approximately 22 nucleotides that act as significant regulators of mRNA translation. In the heart, they control various processes including cardiac cell death, cardiomyocyte regeneration, and cardiac fibroblast transformation into cardiomyocytes. 95 A study by Niu et al. recognized miR‐142‐3p as a contributor to HF after MI. 94 Shah et al. identified lower concentrations of miR‐17‐5p, miR‐20a‐5p, and miR‐106b‐5p to be associated with a higher incidence of HF after MI. 96 In a study by Lakhani et al., miRNA‐24 and 29a levels were reduced in patients with acute MI and low ejection fraction, whereas miRNA‐34a, miRNA‐208b, and miRNA‐126 were increased in these patients. 97
Imaging
Echocardiography
Echocardiography is a commonly used imaging method after MI. In regard to HF prediction, optimal timing of echocardiography is not well defined, as the early post‐MI examination may underestimate systolic function due to myocardial stunning. Therefore, repeated echocardiographic examinations after MI are recommended. 98
Systolic function
Reduced LV ejection fraction (LVEF) is associated with the risk of HF development. 18 , 21 , 24 A 5% decrease in LVEF determined by ventriculography performed during the MI hospitalization increases the risk of HF development after the hospital discharge by 12–18%. 22 , 24 Similarly, a 5% decrease in LVEF evaluated by echocardiography 5–20 months after MI increases the risk of HF by 20%. 18
The wall motion score index (WMSI) reflects wall motion abnormalities better than LVEF because compensatory hyperkinesia of non‐affected regions may compensate for the impaired systolic function. 98 , 99 In a study of 144 patients with MI, WMSI ≥ 1.5 identified people at increased risk of cardiac death, unstable angina, and HF, independent of LVEF. 100 In a study by Møller et al., each 0.2 increase in WMSI was associated with hazard ratio of 1.21 (95% confidence interval 1.07–1.37, P = 0.002) for HF development and hazard ratio of 1.15 (95% confidence interval 1.10–1.21, P < 0.0001) for mortality. 99 A study by Jurado‐Román et al. deemed WMS a more powerful predictor of mortality and HF than LVEF. 101
Right ventricular (RV) dysfunction significantly contributes to HF development after MI. The tricuspid annular plane systolic excursion (TAPSE) is the most commonly used parameter to evaluate RV systolic function. In patients after MI, RV dysfunction defined by TAPSE ≤ 14 mm was able to predict early cardiac events, including cardiogenic shock. 102 However, TAPSE assesses only longitudinal contraction of RV and as such provides only partial information on RV function. Fractional area change reflects RV function better than TAPSE. In several studies among patients after MI, decreased fractional area change was associated with an increased risk of adverse events, including HF. 103 , 104
Diastolic function
Standard Doppler examination of transmitral flow provides valuable information in patients after MI. A meta‐analysis of 12 studies by Møller et al. found that restrictive filling pattern is associated with an increased risk of all‐cause mortality and HF. 105 Tissue Doppler‐derived parameter of E/e′ over 15 is also a strong predictor of mortality and HF development after MI. 106
Left ventricular remodelling
Left ventricular remodelling is commonly defined as a 20% increase in LV end‐diastolic volume. 9 Post‐MI remodelling is exacerbated by a larger infarct size, transmural MI, microvascular obstruction, myocardial haemorrhage, and advanced age. In the contemporary era, almost half of patients after MI demonstrate LV remodelling on echocardiography within 1 year from MI. Among patients with LV remodelling, the risk of hospitalization for HF is 2.7 times higher than in those without LV remodelling. 107
Speckle tracking echocardiography
Speckle tracking echocardiography (STE) measures regional and global myocardial deformation. Global longitudinal (apico‐basal) strain is the most used and is superior to LVEF measurement, especially in the early phases of systolic dysfunction. In a study by Ersbøll et al. among MI patients with LVEF > 40%, global longitudinal (apico‐basal) strain higher than −14 was associated with a five times higher risk of HF and 12 times higher risk of cardiac death. 108 3D STE is a novel method offering more realistic and accurate models of LV than 2D STE. 109 Global area strain, one of the 3D STE parameters, combines both longitudinal and circumferential strains. Among patients after MI, global area strain is an independent predictor of MACE and HF hospitalization, superior to conventional 2D echocardiography parameters. 110 , 111
Myocardial contrast echocardiography
Myocardial contrast echocardiography (MCE) visualizes myocardial perfusion by intravenous or intracoronary infusion of microbubbles. MCE can distinguish reversible and irreversible ischaemia, thus detecting myocardial viability. 98 Lack of perfusion signals caused by microvascular obstruction on MCE is consistent with the results of cardiac magnetic resonance. 98 To detect no‐reflow, MCE should be ideally performed 24–48 h after coronary intervention for MI. In several studies, no‐reflow after MI was an independent predictor of LV recovery and adverse outcomes including HF. 112 , 113
Stress echocardiography
Dobutamine stress echo‐derived parameters such as infarction zone non‐viability and ischaemia/infarction at a distance were identified as independent predictors of adverse outcomes, including HF, in patients 2 to 7 days after MI. 114
Cardiac magnetic resonance
Infarct size
Magnetic resonance imaging is currently the gold standard imaging modality for quantifying infarct size using late gadolinium enhancement. According to a meta‐analysis of studies measuring infarct size by MRI or single‐photon emission computed tomography in patients after STEMI, for every 5% increase in MI size, the risk of hospitalization for HF increases by 20%. 115
On the other hand, a recent prospective study showed no additional long‐term prognostic value of infarct size measured by MRI over LVEF in patients after non‐STEMI. 116 The difference in prognostic value in STEMI and non‐STEMI may be explained by a different magnitude of myocardial damage.
Microvascular obstruction
Microvascular obstruction refers to the lack of perfusion in the coronary microcirculation, despite revascularization of the epicardial vessels. Microvascular obstruction can be identified as a hypointense core within the area of hyperenhancement on either early (referred to as early microvascular obstruction) or late gadolinium enhancement (late microvascular obstruction). Microvascular obstruction is associated with larger MI size and adverse remodelling. In a recent individual patient data meta‐analysis, microvascular obstruction increase by 10% elevated the risk of hospitalization for HF by 80% and all‐cause mortality by 114%. 117
Intramyocardial haemorrhage
If the microvascular injury after MI is severe and the integrity of microcirculation is compromised, extravasation of red blood cells into the myocardium can occur. Red blood cell extravasation is referred to as intramyocardial haemorrhage and can be detected by MRI as a hypointense zone within the MI core on T2* imaging or mapping. Several studies suggest that iron deposits from red blood cells trigger pro‐inflammatory response and lead to adverse LV remodelling. 118 , 119 Smaller studies in patients after STEMI suggested that myocardial haemorrhage was more closely associated with adverse outcomes, including HF, than microvascular obstruction. 120 . 121
Multiple scars
A MRI substudy of the third Danish Study of Optimal Acute Treatment of Patients with ST‐segment Elevation Myocardial Infarction (DANAMI 3) proved that multiple scars (characterized by late gadolinium enhancement in more than one myocardial areas remote from acute infarction area) were associated with almost three‐fold risk of all‐cause mortality and HF hospitalization after adjustment for clinical risk factors and MI size. 122
Molecular imaging
Molecular imaging is an emerging method studying different phases of the post‐MI period at a molecular level. The principle is based on the existence of specific tracers that bind to molecules of interest. Various methods of nuclear medicine have shown potential to predict adverse LV remodelling [e.g. tracers binding to MMP‐2 or MMP‐9 within the infarct zone, angiotensin‐converting enzyme inhibitor (ACEi) and angiotensin receptor antagonist‐based tracers, growth factor receptors, and αvβ3 integrin tracers]. 123 Currently, data showing predictive value of these methods for HF prediction after MI are lacking.
Remote monitoring
Among patients with established HF, various remote monitoring strategies have been tested to detect worsening of HF and reduce the risk of HF readmission. Several implantable 124 (using data from cardioverter defibrillators and cardiac resynchronization therapy) and wearable 125 devices have shown the capability to detect HF exacerbation.
However, most of the studies with implantable devices could not detect the mortality benefit of telemonitoring. 126 , 127 , 128 The exception was IN‐TIME trial (Biotronik Home Monitoring technology), which documented improvement of a composite clinical score and particularly all‐cause mortality in remote monitoring group of patients. 129 Pooled analysis of the three trials with the same monitoring system with daily transmissions (TRUST, ECOST, and IN‐TIME) confirmed 38% and 36% reduction of all‐cause mortality and the composite endpoint of all‐cause mortality or hospitalization for HF worsening, respectively. The benefit of this form of monitoring appears to be driven by the prevention of HF exacerbation, mainly due to early detection of arrhythmias and/or loss of biventricular pacing. 130 Another technology that was capable to reduce HF hospitalizations was the CardioMEMS device—an implantable pulmonary artery pressure monitor. 131 Yet no remote monitoring study has so far targeted at‐risk population after MI.
Guideline‐recommended therapies
Pharmacotherapy
Beta‐blockers
Beta‐blockers interfere with the harmful effects of sustained activation of the sympathetic nervous system, particularly by blocking the β1‐adrenergic receptors. Together with ACEi, angiotensin receptor blockers, and statins, beta‐blockers indirectly inhibit MMPs. 132
The evidence for the favourable effect of beta‐blockers on post‐MI outcomes comes mainly from the pre‐thrombolytic era. In a meta‐analysis of 31 randomized studies, long‐term beta‐blocker use reduced all‐cause mortality after MI by 23%. 133 However, none of the studies specifically involved patients with systolic dysfunction and HF during MI hospitalization. 134
In the SAVE and AIRE studies, which originally analysed the effect of ACEi in MI patients with LV systolic dysfunction, beta‐blocker use reduced the risk of progression to severe HF by 21% and 42%, respectively. 135 , 136 In the CAPRICORN study, carvedilol administered in MI patients with systolic dysfunction (LVEF < 40%) reduced all‐cause mortality by 23% and HF hospitalization by 14% compared with placebo. 134 This effect was additional to ACEi. 134
Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers
Activation of the renin–angiotensin–aldosterone system actively participates in the process of LV remodelling, myocardial fibrosis, and HF development after MI. ACEi by blocking conversion of angiotensin I into angiotensin II supresses vasoconstriction and aldosterone secretion mediated by angiotensin II. Angiotensin receptor blockers block this action of angiotensin II by interfering with the binding of angiotensin II to its receptor.
Early initiation of ACEi within 0–36 h from MI symptom onset reduces 30 day mortality and HF by 7% and 4%, respectively. 137 The absolute benefit is greater in high‐risk groups (such as Killip Class II/III, heart rate >100 b.p.m. at entry) and anterior MI. Importantly, 40% of the survival benefit occurred on the first day of treatment, underscoring the value of initiating ACEIs early, as long as patients have adequate blood pressure. 137 The positive effect of ACEi post‐MI was proved also in long‐term studies. 138 , 139 , 140 ACEIs started between 3 and 16 days post‐MI reduce the relative risk of mortality by 26% and readmission for HF by 27%. 141
Angiotensin receptor blockers are used in patients with intolerance of ACEi. Various studies have shown favourable effect of losartan or valsartan on mortality and HF hospitalization. 39 , 142
Mineralocorticoid receptor antagonist
Aldosterone by its action on distal nephron increases sodium and water reabsorption leading to an expansion of the extracellular fluid. In the heart, mineralocorticoid receptor activation triggers inflammation, hypertrophy, and fibrosis. Mineralocorticoid receptor antagonist eplerenone was tested in the EPHESUS study, in which patients after MI with LVEF < 40% and HF or diabetes were enrolled. As compared with placebo, eplerenone reduced all‐cause mortality and HF hospitalization by 15% and death from cardiovascular causes by 17%. 143 This effect was present only if eplerenone was administered in the first 7 days after MI. 144
Statins
Statins are lipid‐lowering drugs with pleiotropic effects. Besides inhibition of 3‐hydroxy‐3‐methylglutarylcoenzyme A reductase, the key enzyme in cholesterol synthesis, statins exert endothelium‐stabilizing, anti‐inflammatory, and antiproliferative effects on cells involved in atherosclerosis. 145 In the IDEAL and PROVE IT‐TIMI 22 studies, high doses of statin (atorvastatin 80 mg daily) decreased HF risk by 26% and 45% as compared with low to moderate statin dose, respectively. 146 , 147 Early administration of statins (within 24 h of hospitalization) is associated with a 2.5‐fold risk reduction of HF hospitalization and a three‐fold reduction in in‐hospital mortality. 148
Percutaneous coronary intervention
A decrease in HF after MI since the adoption of PCI has been well documented. 10 , 149 , 150 , 151 In population‐based studies from Sweden, Western Australia, Denmark, and Olmsted County in the USA, increase in PCI rates led to 20–41% reduction in HF after MI. 10 , 149 , 150 , 151
Conclusions
Development of HF after MI is associated with adverse events, impaired quality of life, and lower survival. As reviewed in this paper, a wide range of clinical, laboratory, and diagnostic findings are associated with HF development after MI. However, precise, cost‐effective, and accurate scoring system integrating clinical risk factors, genetics, biomarkers, and imaging methods for HF prediction and prognostication after MI is lacking. Better identification of patients at risk of HF development after MI is needed because timely initiation of guideline‐directed HF therapy can reduce the risk of further LV remodelling, morbidity, and mortality.
Conflict of interest
None declared.
Funding
This study was supported by the Ministry of Health of the Czech Republic (Ministerstvo Zdravotnictví Ceské Republiky), Grant No. NV 19‐09‐00125. All rights reserved.
Author contributions
D.J. performed the literature search and wrote the original draft. P.W. performed the literature search and critically revised the work. J.S., J. Kautzner, V.S., V.A., V.M., and J. Kettner critically revised the work.
Jenča, D. , Melenovský, V. , Stehlik, J. , Staněk, V. , Kettner, J. , Kautzner, J. , Adámková, V. , and Wohlfahrt, P. (2021) Heart failure after myocardial infarction: incidence and predictors. ESC Heart Failure, 8: 222–237. 10.1002/ehf2.13144.
References
- 1. Roger VL. Epidemiology of heart failure. Circ Res 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no‐reflow in humans. J Am Coll Cardiol 2009; 54: 281–292. [DOI] [PubMed] [Google Scholar]
- 3. Ali AS, Rybicki BA, Alam M, Wulbrecht N, Richer‐Cornish K, Khaja F, Sabbah HN, Goldstein S. Clinical predictors of heart failure in patients with first acute myocardial infarction. Am Heart J 1999; 138: 1133–1139. [DOI] [PubMed] [Google Scholar]
- 4. Shah RV, Holmes D, Anderson M, Wang TY, Kontos MC, Wiviott SD, Scirica BM. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: insights from the National Cardiovascular Data ACTION Registry. Circ Heart Fail 2012; 5: 693–702. [DOI] [PubMed] [Google Scholar]
- 5. Steg PG, Dabbous OH, Feldman LJ, Cohen‐Solal A, Aumont MC, Lopez‐Sendon J, Budaj A, Goldberg RJ, Klein W, Anderson FA Jr. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004; 109: 494–499. [DOI] [PubMed] [Google Scholar]
- 6. Desta L, Jernberg T, Lofman I, Hofman‐Bang C, Hagerman I, Spaak J, Persson H. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail 2015; 3: 234–242. [DOI] [PubMed] [Google Scholar]
- 7. Salomaa V, Ketonen M, Koukkunen H, Immonen‐Räihä P, Jerkkola T, Kärjä‐Koskenkari P, Mähönen M, Niemelä M, Kuulasmaa K, Palomäki P, Mustonen J, Arstila M, Vuorenmaa T, Lehtonen A, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesäniemi YA, Pyörälä K. Decline in out‐of‐hospital coronary heart disease deaths has contributed the main part to the overall decline in coronary heart disease mortality rates among persons 35 to 64 years of age in Finland. Circulation 2003; 108: 691–696. [DOI] [PubMed] [Google Scholar]
- 8. Dudas K, Lappas G, Stewart S, Rosengren A. Trends in out‐of‐hospital deaths due to coronary heart disease in Sweden (1991 to 2006). Circulation 2011; 123: 46–52. [DOI] [PubMed] [Google Scholar]
- 9. Flachskampf FA, Schmid M, Rost C, Achenbach S, DeMaria AN, Daniel WG. Cardiac imaging after myocardial infarction. Eur Heart J 2011; 32: 272–283. [DOI] [PubMed] [Google Scholar]
- 10. Hung J, Teng TH, Finn J, Knuiman M, Briffa T, Stewart S, Sanfilippo FM, Ridout S, Hobbs M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population‐based study of 20,812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc 2013; 2: e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sulo G, Igland J, Vollset SE, Nygård O, Ebbing M, Sulo E, Egeland GM, Tell GS. Heart failure complicating acute myocardial infarction; burden and timing of occurrence: a nation‐wide analysis including 86 771 patients from the cardiovascular disease in Norway (CVDNOR) project. J Am Heart Assoc 2016; 5: e002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerber Y, Weston SA, Enriquez‐Sarano M, Berardi C, Chamberlain AM, Manemann SM, Jiang R, Dunlay SM, Roger VL. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail 2016; 9: e002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC heart fail 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 14. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y, American Heart Association Council on Epidemiology and Prevention , American Heart Association Council on Clinical Cardiology , American Heart Association Council on Cardiovascular Nursing , American Heart Association Council on High Blood Pressure Research , Quality of Care and Outcomes Research Interdisciplinary Working Group , Functional Genomics and Translational Biology Interdisciplinary Working Group . Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 2008; 117: 2544–2565. [DOI] [PubMed] [Google Scholar]
- 15. Daugherty SL, Ho PM, Spertus JA, Jones PG, Bach RG, Krumholz HM, Peterson ED, Rumsfeld JS, Masoudi FA. Association of early follow‐up after acute myocardial infarction with higher rates of medication use. Arch Intern Med 2008; 168: 485–491. [DOI] [PubMed] [Google Scholar]
- 16. Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow‐up and medication adherence after acute myocardial infarction. JAMA Cardiol 2016; 1: 147–155. [DOI] [PubMed] [Google Scholar]
- 17. Tung YC, Chang GM, Chang HY, Yu TH. Relationship between early physician follow‐up and 30‐day readmission after acute myocardial infarction and heart failure. PLoS ONE 2017; 12: e0170061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis EF, Velazquez EJ, Solomon SD, Hellkamp AS, McMurray JJ, Mathias J, Rouleau JL, Maggioni AP, Swedberg K, Kober L, White H, Dalby AJ, Francis GS, Zannad F, Califf RM, Pfeffer MA. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J 2008; 29: 748–756. [DOI] [PubMed] [Google Scholar]
- 19. Gho JMIH, Postema PG, Conijn M, Bruinsma N, de Jong JSSG, Bezzina CR, Wilde AAM, Asselbergs FW. Heart failure following STEMI: a contemporary cohort study of incidence and prognostic factors. Open Heart 2017; 4: e000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wellings J, Kostis JB, Sargsyan D, Cabrera J, Kostis WJ. Risk factors and trends in incidence of heart failure following acute myocardial infarction. Am J Cardiol 2018; 122: 1–5. [DOI] [PubMed] [Google Scholar]
- 21. Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA, CARE Study . Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol 2003; 42: 1446–1453. [DOI] [PubMed] [Google Scholar]
- 22. O'Connor CM, Hathaway WR, Bates ER, Leimberger JD, Sigmon KN, Kereiakes DJ, George BS, Samaha JK, Abbottsmith CW, Candela RJ, Topol EJ, Califf RM. Clinical characteristics and long‐term outcome of patients in whom congestive heart failure develops after thrombolytic therapy for acute myocardial infarction: development of a predictive model. Am Heart J 1997; 133: 663–673. [DOI] [PubMed] [Google Scholar]
- 23. Shih J‐Y, Chen Z‐C, Chang H‐Y, Liu Y‐W, Ho C‐H, Chang W‐T. Risks of age and sex on clinical outcomes post myocardial infarction. Int J Cardiol Heart Vasc 2019; 23: 100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly DJ, Gershlick T, Witzenbichler B, Guagliumi G, Fahy M, Dangas G, Mehran R, Stone GW. Incidence and predictors of heart failure following percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the HORIZONS‐AMI trial. Am Heart J 2011; 162: 663–670. [DOI] [PubMed] [Google Scholar]
- 25. Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G, WISE Investigators . Insights from the NHLBI‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender‐optimized diagnostic strategies. J Am Coll Cardiol 2006; 47: S4–S20. [DOI] [PubMed] [Google Scholar]
- 26. Thuresson M, Jarlöv MB, Lindahl B, Svensson L, Zedigh C, Herlitz J. Symptoms and type of symptom onset in acute coronary syndrome in relation to ST elevation, sex, age, and a history of diabetes. Am Heart J 2005; 150: 234–242. [DOI] [PubMed] [Google Scholar]
- 27. Arora S, Stouffer GA, Kucharska‐Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL, Caughey MC. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation 2019; 139: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M. Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction‐I. Arch Intern Med 1998; 158: 981–988. [DOI] [PubMed] [Google Scholar]
- 29. Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, Stuckey T, Tcheng JE, Mehran R, Lansky AJ, Grines CL, Stone GW. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007; 28: 1709–1716. [DOI] [PubMed] [Google Scholar]
- 30. Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation 1993; 87: 755–763. [DOI] [PubMed] [Google Scholar]
- 31. Santoro GM, Carrabba N, Migliorini A, Parodi G, Valenti R. Acute heart failure in patients with acute myocardial infarction treated with primary percutaneous coronary intervention☆ . Eur J Heart Fail 2008; 10: 780–785. [DOI] [PubMed] [Google Scholar]
- 32. Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, Barison A, Carbone I, Lombardi M, Agati L, Janssens S, Bogaert J. Relationship between location and size of myocardial infarction and their reciprocal influences on post‐infarction left ventricular remodelling. Eur Heart J 2011; 32: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 33. Chadda K, Goldstein S, Byington R, Curb JD. Effect of propranolol after acute myocardial infarction in patients with congestive heart failure. Circulation 1986; 73: 503–510. [DOI] [PubMed] [Google Scholar]
- 34. Myftiu S, Bara P, Sharka I, Shkoza A, Belshi X, Rruci E, Vyshka G. Heart failure predictors in a group of patients with myocardial infarction. Open access Maced J Med Sci 2016; 4: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carrick D, Haig C, Maznyczka AM, Carberry J, Mangion K, Ahmed N, Yue May VT, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Hood S, Watkins S, Davie A, Mahrous A, Mordi I, Ford I, Radjenovic A, Welsh P, Sattar N, Wetherall K, Oldroyd KG, Berry C. Hypertension, microvascular pathology, and prognosis after an acute myocardial infarction. Hypertension 2018; 72: 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richards AM, Nicholls MG, Troughton RW, Lainchbury JG, Elliott J, Frampton C, Espiner EA, Crozier IG, Yandle TG, Turner J. Antecedent hypertension and heart failure after myocardial infarction. J Am Coll Cardiol 2002; 39: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 37. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 2008; 30: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 38. Melchior T, Rask‐Madsen C, Torp‐Pedersen C, Hildebrandt P, Kober L, Jensen G. The impact of heart failure on prognosis of diabetic and non‐diabetic patients with myocardial infarction: a 15‐year follow‐up study. Eur J Heart Fail 2001; 3: 83–90. [DOI] [PubMed] [Google Scholar]
- 39. Velazquez EJ, Francis GS, Armstrong PW, Aylward PE, Diaz R, O'Connor CM, White HD, Henis M, Rittenhouse LM, Kilaru R, van Gilst W, Ertl G, Maggioni AP, Spac J, Weaver WD, Rouleau JL, McMurray J, Pfeffer MA, Califf RM, VALIANT registry . An international perspective on heart failure and left ventricular systolic dysfunction complicating myocardial infarction: the VALIANT registry. Eur Heart J 2004; 25: 1911–1919. [DOI] [PubMed] [Google Scholar]
- 40. Wu AH, Parsons L, Every NR, Bates ER. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI‐2). J Am Coll Cardiol 2002; 40: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 41. Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O'Connor CM, Diaz R, Belenkov YN. Newly diagnosed and previously known diabetes mellitus and 1‐year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation 2004; 110: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 42. Prasad A, Stone GW, Stuckey TD, Costantini CO, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox DA, Grines CL, Lansky AJ, Gersh BJ. Impact of diabetes mellitus on myocardial perfusion after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol 2005; 45: 508–514. [DOI] [PubMed] [Google Scholar]
- 43. Solomon SD, St John Sutton M, Lamas GA, Plappert T, Rouleau JL, Skali H, Moyé L, Braunwald E, Pfeffer MA, Survival And Ventricular Enlargement (SAVE) Investigators . Ventricular remodeling does not accompany the development of heart failure in diabetic patients after myocardial infarction. Circulation 2002; 106: 1251–1255. [DOI] [PubMed] [Google Scholar]
- 44. Thune JJ, Solomon SD. Left ventricular diastolic function following myocardial infarction. Curr Heart Fail Rep 2006; 3: 170–174. [DOI] [PubMed] [Google Scholar]
- 45. Ertelt K, Brener SJ, Mehran R, Ben‐Yehuda O, McAndrew T, Stone GW. Comparison of outcomes and prognosis of patients with versus without newly diagnosed diabetes mellitus after primary percutaneous coronary intervention for ST‐elevation myocardial infarction (the HORIZONS‐AMI study). Am J Cardiol 2017; 119: 1917–1923. [DOI] [PubMed] [Google Scholar]
- 46. Shroff GR, Frederick PD, Herzog CA. Renal failure and acute myocardial infarction: clinical characteristics in patients with advanced chronic kidney disease, on dialysis, and without chronic kidney disease. A collaborative project of the United States Renal Data System/National Institutes of Health and the National Registry of Myocardial Infarction. Am Heart J 2012; 163: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol: JASN 2006; 17: 2034–2047. [DOI] [PubMed] [Google Scholar]
- 48. Ezekowitz J, McAlister FA, Humphries KH, Norris CM, Tonelli M, Ghali WA, Knudtson ML, APPROACH Investigators . The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol 2004; 44: 1587–1592. [DOI] [PubMed] [Google Scholar]
- 49. Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, Burstein S, Gibson M, Poole WK, Cannon CP. Previous angina alters in‐hospital outcome in TIMI 4: a clinical correlate to preconditioning? Circulation 1995; 91: 37–45. [DOI] [PubMed] [Google Scholar]
- 50. Nakagawa Y, Ito H, Kitakaze M, Kusuoka H, Hori M, Kuzuya T, Higashino Y, Fujii K, Minamino T. Effect of angina pectoris on myocardial protection in patients with reperfused anterior wall myocardial infarction: retrospective clinical evidence of “preconditioning”. J Am Coll Cardiol 1995; 25: 1076–1083. [DOI] [PubMed] [Google Scholar]
- 51. Solomon SD, Anavekar NS, Greaves S, Rouleau JL, Hennekens C, Pfeffer MA. Angina pectoris prior to myocardial infarction protects against subsequent left ventricular remodeling. J Am Coll Cardiol 2004; 43: 1511–1514. [DOI] [PubMed] [Google Scholar]
- 52. Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010; 375: 727–734. [DOI] [PubMed] [Google Scholar]
- 53. Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, Panagopoulou V, Tsarouchas K, Vavetsi S, Pyrgakis V, Deftereos S. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv 2010; 3: 49–55. [DOI] [PubMed] [Google Scholar]
- 54. Hausenloy DJ, Kharbanda RK, Møller UK, Ramlall M, Aarøe J, Butler R, Bulluck H, Clayton T, Dana A, Dodd M, Engstrom T, Evans R, Lassen JF, Christensen EF, Garcia‐Ruiz JM, Gorog DA, Hjort J, Houghton RF, Ibanez B, Knight R, Lippert FK, Lønborg JT, Maeng M, Milasinovic D, More R, Nicholas JM, Jensen LO, Perkins A, Radovanovic N, Rakhit RD, Ravkilde J, Ryding AD, Schmidt MR, Riddervold IS, Sørensen HT, Stankovic G, Varma M, Webb I, Terkelsen CJ, Greenwood JP, Yellon DM, Bøtker HE, Junker A, Kaltoft A, Madsen M, Christiansen EH, Jakobsen L, Carstensen S, Kristensen SD, Thim T, Pedersen KM, Korsgaard MT, Iversen A, Jørgensen E, Joshi F, Pedersen F, Tilsted HH, Alzuhairi K, Saunamäki K, Holmvang L, Ahlehof O, Sørensen R, Helqvist S, Mark BL, Villadsen AB, Raungaard B, Thuesen L, Christiansen MK, Freeman P, Jensen SE, Skov CS, Aziz A, Hansen HS, Ellert J, Veien K, Pedersen KE, Hansen KN, Ahlehoff O, Cappelen H, Wittrock D, Hansen PA, Ankersen JP, Hedegaard KW, Kempel J, Kaus H, Erntgaard D, Pedersen DM, Giebner M, Hansen TMH, Radosavljevic‐Radovanovic M, Prodanovic M, Savic L, Pejic M, Matic D, Uscumlic A, Subotic I, Lasica R, Vukcevic V, Suárez A, Samaniego B, Morís C, Segovia E, Hernández E, Lozano I, Pascual I, Vegas‐Valle JM, Rozado J, Rondán J, Avanzas P, del Valle R, Padrón R, García‐Castro A, Arango A, Medina‐Cameán AB, Fente AI, Muriel‐Velasco A, Pomar‐Amillo Á, Roza CL, Martínez‐Fernández CM, Buelga‐Díaz C, Fernández‐Gonzalo D, Fernández E, Díaz‐González E, Martinez‐González E, Iglesias‐Llaca F, Viribay FM, Fernández‐Mallo FJ, Hermosa FJ, Martínez‐Bastida G, Goitia‐Martín J, Vega‐Fernández JL, Tresguerres JM, Rodil‐Díaz JA, Villar‐Fernández L, Alberdi L, Abella‐Ovalle L, de la Roz M, Fernández‐Carral MFC, Naves MC, Peláez MC, Fuentes MD, García‐Alonso M, Villanueva MJ, Vinagrero MS, Vázquez‐Suárez M, Martínez‐Valle M, Nonide M, Pozo‐López M, Bernardo‐Alba P, Galván‐Núñez P, Martínez‐Pérez PJ, Castro R, Suárez‐Coto R, Suárez‐Noriega R, Guinea R, Quintana RB, de Cima S, Hedrera SA, Laca SI, Llorente‐Álvarez S, Pascual S, Cimas T, Mathur A, McFarlane‐Henry E, Leonard G, Veerapen J, Westwood M, Colicchia M, Prossora M, Andiapen M, Mohiddin S, Lenzi V, Chong J, Francis R, Pine A, Jamieson‐Leadbitter C, Neal D, Din J, McLeod J, Roberts J, Polokova K, Longman K, Penney L, Lakeman N, Wells N, Hopper O, Coward P, O'Kane P, Harkins R, Guyatt S, Kennard S, Orr S, Horler S, Morris S, Walvin T, Snow T, Cunnington M, Burd A, Gowing A, Krishnamurthy A, Harland C, Norfolk D, Johnstone D, Newman H, Reed H, O'Neill J, Greenwood J, Cuxton J, Corrigan J, Somers K, Anderson M, Burtonwood N, Bijsterveld P, Brogan R, Ryan T, Kodoth V, Khan A, Sebastian D, Gorog D, Boyle G, Shepherd L, Hamid M, Farag M, Spinthakis N, Waitrak P, de Sousa P, Bhatti R, Oliver V, Walshe S, Odedra T, Gue Y, Kanji R, Ryding A, Ratcliffe A, Merrick A, Horwood C, Sarti C, Maart C, Moore D, Dockerty F, Baucutt K, Pitcher L, Ilsley M, Clarke M, Germon R, Gomes S, Clare T, Nair S, Staines J, Nicholson S, Watkinson O, Gallagher I, Nelthorpe F, Musselwhite J, Grosser K, Stimson L, Eaton M, Heppell R, Turney S, Horner V, Schumacher N, Moon A, Mota P, O'Donnell J, Panicker AS, Musa A, Tapp L, Krishnamoorthy S, Ansell V, Ali D, Hyndman S, Banerjee P, Been M, Mackenzie A, McGregor A, Hildick‐Smith D, Champney F, Ingoldby F, Keate K, Bennett L, Skipper N, Gregory S, Harfield S, Mudd A, Wragg C, Barmby D, Grech E, Hall I, Middle J, Barker J, Fofie J, Gunn J, Housley K, Cockayne L, Weatherlley L, Theodorou N, Wheeldon N, Fati P, Storey RF, Richardson J, Iqbal J, Adam Z, Brett S, Agyemang M, Tawiah C, Hogrefe K, Raju P, Braybrook C, Gracey J, Waldron M, Holloway R, Burunsuzoglu S, Sidgwick S, Hetherington S, Beirnes C, Fernandez O, Lazar N, Knighton A, Rai A, Hoare A, Webb I, Breeze J, Martin K, Andrews M, Patale S, Bennett A, Smallwood A, Radford E, Cotton J, Martins J, Wallace L, Milgate S, Munir S, Metherell S, Cottam V, Massey I, Copestick J, Delaney J, Wain J, Sandhu K, Emery L, Butler R, Hall C, Bucciarelli‐Ducci C, Besana R, Hussein J, Bell S, Gill A, Bales E, Polwarth G, East C, Smith I, Oliveira J, Victor S, Woods S, Hoole S, Ramos A, Sevillano A, Nicholson A, Solieri A, Redman E, Byrne J, Joyce J, Riches J, Davies J, Allen K, Saclot L, Ocampo M, Vertue M, Christmas N, Koothoor R, Gamma R, Alvares W, Pepper S, Kobson B, Reeve C, Malik I, Chester E, Saunders H, Mojela I, Smee J, Davies J, Davies N, Clifford P, Dias P, Kaur R, Moreira S, Ahmad Y, Tomlinson L, Pengelley C, Bidle A, Spence S, al‐Lamee R, Phuyal U, Abbass H, Bose T, Elliott R, Foundun A, Chung A, Freestone B, Lee DK, Elshiekh DM, Pulikal G, Bhatre G, Douglas J, Kaeng L, Pitt M, Watkins R, Gill S, Hartley A, Lucking A, Moreby B, Darby D, Corps E, Parsons G, de Mance G, Fahrai G, Turner J, Langrish J, Gaughran L, Wolyrum M, Azkhalil M, Bates R, Given R, Kharbanda R, Douthwaite R, Lloyd S, Neubauer S, Barker D, Dana A, Suttling A, Turner C, Smith C, Longbottom C, Ross D, Cunliffe D, Cox E, Whitehead H, Hudson K, Jones L, Drew M, Chant N, Haworth P, Capel R, Austin R, Howe S, Smith T, Hobson A, Strike P, Griffiths H, Anantharam B, Jack P, Thornton E, Hodgson A, Jennison A, McSkeane A, Smith B, Shaw C, Leathers C, Armstrong E, Carruthers G, Simpson H, Smith J, Hodierne J, Kelly J, Barclay J, Scott K, Gregson L, Buchanan L, McCormick L, Varma M, Kelsall N, Mcarthy R, Taylor R, Thompson R, Shelton R, Moore R, Tomlinson S, Thambi S, Cooper T, Oakes T, Deen Z, Relph C, prentice S, Hall L, Dillon A, Meadows D, Frank E, Markham‐Jones H, Thomas I, Gale J, Denman J, Gale J, O'Connor J, Hindle J, Jackson‐Lawrence K, Warner K, Lee K, Upton R, Elston R, Lee S, Venugopal V, Finch A, Fleming C, Whiteside C, Pemberton C, Wilkinson C, Sebastian D, Riedel E, Giuffrida G, Burnett G, Spickett H, Glen J, Brown J, Thornborough L, Pedley L, Morgan M, Waddington N, Brennan O, More R, Brady R, Preston S, Loder C, Vlad I, Laurence J, Smit A, Dimond K, Hayes M, Paddy L, Crause J, Amed N, Kaur‐Babooa P, Rakhit R, Kotecha T, Fayed H, Francis R, Pavlidis A, Prendergast B, Clapp B, Perara D, Atkinson E, Ellis H, Wilson K, Gibson K, Smith M, Khawaja MZ, Sanchez‐Vidal R, Redwood S, Jones S, Tipping A, Oommen A, Hendry C, Fath‐Orboubadi DRF, Phillips H, Kolakaluri L, Sherwood M, Mackie S, Aleti S, Charles T, Roy L, Henderson R, Stables R, Redwood S, Marber M, Berry A, Redington A, Thygesen K, Andersen HR, Berry C, Copas A, Meade T, Kelbæk H, Bueno H, von Weitzel‐Mudersbach P, Andersen G, Ludman A, Cruden N, Topic D, Mehmedbegovic Z, de la Hera Galarza JM, Robertson S, van Dyck L, Chu R, Astarci J, Jamal Z, Hetherington D, Collier L. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI‐2/ERIC‐PPCI): a single‐blind randomised controlled trial. The Lancet 2019; 394: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ranard LS, Fried JA, Abdalla M, Anstey DE, Givens RC, Kumaraiah D, Kodali SK, Takeda K, Karmpaliotis D, Rabbani LE, Sayer G, Kirtane AJ, Leon MB, Schwartz A, Uriel N, Masoumi A. Approach to acute cardiovascular complications in COVID‐19 infection. Circ Heart Fail 2020; 13: e007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R. Reduction of hospitalizations for myocardial infarction in Italy in the COVID‐19 era. Eur Heart J 2020; 41: 2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu J, Mamas M, Rashid M, Weston C, Hains J, Luescher T, de Belder MA, Deanfield JE, Gale CP. Patient response, treatments, and mortality for acute myocardial infarction during the COVID‐19 pandemic. Eur Heart J‐Qual Care Clin Outcomes 2020. 10.1093/ehjqcco/qcaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung S‐H, Ambrosy AP, Sidney S, Go AS. The Covid‐19 pandemic and the incidence of acute myocardial infarction. N Engl J Med 2020; 383: 691–693. [DOI] [PubMed] [Google Scholar]
- 59. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, van Vleck T, Vaid A, Chaudhry F, de Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount Sinai COVID Informatics Center . Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol 2020; 76: 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, Vehreschild M. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020: e203557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nguyen TL, Phan JA, Hee L, Moses DA, Otton J, Terreblanche OD, Xiong J, Premawardhana U, Rajaratnam R, Juergens CP, Dimitri HR, French JK, Richards DA, Thomas L. High‐sensitivity troponin T predicts infarct scar characteristics and adverse left ventricular function by cardiac magnetic resonance imaging early after reperfused acute myocardial infarction. Am Heart J 2015; 170: 715–25.e2. [DOI] [PubMed] [Google Scholar]
- 62. Tzivoni D, Koukoui D, Guetta V, Novack L, Cowing G. Comparison of Troponin T to creatine kinase and to radionuclide cardiac imaging infarct size in patients with ST‐elevation myocardial infarction undergoing primary angioplasty. Am J Cardiol 2008; 101: 753–757. [DOI] [PubMed] [Google Scholar]
- 63. Gerber Y, Jaffe AS, Weston SA, Jiang R, Roger VL. Prognostic value of cardiac troponin T after myocardial infarction: a contemporary community experience. Mayo Clin Proc 2012; 87: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hassan AK, Bergheanu SC, Hasan‐Ali H, Liem SS, van der Laarse A, Wolterbeek R, Atsma DE, Schalij MJ, Jukema JW. Usefulness of peak troponin‐T to predict infarct size and long‐term outcome in patients with first acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol 2009; 103: 779–784. [DOI] [PubMed] [Google Scholar]
- 65. Stelzle D, Shah ASV, Anand A, Strachan FE, Chapman AR, Denvir MA, Mills NL, McAllister D. High‐sensitivity cardiac troponin I and risk of heart failure in patients with suspected acute coronary syndrome: a cohort study. Eur Heart J‐Qual Care Clin Outcomes 2018; 4: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu M, Yan L, Xu J, Yang X, Jiang T. Predictors and prognosis for incident in‐hospital heart failure in patients with preserved ejection fraction after first acute myocardial infarction: an observational study. Medicine (Baltimore) 2018; 97: e11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Niu JM, Ma ZL, Xie C, Zhang ZQ. Association of plasma B‐type natriuretic peptide concentration with myocardial infarct size in patients with acute myocardial infarction. Gen Mol Res: GMR 2014; 13: 6177–6183. [DOI] [PubMed] [Google Scholar]
- 68. Mayr A, Mair J, Schocke M, Klug G, Pedarnig K, Haubner BJ, Nowosielski M, Grubinger T, Pachinger O, Metzler B. Predictive value of NT‐pro BNP after acute myocardial infarction: relation with acute and chronic infarct size and myocardial function. Int J Cardiol 2011; 147: 118–123. [DOI] [PubMed] [Google Scholar]
- 69. Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG. B‐type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 2003; 107: 2786–2792. [DOI] [PubMed] [Google Scholar]
- 70. Morita E, Yasue H, Yoshimura M, Ogawa H, Jougasaki M, Matsumura T, Mukoyama M, Nakao K. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993; 88: 82–91. [DOI] [PubMed] [Google Scholar]
- 71. Carvalho LSF, Bogniotti LAC, de Almeida OLR, e Silva JC, Nadruz W, Coelho OR, Sposito AC. Change of BNP between admission and discharge after ST‐elevation myocardial infarction (Killip I) improves risk prediction of heart failure, death, and recurrent myocardial infarction compared to single isolated measurement in addition to the GRACE score. Eur Heart J Acute Cardiovasc Care 2019; 8: 643–651. [DOI] [PubMed] [Google Scholar]
- 72. Mok Y, Sang Y, Ballew SH, Hoogeveen RC, Ballantyne CM, Rosamond W, Coresh J, Selvin E, Matsushita K. Premorbid levels of high‐sensitivity cardiac troponin T and natriuretic peptide and prognosis after incident myocardial infarction. Am Heart J 2019; 216: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012; 110: 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Al Aseri ZA, Habib SS, Marzouk A. Predictive value of high sensitivity C‐reactive protein on progression to heart failure occurring after the first myocardial infarction. Vasc Health Risk Manag 2019; 15: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu M, Yan L, Xu J, Yang X, Jiang T. Predictors and prognosis for incident in‐hospital heart failure in patients with preserved ejection fraction after first acute myocardial infarction: an observational study. Medicine 2018; 97: e11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stumpf C, Sheriff A, Zimmermann S, Schaefauer L, Schlundt C, Raaz D, Garlichs CD, Achenbach S. C‐reactive protein levels predict systolic heart failure and outcome in patients with first ST‐elevation myocardial infarction treated with coronary angioplasty. Arch Med Sci 2017; 13: 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Scirica BM, Cannon CP, Sabatine MS, Jarolim P, Sloane S, Rifai N, Braunwald E, Morrow DA, PROVE IT‐TIMI 22 Investigators . Concentrations of C‐reactive protein and B‐type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem 2009; 55: 265–273. [DOI] [PubMed] [Google Scholar]
- 78. Zhang S, Diao J, Qi C, Jin J, Li L, Gao X, Gong L, Wu W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta‐analysis. BMC Cardiovasc Disord 2018; 18: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, Hochman JS, Goodrich EL, Braunwald E, O'Donoghue ML. Interleukin‐6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID‐TIMI 52 (Stabilization of Plaque Using Darapladib—Thrombolysis in Myocardial Infarction 52) trial. J Am Heart Assoc 2017; 6: e005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xuan W, Huang W, Wang R, Chen C, Chen Y, Wang Y, Tan X. Elevated circulating IL‐32 presents a poor prognostic outcome in patients with heart failure after myocardial infarction. Int J Cardiol 2017; 243: 367–373. [DOI] [PubMed] [Google Scholar]
- 81. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD, Acute Coronary Treatment and Intervention Outcomes Network registry . Use of evidence‐based therapies in short‐term outcomes of ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 2010; 121: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Correa S, Morrow DA, Braunwald E, Davies RY, Goodrich EL, Murphy SA, Cannon CP, O'Donoghue ML. Cystatin C for risk stratification in patients after an acute coronary syndrome. J Am Heart Assoc 2018; 7: e009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ibrahim NE, Januzzi JL Jr. Established and emerging roles of biomarkers in heart failure. Circ Res 2018; 123: 614–629. [DOI] [PubMed] [Google Scholar]
- 84. Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 2015; 443: 71–77. [DOI] [PubMed] [Google Scholar]
- 85. Jenkins WS, Roger VL, Jaffe AS, Weston SA, AbouEzzeddine OF, Jiang R, Manemann SM, Enriquez‐Sarano M. Prognostic value of soluble ST2 after myocardial infarction: a community perspective. Am J Med 2017; 130: 1112.e9–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van Kimmenade RR, Januzzi JL Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino‐terminal pro‐brain natriuretic peptide, galectin‐3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 2006; 48: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 87. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 88. Asleh R, Enriquez‐Sarano M, Jaffe AS, Manemann SM, Weston SA, Jiang R, Roger VL. Galectin‐3 levels and outcomes after myocardial infarction: a population‐based study. J Am Coll Cardiol 2019; 73: 2286–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fertin M, Lemesle G, Turkieh A, Beseme O, Chwastyniak M, Amouyel P, Bauters C, Pinet F. Serum MMP‐8: a novel indicator of left ventricular remodeling and cardiac outcome in patients after acute myocardial infarction. PLoS ONE 2013; 8: e71280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM. Plasma MMP‐9 and MMP‐2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail 2004; 10: 328–333. [DOI] [PubMed] [Google Scholar]
- 91. Wagner DR, Delagardelle C, Ernens I, Rouy D, Vaillant M, Beissel J. Matrix metalloproteinase‐9 is a marker of heart failure after acute myocardial infarction. J Card Fail 2006; 12: 66–72. [DOI] [PubMed] [Google Scholar]
- 92. Turkieh A, Fertin M, Bouvet M, Mulder P, Drobecq H, Lemesle G, Lamblin N, de Groote P, Porouchani S, Chwastyniak M, Beseme O, Amouyel P, Mouquet F, Balligand JL, Richard V, Bauters C, Pinet F. Expression and implication of clusterin in left ventricular remodeling after myocardial infarction. Circ Heart Fail 2018; 11: e004838. [DOI] [PubMed] [Google Scholar]
- 93. Reinstadler SJ, Feistritzer H‐J, Reindl M, Klug G, Mayr A, Mair J, Jaschke W, Metzler B. Combined biomarker testing for the prediction of left ventricular remodelling in ST‐elevation myocardial infarction. Open Heart 2016; 3: e000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Niu X, Zhang J, Zhang L, Hou Y, Pu S, Chu A, Bai M. Weighted gene co‐expression network analysis identifies critical genes in the development of heart failure after acute myocardial infarction. Front Genet 2019; 10: 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sun T, Dong Y‐H, Du W, Shi C‐Y, Wang K, Tariq M‐A, Wang JX, Li PF. The role of microRNAs in myocardial infarction: from molecular mechanism to clinical application. Int J Mol Sci 2017; 18: 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shah RV, Rong J, Larson MG, Yeri A, Ziegler O, Tanriverdi K, Murthy V, Liu X, Xiao C, Pico AR, Huan T, Levy D, Lewis GD, Rosenzweig A, Vasan RS, Das S, Freedman JE. Associations of circulating extracellular RNAs with myocardial remodeling and heart failure. JAMA Cardiol 2018; 3: 871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lakhani HV, Khanal T, Gabi A, Yousef G, Alam MB, Sharma D, Aljoudi H, Puri N, Thompson E, Shapiro JI, Sodhi K. Developing a panel of biomarkers and miRNA in patients with myocardial infarction for early intervention strategies of heart failure in West Virginian population. PLoS ONE 2018; 13: e0205329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prastaro M, Pirozzi E, Gaibazzi N, Paolillo S, Santoro C, Savarese G, Losi MA, Esposito G, Perrone Filardi P, Trimarco B, Galderisi M. Expert review on the prognostic role of echocardiography after acute myocardial infarction. J Am Soc Echocardiogr 2017; 30: 431–43.e2. [DOI] [PubMed] [Google Scholar]
- 99. Møller JE, Hillis GS, Oh JK, Reeder GS, Gersh BJ, Pellikka PA. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J 2006; 151: 419–425. [DOI] [PubMed] [Google Scholar]
- 100. Carluccio E, Tommasi S, Bentivoglio M, Buccolieri M, Prosciutti L, Corea L. Usefulness of the severity and extent of wall motion abnormalities as prognostic markers of an adverse outcome after a first myocardial infarction treated with thrombolytic therapy. Am J Cardiol 2000; 85: 411–415. [DOI] [PubMed] [Google Scholar]
- 101. Jurado‐Román A, Agudo‐Quílez P, Rubio‐Alonso B, Molina J, Díaz B, García‐Tejada J, Martín R, Tello R. Superiority of wall motion score index over left ventricle ejection fraction in predicting cardiovascular events after an acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2019; 8: 78–85. [DOI] [PubMed] [Google Scholar]
- 102. Hayrapetyan HG, Adamyan KG. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with acute inferior myocardial infarction. Eur Heart J 2013; 34. [Google Scholar]
- 103. Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moyé LA, Lewis SJ, Braunwald E, Solomon SD, SAVE Investigators . Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002; 39: 1450–1455. [DOI] [PubMed] [Google Scholar]
- 104. Anavekar NS, Skali H, Bourgoun M, Ghali JK, Kober L, Maggioni AP, McMurray JJ, Velazquez E, Califf R, Pfeffer MA, Solomon SD. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am J Cardiol 2008; 101: 607–612. [DOI] [PubMed] [Google Scholar]
- 105. Møller JE, Whalley GA, Dini FL, Doughty RN, Gamble GD, Klein AL, Quintana M, Yu CM. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta‐analysis: Meta‐Analysis Research Group in Echocardiography acute myocardial infarction. Circulation 2008; 117: 2591–2598. [DOI] [PubMed] [Google Scholar]
- 106. Hillis GS, Møller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, Reeder GS, Oh JK. Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 2004; 43: 360–367. [DOI] [PubMed] [Google Scholar]
- 107. van der Bijl P, Abou R, Goedemans L, Gersh BJ, Holmes DR Jr, Ajmone Marsan N, Delgado V, Bax JJ. Left ventricular post‐infarct remodeling: implications for systolic function improvement and outcomes in the modern era. JACC Heart Fail 2020; 8: 131–140. [DOI] [PubMed] [Google Scholar]
- 108. Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C, Søgaard P, Køber L. Prediction of all‐cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013; 61: 2365–2373. [DOI] [PubMed] [Google Scholar]
- 109. Xu L, Huang X, Ma J, Huang J, Fan Y, Li H, Qiu J, Zhang H, Huang W. Value of three‐dimensional strain parameters for predicting left ventricular remodeling after ST‐elevation myocardial infarction. Int J Cardiovasc Imaging 2017; 33: 663–673. [DOI] [PubMed] [Google Scholar]
- 110. Cai W, Dong Y, Tian L, Cao CX, Niu XL, Liu XL, Liu JX, Ji WJ, Zhang Z, Zhou X, Li YM. Predictive value of four‐dimensional strain echocardiography for adverse cardiovascular outcomes in ST‐elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Cardiology 2018; 139: 255–264. [DOI] [PubMed] [Google Scholar]
- 111. Shin SH, Suh YJ, Baek YS, Lee MJ, Park SD, Kwon SW, Woo SI, Kim DH, Park KS, Kwan J. Impact of area strain by 3D speckle tracking on clinical outcome in patients after acute myocardial infarction. Echocardiography 2016; 33: 1854–1859. [DOI] [PubMed] [Google Scholar]
- 112. Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schömig A, Kastrati A. 5‐year prognostic value of no‐reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 2010; 55: 2383–2389. [DOI] [PubMed] [Google Scholar]
- 113. Aggarwal S, Xie F, High R, Pavlides G, Porter TR. Prevalence and predictive value of microvascular flow abnormalities after successful contemporary percutaneous coronary intervention in acute ST‐segment elevation myocardial infarction. J Am Soc Echocardiogr 2018; 31: 674–682. [DOI] [PubMed] [Google Scholar]
- 114. Carlos ME, Smart SC, Wynsen JC, Sagar KB. Dobutamine stress echocardiography for risk stratification after myocardial infarction. Circulation 1997; 95: 1402–1410. [DOI] [PubMed] [Google Scholar]
- 115. Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben‐Yehuda O. Relationship between infarct size and outcomes following primary PCI: patient‐level analysis from 10 randomized trials. J Am Coll Cardiol 2016; 67: 1674–1683. [DOI] [PubMed] [Google Scholar]
- 116. Feistritzer HJ, Nanos M, Eitel I, Jobs A, de Waha‐Thiele S, Meyer‐Saraei R, Freund A, Stiermaier T, Abdel‐Wahab M, Lurz P, Reinstadler SJ, Reindl M, Klug G, Metzler B, Desch S, Thiele H. Determinants and prognostic value of cardiac magnetic resonance imaging‐derived infarct characteristics in non‐ST‐elevation myocardial infarction. Eur Heart J Cardiovasc Imaging 2020; 21: 67–76. [DOI] [PubMed] [Google Scholar]
- 117. de Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, Ben‐Yehuda O, Jenkins P, Thiele H, Stone GW. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J 2017; 38: 3502–3510. [DOI] [PubMed] [Google Scholar]
- 118. Kali A, Cokic I, Tang R, Dohnalkova A, Kovarik L, Yang HJ, Kumar A, Prato FS, Wood JC, Underhill D, Marbán E. Persistent microvascular obstruction after myocardial infarction culminates in the confluence of ferric iron oxide crystals, proinflammatory burden, and adverse remodeling. Circ Cardiovasc Imaging 2016; 9: e004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kali A, Kumar A, Cokic I, Tang RL, Tsaftaris SA, Friedrich MG, Dharmakumar R. Chronic manifestation of postreperfusion intramyocardial hemorrhage as regional iron deposition: a cardiovascular magnetic resonance study with ex vivo validation. Circ Cardiovasc Imaging 2013; 6: 218–228. [DOI] [PubMed] [Google Scholar]
- 120. Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A, Mahrous A, Mordi I, Rauhalammi S, Sattar N, Welsh P, Radjenovic A, Ford I, Oldroyd KG, Berry C. Myocardial hemorrhage after acute reperfused ST‐segment‐elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging 2016; 9: e004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Reinstadler SJ, Stiermaier T, Reindl M, Feistritzer HJ, Fuernau G, Eitel C, Desch S, Klug G, Thiele H, Metzler B, Eitel I. Intramyocardial haemorrhage and prognosis after ST‐elevation myocardial infarction. Eur Heart J Cardiovasc Imaging 2019; 20: 138–146. [DOI] [PubMed] [Google Scholar]
- 122. Ekström K, Nepper‐Christensen L, Ahtarovski KA, Kyhl K, Göransson C, Bertelsen L, Ghotbi AA, Kelbæk H, Helqvist S, Høfsten DE, Køber L, Schoos MM, Vejlstrup N, Lønborg J, Engstrøm T. Impact of multiple myocardial scars detected by CMR in patients following STEMI. J Am Coll Cardiol Img 2019; 12: 2168–2178. [DOI] [PubMed] [Google Scholar]
- 123. Curley D, Lavin Plaza B, Shah AM, Botnar RM. Molecular imaging of cardiac remodelling after myocardial infarction. Basic Res Cardiol 2018; 113: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA, Wariar R, Zhang Y, Singh JP. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail 2017; 5: 216–225. [DOI] [PubMed] [Google Scholar]
- 125. Stehlik J, Schmalfuss C, Bozkurt B, Nativi‐Nicolau J, Wohlfahrt P, Wegerich S, Rose K, Ray R, Schofield R, Deswal A, Sekaric J, Anand S, Richards D, Hanson H, Pipke M, Pham M. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK‐HF multicenter study. Circ Heart Fail 2020; 13: e006513. [DOI] [PubMed] [Google Scholar]
- 126. Veldhuisen DJV, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Munoz Aguilera R, Lunati M, Yu CM, Gerritse B. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 127. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Harris S, Cowie MR. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017; 38: 2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, Clementy N, Amori V, di Mangoni S, Stefano L, Burri H. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE‐CARE multicentre randomized controlled trial. Eur J Heart Fail 2017; 19: 416–425. [DOI] [PubMed] [Google Scholar]
- 129. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Søgaard P, IN‐TIME study group* . Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet 2014; 384: 583–590. [DOI] [PubMed] [Google Scholar]
- 130. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon‐Moreau L, Proff J, Gerds TA, Anker SD, Torp‐Pedersen C. Daily remote monitoring of implantable cardioverter‐defibrillators: insights from the pooled patient‐level data from three randomized controlled trials (IN‐TIME, ECOST, TRUST). Eur Heart J 2017; 38: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 132. DeLeon‐Pennell KY, Meschiari CA, Jung M, Lindsey ML. Matrix metalloproteinases in myocardial infarction and heart failure. Prog Mol Biol Transl Sci 2017; 147: 75–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Freemantle N, Cleland J, Young P, Mason J, Harrison J. β Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999; 318: 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357: 1385–1390. [DOI] [PubMed] [Google Scholar]
- 135. Vantrimpont P, Rouleau JL, Wun CC, Ciampi A, Klein M, Sussex B, Arnold JM, Moyé L, Pfeffer M. Additive beneficial effects of beta‐blockers to angiotensin‐converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. J Am Coll Cardiol 1997; 29: 229–236. [DOI] [PubMed] [Google Scholar]
- 136. Spargias KS, Hall AS, Greenwood DC, Ball SG. β Blocker treatment and other prognostic variables in patients with clinical evidence of heart failure after acute myocardial infarction: evidence from the AIRE study. Heart 1999; 81: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. ACE Inhibitor Myocardial Infarction Collaborative Group . Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. Circulation 1998; 97: 2202–2212. [DOI] [PubMed] [Google Scholar]
- 138. Køber L, Torp‐Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC, Trandolapril Cardiac Evaluation (TRACE) Study Group . A clinical trial of the angiotensin‐converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1995; 333: 1670–1676. [DOI] [PubMed] [Google Scholar]
- 139. AIREAS Investigators . Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993; 342: 821–828. [PubMed] [Google Scholar]
- 140. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, The SAVE Investigators . Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction—results of the survival and ventricular enlargement trial. N Engl J Med 1992; 327: 669–677. [DOI] [PubMed] [Google Scholar]
- 141. Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray G, Torp‐Pedersen C, Ball S, Pogue J, Moyé L, Braunwald E. Long‐term ACE‐inhibitor therapy in patients with heart failure or left‐ventricular dysfunction: a systematic overview of data from individual patients. Lancet 2000; 355: 1575–1581. [DOI] [PubMed] [Google Scholar]
- 142. Dickstein K, Kjekshus J, OPTIMAAL Steering Committee, for the OPTIMAAL Study Group . Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Lancet 2002; 360: 752–760. [DOI] [PubMed] [Google Scholar]
- 143. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 144. Adamopoulos C, Ahmed A, Fay R, Angioi M, Filippatos G, Vincent J, Pitt B, Zannad F, EPHESUS Investigators . Timing of eplerenone initiation and outcomes in patients with heart failure after acute myocardial infarction complicated by left ventricular systolic dysfunction: insights from the EPHESUS trial. Eur J Heart Fail 2009; 11: 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chen H, Ikeda U, Shimpo M, Shimada K. Direct effects of statins on cells primarily involved in atherosclerosis. Hypertens Res 2000; 23: 187–192. [DOI] [PubMed] [Google Scholar]
- 146. Strandberg TE, Holme I, Faergeman O, Kastelein JJ, Lindahl C, Larsen ML, Olsson AG, Pedersen TR, Tikkanen MJ, IDEAL Study Group . Comparative effect of atorvastatin (80 mg) versus simvastatin (20 to 40 mg) in preventing hospitalizations for heart failure in patients with previous myocardial infarction. Am J Cardiol 2009; 103: 1381–1385. [DOI] [PubMed] [Google Scholar]
- 147. Scirica BM, Morrow DA, Cannon CP, Ray KK, Sabatine MS, Jarolim P, Shui A, McCabe C, Braunwald E, PROVE IT‐TIMI 22 Investigators . Intensive statin therapy and the risk of hospitalization for heart failure after an acute coronary syndrome in the PROVE IT‐TIMI 22 study. J Am Coll Cardiol 2006; 47: 2326–2331. [DOI] [PubMed] [Google Scholar]
- 148. Wright RS, Bybee K, Miller WL, Laudon DA, Murphy JG, Jaffe AS. Reduced risks of death and CHF are associated with statin therapy administered acutely within the first 24 h of AMI. Int J Cardiol 2006; 108: 314–319. [DOI] [PubMed] [Google Scholar]
- 149. Desta L, Jernberg T, Spaak J, Hofman‐Bang C, Persson H. Risk and predictors of readmission for heart failure following a myocardial infarction between 2004 and 2013: a Swedish nationwide observational study. Int J Cardiol 2017; 248: 221–226. [DOI] [PubMed] [Google Scholar]
- 150. Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, Redfield MM, Roger VL. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol 2013; 178: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gjesing A, Gislason GH, Køber L, Gustav Smith J, Christensen SB, Gustafsson F, Olsen AM, Torp‐Pedersen C, Andersson C. Nationwide trends in development of heart failure and mortality after first‐time myocardial infarction 1997–2010: a Danish cohort study. Eur J Intern Med 2014; 25: 731–738. [DOI] [PubMed] [Google Scholar]


