Abstract
Aims
We investigated the 2 year rate of ischaemic stroke/transient ischaemic attack (IS) in patients with heart failure (HF) who were in sinus rhythm (HF‐SR) and aimed to develop a score for stratifying risk of IS in this population.
Methods and results
A total of 15 425 patients (mean age 71.5 years, 39% women) with HF‐SR enrolled in the Swedish Heart Failure Register were included; 28 815 age‐matched and sex‐matched controls, without a registered diagnosis of HF, were selected from the Swedish Population Register. The 2 year rate of IS was 3.0% in patients and 1.4% in controls. In the patient group, a risk score including age (1p for 65–74 years; 2p for 75–84 years; 3p for ≥85 years), previous IS (2p), ischaemic heart disease, diabetes, hypertension, kidney dysfunction, and New York Heart Association III/IV class (1p each) was generated. Over a mean follow‐up of 20.1 (SD 7.5) months, the cumulative incidences (per 1000 person‐years) of IS in patients with score 0 to ≥7 were 2.2, 5.3, 8.9, 13.2, 15.7, 20.4, 26.4, and 33.0, with hazard ratios for score 1 to ≥7 (with 0 as reference): 2.4, 4.1, 6.1, 7.2, 9.4, 12.2, and 15.3. The risk score performed modestly (area under the curve 63.7%; P = 0.4711 for lack of fit with a logistic model; P = 0.7062 with Poisson, scaled by deviance).
Conclusions
In terms of absolute risk, only 27.6% of patients had an annual IS incidence of ≤1%. To which extent this would be amenable to anticoagulant treatment remains conjectural. A score compiling age and specific co‐morbidities identified HF‐SR patients with increased risk of IS with modest discriminative ability.
Keywords: Heart failure, Haemorrhagic stroke, Ischaemic stroke, Risk, Prognosis
Introduction
Heart failure (HF) of any aetiology has been linked to endothelial dysfunction, activation of thrombin‐related pathways, hypercoagulability, and inflammation. 1 These mechanisms are known to increase the risk of systemic and venous thromboembolic complications even in the absence of atrial fibrillation (AF) or flutter. 2
During the first 1 to 6 months after a diagnosis of HF, there is a high risk of ischaemic stroke, which seems to attenuate over time. 3 In a meta‐analysis 4 published in 2013, which included 4386 patients from four randomized controlled trials (WASH, 5 WATCH, 6 WARCHEF, 7 and HELAS 8 ), the authors found a benefit in using oral anticoagulants compared with aspirin in reducing the risk of stroke but no survival improvement in patients with HF with reduced ejection fraction and sinus rhythm (HFrEF‐SR); at the same time, the risk of major bleeding associated with anticoagulant use outweighed the anti‐thromboembolic benefit. With the new oral anticoagulants available on the market, however, the risk of bleeding is reported to be lower than previously resported. 9
The COMMANDER‐HF study published in 2018 10 concluded that rivaroxaban gave no benefit for the composite endpoint of death from any cause, myocardial infarction, or stroke in patients with HFrEF‐SR and coronary artery disease; however, in a post hoc analysis, 11 the authors found reduced rates of ischaemic stroke as compared with placebo in this population. Very few studies reporting real‐life data have used risk stratification models for estimating the incidence of thromboembolic complications in the context of HF‐SR. Also, the selection of patients, inclusion criteria, type of treatment, definition of endpoints, and historical period differed between studies. Moreover, definitions of HF have been heterogeneous and based mostly on reported symptoms and only occasionally on left ventricular ejection fraction (LVEF). 12
The principal aim of this study was to investigate the cumulative 2 year rate of incident ischaemic stroke or transient ischaemic attack (IS), and haemorrhagic stroke (HS) in HF‐SR patients registered in the Swedish Heart Failure Register (Swede‐HF) in comparison with a matched control population, selected from the Swedish Population Register. A secondary aim was to develop a risk stratification model that would help identify HF‐SR patients at high risk for IS.
Methods
Data sources and study cohort
Swede‐HF is a nationwide register, launched in 2003. 13 Approximately 80 variables are recorded and the inclusion criterion is clinician‐diagnosed HF, irrespective of LVEF. Patients are included either at hospital discharge or as outpatients, either hospital‐based or in primary care. Baseline variables are recorded online into a database managed by the Uppsala Clinical Research Centre, Sweden, where the protocol, registration form, and annual report are available. 14 Individual written patient consent is not required, according to Swedish law, but patients are informed about the register and allowed to opt out. Patients who were registered in Swede‐HF between 1 January 2003 and 31 December 2013 were eligible for inclusion in the present study if their LVEF had been recorded.
For each case, a maximum of two age‐matched and sex‐matched controls without HF were randomly selected from the Swedish Population Register. Information regarding use of medication was extracted from the National Drug Register (NDR), covering all prescriptions dispensed from Swedish pharmacies, coded according to the Anatomical Therapeutic Chemical Classification. For controls, information on baseline co‐morbidities was collected from the National Patient Register (NPR). In order to obtain as complete information as possible regarding co‐morbidities and baseline medication for cases, information from the NPR and from the NDR complemented the data registered in Swede‐HF. All diagnoses were coded according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD‐10) (Table S1 ). For patients only, creatinine levels were used to obtain estimated glomerular filtration rate (eGFR) according to the Modification of Diet in Renal Disease study equation. 15 Kidney dysfunction was defined as an eGFR < 60 mL/min/1.73 m2, and results were supplemented with information on kidney failure reported to the NPR. LVEF measured by echocardiography was recorded at baseline. Baseline was defined as the date of index registration in Swede‐HF.
A retrospective matched‐cohort study design was used, where 45 024 patients with HF were identified. All study subjects who had ever been diagnosed with AF and/or those who received treatment with anticoagulants within the previous 6 months from index and/or during the follow‐up period were excluded from the study; for details regarding inclusion, see flow chart (Figure 1 ). For inclusion, patients were additionally required to have been free from IS and HS diagnoses within the previous 2 years but could have had any of these diagnoses preceding this period. After exclusions, the final study population (15 425 patients with HF and 28 815 matched controls) was divided into four age groups (<65; 65–74; 75–84; and ≥85 years), as shown in Table 1 . The present study complies with the Declaration of Helsinki and was approved by the Regional Ethical Review Board of the Swedish Ethical Review Authority (Dnr 026‐16).
Figure 1.
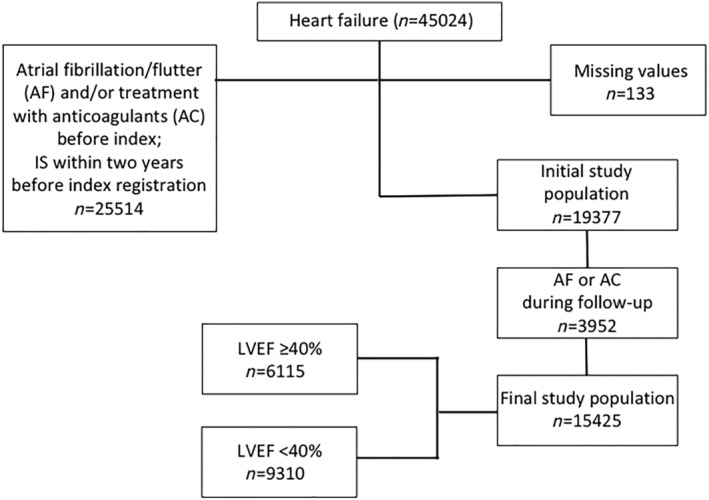
Flow chart showing selection of the study population. LVEF, left ventricular ejection fraction.
Table 1.
Characteristics of the study population at baseline by age group
| Age groups | <65 years | 65–74 years | 75–84 years | >84 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (n = 4283) | Control (n = 8562) | P‐value | Case (n = 3850) | Control (n = 7666) | P‐value | Case (n = 4777) | Control (n = 9079) | P‐value | Case (n = 2515) | Control (n = 3508) | P‐value | |
| Clinical characteristics | ||||||||||||
| Age, mean (SD) | 54.4 (8.9) | 54.4 (8.9) | 0.9806 | 69.7 (2.9) | 69.7 (2.9) | 0.8926 | 79.7 (2.8) | 79.6 (2.8) | 0.0763 | 88.2 (2.9) | 87.5 (2.3) | <0.0001 |
| Age, median (Q1, Q3) | 57 (50; 61) | 57 (50; 61) | 0.9732 | 70 (67; 72) | 70 (67; 72) | 0.8920 | 80 (77; 82) | 80 (77; 82) | 0.0750 | 88 (86; 90) | 87 (86; 89) | <0.001 |
| Women, % | 1268 (29.6) | 2536 (29.6) | 0.9871 | 1316 (34.2) | 2626 (34.3) | 0.9376 | 2009 (42.1) | 3934 (43.3) | 0.1495 | 1369 (54.4) | 2137 (60.9) | <0.001 |
| Obesity | 297 (6.9) | 99 (1.2) | <0.001 | 211 (5.5) | 80 (1.0) | <0.001 | 123 (2.6) | 31 (0.3) | <0.001 | 11 (0.4) | 4 (0.1) | 0.0130 |
| Ischaemic heart disease, % | 1651 (38.5) | 373 (4.4) | <0.001 | 2164 (56.2) | 806 (10.5) | <0.001 | 2864 (60.0) | 1268 (14.0) | <0.001 | 1403 (55.8) | 503 (14.3) | <0.001 |
| Dilated cardiomyopathy, % | 904 (21.1) | 83 (1.0) | <0.001 | 359 (9.3) | 26 (0.3) | <0.001 | 196 (4.1) | 19 (0.2) | <0.001 | 35 (1.4) | 1 (0.0) | <0.001 |
| Hypertrophic cardiomyopathy, % | 78 (1.8) | 38 (0.4) | <0.001 | 42 (1.1) | 11 (0.1) | <0.001 | 31 (0.6) | 8 (0.1) | <0.001 | 14 (0.6) | 1 (0.0) | 0.0001 |
| Co‐morbidities, n (%) | ||||||||||||
| Hypertension | 1530 (35.7) | 706 (8.2) | <0.001 | 1785 (46.4) | 1346 (17.6) | <0.001 | 2378 (49.8) | 2071 (22.8) | <0.001 | 1282 (51.0) | 949 (27.1) | <0.001 |
| Valvulopathy | 189 (4.4) | 18 (0.2) | <0.001 | 217 (5.6) | 33 (0.4) | <0.001 | 364 (7.6) | 49 (0.5) | <0.001 | 160 (6.4) | 11 (0.3) | <0.001 |
| Aortic or mitral | ||||||||||||
| Diabetes mellitus | 971 (22.7) | 417 (4.9) | <0.001 | 1217 (31.6) | 594 (7.7) | <0.001 | 1248 (26.1) | 788 (8.7) | <0.001 | 465 (18.5) | 290 (8.3) | <0.001 |
| Lung disease | 585 (13.7) | 278 (3.2) | <0.001 | 752 (19.5) | 384 (5.0) | <0.001 | 881 (18.4) | 514 (5.7) | <0.001 | 370 (14.7) | 184 (5.2) | <0.001 |
| Ischaemic stroke/TIA | 126 (2.9) | 75 (0.9) | <0.001 | 264 (6.9) | 277 (3.6) | <0.001 | 478 (10.0) | 528 (5.8) | <0.001 | 304 (12.1) | 263 (7.5) | <0.001 |
| Kidney dysfunction | 206 (4.8) | 61 (0.7) | <0.001 | 317 (8.2) | 70 (0.9) | <0.001 | 481 (10.1) | 111 (1.2) | <0.001 | 283 (11.3) | 70 (2.0) | <0.001 |
| Outcome (2 year follow‐up), n (%) | ||||||||||||
| Ischaemic stroke/TIA | 59 (1.4) | 38 (0.4) | <0.001 | 115 (3.0) | 92 (1.2) | <0.001 | 189 (4.0) | 203 (2.2) | <0.001 | 99 (3.9) | 79 (2.3) | 0.0001 |
| Haemorrhagic stroke | 5 (0.1) | 8 (0.1) | 0.6953 | 10 (0.3) | 12 (0.2) | 0.2315 | 16 (0.3) | 47 (0.5) | 0.1286 | 12 (0.5) | 13 (0.4) | 0.5259 |
| Cardiovascular mortality | 104 (2.4) | 8 (0.1) | <0.001 | 189 (4.9) | 22 (0.3) | <0.001 | 480 (10.0) | 127 (1.4) | <0.001 | 453 (18.0) | 128 (3.6) | <0.001 |
| All‐cause mortality | 349 (8.1) | 61 (0.7) | <0.001 | 653 (17.0) | 154 (2.0) | <0.001 | 1453 (30.4) | 566 (6.2) | <0.001 | 1333 (53.0) | 580 (16.5) | <0.001 |
| Per 1000 person‐years, IR (95% CI) | ||||||||||||
| Ischaemic stroke/TIA | 7.29 (5.65–9.41) | 2.23 (1.62–3.07) | <0.001 | 16.93 (14.11–20.33) | 6.09 (4.97–7.47) | <0.001 | 24.94 (21.62–28.76) | 11.66 (10.17–13.38) | <0.001 | 30.97 (25.43–37.72) | 12.46 (10.00–15.54) | <0.001 |
| Haemorrhagic stroke | 0.61 (0.26–1.48) | 0.47 (0.24–0.94) | 0.6371 | 1.45 (0.78–2.70) | 0.79 (0.45–1.39) | 0.1564 | 2.08 (1.27–3.39) | 2.68 (2.01–3.56) | 0.3794 | 3.68 (2.09–6.48) | 2.03 (1.18–3.50) | 0.1383 |
| Per 1000 person‐years, MR (95% CI) | ||||||||||||
| Cardiovascular mortality | 12.76 (10.53–15.47) | 0.47 (0.23–0.94) | <0.001 | 27.41 (23.77–31.61) | 1.45 (0.95–2.20) | <0.001 | 62.15 (56.83–67.97) | 7.22 (6.07–8.59) | <0.001 | 138.9 (126.6–152.2) | 20.00 (16.82–23.79) | <0.001 |
| All‐cause mortality | 42.83 (38.56–47.57) | 3.58 (2.78–4.59) | <0.001 | 94.71 (87.72–102.3) | 10.14 (8.66–11.88) | <0.001 | 188.1 (178.7–198.1) | 32.17 (29.63–34.94) | <0.001 | 408.6 (387.2–431.1) | 90.63 (83.55–98.32) | <0.001 |
Outcomes
Outcome was defined as any incident IS (ICD‐10 codes G45 and I63) that occurred within the following 2 years after the index registration of HF. Additionally, information on incident HS (ICD‐10 codes I61 and I62) was collected. Throughout the course of the study, computed tomography scan has been part of the routine workup of patients with neurological deficit in Sweden; thus, when a diagnosis of stroke is reported in the NPR, it is always coded according to type, that is, ischaemic or haemorrhagic. Cardiovascular and all‐cause mortality data were collected from the Cause of Death Register, National Board of Health and Welfare in Sweden.
Risk score
Factors associated with the outcome (IS) in univariate Cox regression (P < 0.1) were included in a multivariable regression analysis. A risk score was created by combining those variables that were found to significantly increase the risk of IS. Variables in the final model were tested for interactions, if any. The variables were weighted based on hazard ratio (HR) values, as follows: age (1p for 65–74 years; 2p for 75–84 years; 3p for ≥85 years), previous ischaemic heart disease (1p), hypertension (1p), diabetes mellitus (1p), previous IS (2p), kidney dysfunction (1p), and New York Heart Association (NYHA) class III or IV (1p).
Statistics
Data on vital status were censored on 31 December 2015. All data management and statistical analyses were carried out in SAS 9.4; graphics were drawn in R 3.5.3. Significance level was pre‐set at p < 0.05 unless stated otherwise. Baseline characteristics are presented as counts (per cent) for categorical and as means (standard deviation), or medians (first and third quartiles) for numeric data. Differences between study groups were assessed by χ 2 test for dichotomous and t‐test for continuous variables. Non‐parametric Wilcoxon test was used for comparison of medians.
Crude incidence rates (IRs) of IS by risk score are shown as counts (per cent). Cumulative incidence per 1000 person‐years was obtained by Cumulative Incidence Function from Cox proportional hazard model with any cause (but outcome of interest) of death as competing risk. IR per 1000 person‐years was calculated under assumption of Poisson distribution. Unadjusted Cox models and logistic regression were used to obtain hazard ratios (HRs) and odds ratios (ORs) with the lowest risk score (0) as reference in order to describe the relationship between risk categories.
The event count was then modelled by logistic (1) and Poisson regression, scaled by deviance (2) in search for the best fit for the data. When appropriate, lack of fit (1) or test of the model form (2) was performed. Akaike information criterion (AIC) (3) was used for model selection.
| Model | Expression | Parameter | Test | Required P‐value/pre‐set evaluation criteria |
|---|---|---|---|---|
| 1. Logit | (p) = ln ( ) | p is a probability of an event |
Lack of fit C‐statistics |
≥0.0565.0–74.0 satisfying; 75.0–84.0 good; 85.0 and higher excellent |
| 2. Poisson | μ = E(Y i), Var(Y i) = φμ | φ is correction term for overdispersion | Model form | ≥0.05 |
| 3. AIC | 2k − 2log(L(θ|y)) |
k is the number of estimated parameters; log(L(θ|y)) is the log‐likelihood at its maximum |
Quantity of information lost during a statistical procedure | The smallest value is preferred |
Results
Study population
After exclusions, the final study population consisted of 15 425 HF‐SR patients and 28 815 age‐matched and gender‐matched controls (Figure 1 ). The mean age of the patients was 71.5 (SD 13.3) years, and 38.7% were women. Even the youngest patients (<65 years) had a high prevalence of cardiovascular and other disorders. Ischaemic heart disease was present in 38.5%, hypertension in 35.7%, diabetes in 22.7%, and dilated cardiomyopathy in 21.1%. Older patients had a lower prevalence of cardiomyopathy, but more often hypertension, kidney dysfunction, ischaemic heart disease, and a history of IS. The control population had markedly fewer co‐morbidities than the HF‐SR patients (Table 1 ).
Outcome
During a mean follow‐up of 20.1 (SD 7.5) months, 462 (3.0%) patients and 412 (1.4%) controls had an IS, P < 0.0001; the corresponding numbers for HS were 43 (0.3%) and 80 (0.3%), P = 0.983, while all‐cause mortality was 24.6% among patients and 4.7% among controls, P < 0.0001.
The crude IS IR per 1000 person‐years increased across age groups among both patients and controls; patients < 65 years had an IR of 7.29 (CI 5.65–9.41), while for age‐matched controls, the IR was 2.23 (CI 1.62–3.07), P < 0.0001. The IR was 30.97 (CI 25.43–37.72) and 12.46 (CI 10.00–15.54) for patients and controls >84 years, respectively, P < 0.0001. The IR for HS followed a similar age‐related trend, but no significant difference was observed between patients and controls (Table 1 ).
Of the patients, 9310 (60.4%) had LVEF < 40% (Table 2 ). Patients with LVEF ≥ 40% (n = 6115, 39.6%) were older, more often women, and had a higher co‐morbidity burden. Although the all‐cause mortality rate in an unadjusted analysis was higher among patients with LVEF ≥ 40% than among patients with LVEF < 40% (25.8% vs. 23.7%, P = 0.003), no difference in risk of IS by LVEF was found.
Table 2.
Characteristics of the study population and outcomes by left ventricular ejection fraction
| Total | LVEF ≥ 40% | LVEF < 40% | ||
|---|---|---|---|---|
| LVEF groups | (n = 15 425) | (n = 6115) | (n = 9310) | P‐value |
| Clinical characteristics | ||||
| Age, mean (SD) | 71.5 (13.3) | 73.6 (13.1) | 70.2 (13.3) | <0.0001 |
| Age, median (Q1, Q3) | 73 (63; 82) | 76 (66; 83) | 72 (62; 81) | <0.001 |
| Women, % | 5962 (38.7) | 2906 (47.5) | 3056 (32.8) | <0.001 |
| Obesity | 642 (4.2) | 328 (5.4) | 314 (3.4) | <0.001 |
| Smoking | 2126 (13.8) | 686 (11.2) | 1440 (15.5) | <0.001 |
| Duration of heart failure | ||||
| Up to 6 months | 8774 (56.9) | 3386 (55.4) | 5388 (57.9) | 0.0087 |
| >6 months | 6543 (42.4) | 2686 (43.9) | 3857 (41.4) | |
| Ischaemic heart disease | 9443 (61.2) | 3758 (61.5) | 5685 (61.1) | 0.6250 |
| Dilated cardiomyopathy, % | 1494 (9.7) | 415 (6.8) | 1079 (11.6) | <0.001 |
| Hypertrophic cardiomyopathy, % | 165 (1.1) | 110 (1.8) | 55 (0.6) | <0.001 |
| NTproBNP, median (Q1; Q3) | 2110 (740; 5805) | 1407 (506; 4091) | 2632 (982; 7000) | <0.001 |
| NTproBNP (n missing) | 10 382 (67.3) | 4161 (68.0) | 6221 (66.8) | |
| Co‐morbidities, n (%) | ||||
| Hypertension | 9026 (58.5) | 4058 (66.4) | 4968 (53.4) | <0.001 |
| Valvulopathy (aortic or mitral) | 930 (6.0) | 421 (6.9) | 509 (5.5) | 0.0003 |
| Diabetes mellitus | 4454 (28.9) | 1843 (30.1) | 2611 (28.0) | 0.0050 |
| Lung disease | 2588 (16.8) | 1160 (19.0) | 1428 (15.3) | <0.001 |
| Ischaemic stroke/TIA | 1383 (9.0) | 650 (10.6) | 733 (7.9) | <0.001 |
| Kidney dysfunction | 1583 (10.3) | 712 (11.6) | 871 (9.4) | <0.001 |
| Baseline medication | ||||
| RAS inhibition | 13 049 (84.6) | 4738 (77.5) | 8311 (89.3) | <0.001 |
| Beta‐blockers | 13 066 (84.7) | 4772 (78.0) | 8294 (89.1) | <0.001 |
| MRA | 4030 (26.1) | 1235 (20.2) | 2795 (30.0) | <0.001 |
| Platelet inhibitor | 11 223 (72.8) | 4337 (70.9) | 6886 (74.0) | <0.001 |
| Statins | 8116 (52.6) | 3189 (52.2) | 4927 (52.9) | 0.3481 |
| Diuretics | 11 163 (72.4) | 4323 (70.7) | 6840 (73.5) | 0.0002 |
| Outcome (2 year follow‐up), n (%) | ||||
| Ischaemic stroke/TIA | 462 (3.0) | 181 (3.0) | 281 (3.0) | 0.8353 |
| Time to event, months, median (Q1; Q3) | 8.4 (2.6; 16.4) | 8.0 (2.5; 16.3) | 8.8 (2.8; 17.0) | 0.4464 |
| Haemorrhagic stroke | 43 (0.3) | 23 (0.4) | 20 (0.2) | 0.0631 |
| Time to event, months, median (Q1; Q3) | 10.9 (4.5; 19.5) | 11.7 (5.2; 19.5) | 10.5 (4.3; 19.3) | 0.6612 |
| Cardiovascular mortality | 1226 (8.0) | 460 (7.5) | 766 (8.2) | 0.1132 |
| All‐cause mortality | 3788 (24.6) | 1579 (25.8) | 2209 (23.7) | 0.0031 |
| Per 1000 person‐years | ||||
| Ischaemic stroke/TIA | 18.01 (16.44–19.72) | 17.93 (15.50–20.74) | 18.05 (16.06–20.29) | 0.9436 |
| Haemorrhagic stroke | 1.65 (1.23–2.23) | 2.25 (1.50–3.39) | 1.27 (0.82–1.96) | 0.0603 |
| Cardiovascular mortality | 47.10 (44.54–49.81) | 44.94 (41.01–49.24) | 48.51 (45.19–52.06) | 0.1954 |
| All‐cause mortality | 145.5 (141.0–150.2) | 154.3 (146.8–162.1) | 139.9 (134.2–145.8) | 0.0030 |
MRA, magnetic resonance angiography; NTproBNP, N‐terminal pro‐brain natriuretic peptide.
In a multivariable regression of the HF‐SR patients without any reported co‐morbidity (n = 2368; 69% with LVEF < 40%, mean age 65.5 (SD 18.6) years; 31% with LVEF ≥ 40%, mean age 63.5 ± 15.6 years; P = 0.013), gender (HR 1.13; CI 0.60–2.25; P = 0.72) and LVEF were not independent predictors of IS (HR 0.79; CI 0.39–1.59, P = 0.50) in contrast to age (HR 1.07; CI 1.04–1.10, P < 0.0001).
Risk score in the patient population
Table 3 shows ORs for IS associated with individual baseline variables. With death as competing risk, an incremental HR for IS was observed for patients with score 1 to ≥7: 2.4, 4.2, 6.0, 7.4, 9.3, 12.4, and 14.9; while the IS cumulative incidence per 1000 person‐years was 2.2, 5.3, 9.0, 13.0, 15.9, 19.8, 26.5, and 31.7, respectively. Narrower confidence intervals and lower AIC were obtained by using the Poisson model in comparison with the logistic model (Figure 2 and Table 4 ). With the use of a χ 2‐test, no statistical difference was found between the observed and predicted IS events (P = 0.64 for the Poisson model and P = 0.30 for the logistic model). The risk score performed modestly (C‐statistic 63.7%; P = 0.47 for lack of fit with a logistic model and P = 0.71 with Poisson, scaled by deviance).
Table 3.
Odds ratios and corresponding number of points for variables included in the risk score
| Tested variables | OR for ischaemic stroke | P‐value | Risk points |
|---|---|---|---|
| Age | |||
| <65 | 1.00 (ref.) | 0 | |
| 65–74 | 2.30 (1.68–3.15) | <0.0001 | 1 |
| 75–84 | 3.35 (2.50–4.48) | <0.001 | 2 |
| 85 + | 4.02 (2.91–5.55) | <0.001 | 3 |
| Smoking | 1.18 (0.98–1.42) | 0.0773 | 0 |
| Co‐morbidity | |||
| Ischaemic heart disease | 1.53 (1.26–1.87) | <0.001 | 1 |
| Hypertension | 1.58 (1.30–1.92) | <0.001 | 1 |
| Diabetes mellitus | 1.64 (1.36–1.98) | <0.001 | 1 |
| Ischaemic stroke/TIA | 2.51 (1.98–3.18) | <0.001 | 2 |
| Kidney dysfunction a | 1.62 (1.35–1.95) | 0.0002 | 1 |
| Left ventricle ejection fraction | |||
| LVEF > 40 | 1.00 (ref.) | 0 | |
| LVEF ≤ 40 | 1.01 (0.84–1.22) | 0.9256 | 0 |
| NYHA class a | |||
| I | 1.00 (ref.) | 0 | |
| II | 1.11 (0.84–1.48) | 0.4650 | |
| III | 1.52 (1.14–2.02) | 0.0047 | 1 |
| IV | 1.98 (1.39–2.81) | 0.0002 | |
Missing values: kidney dysfunction (0.06%) and NYHA class (0.01%).
Figure 2.
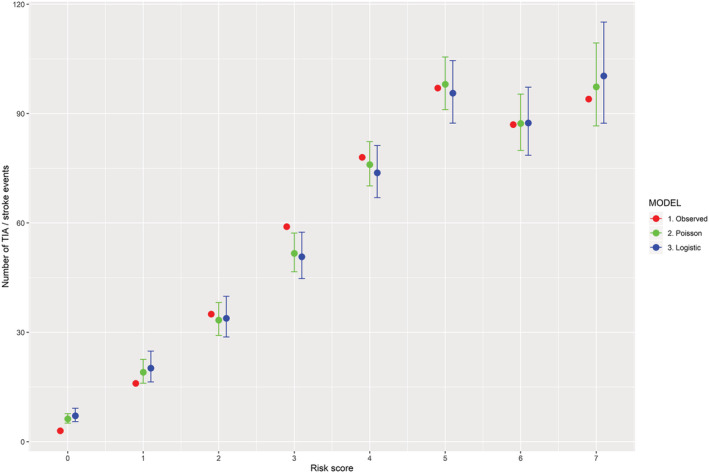
Performance of risk score: observed and predicted ischaemic stroke events by number of risk points.
Table 4.
Goodness of fit (observed vs. predicted ischaemic stroke/TIA events) by number of risk points according to ordinal logistic model and Poisson, scaled by deviance
| Score points | Study population | Observed events | Predicted values | |
|---|---|---|---|---|
| n (%) | n (%) | Logistic | Poisson | |
| 0 | 707 (4.6) | 3 (0.4) | 7.0 (5.5–9.1) | 6.2 (5.0–7.6) |
| 1 | 1548 (10.0) | 16 (1.0) | 20.1 (16.3–24.7) | 19.0 (16.0–22.4) |
| 2 | 2008 (13.0) | 35 (1.7) | 34.0 (28.8–40.0) | 33.5 (29.3–38.2) |
| 3 | 2329 (15.1) | 59 (2.5) | 50.6 (44.7–57.4) | 51.5 (46.5–57.0) |
| 4 | 2625 (17.0) | 77 (2.9) | 73.7 (66.9–81.2) | 76.0 (70.3–82.2) |
| 5 | 2642 (17.1) | 98 (3.7) | 95.7 (87.5–104.7) | 98.1 (91.3–105.5) |
| 6 | 1881 (12.2) | 87 (4.6) | 87.4 (78.5–97.2) | 87.2 (80.0–95.2) |
| ≥7 | 1685 (10.9) | 94 (5.6) | 100.5 (87.5–115.3) | 97.5 (87.0–109.4) |
| AUC | 63.7 (61.4–66.1) | |||
| Wald χ 2 (global null hypothesis: β = 0) | <0.0001 | <0.0001 | ||
| Lack of fit/test of the model form | 0.4711 | 0.7062 | ||
| Akaike information criterion | 53.9 | 52.0 | ||
AUC, area under the curve.
Discussion
In this nationwide study, we found that the cumulative risk of IS among HF‐SR patients during the first 2 years after index registration was 3%, about twice that of matched controls. The risk of HS, however, was roughly 10 times lower and remarkably similar in patients and controls (0.3%). A risk score compiling age and co‐morbidity was shown to identify patients at high risk for IS with moderate discriminative ability.
As expected, the co‐morbidity burden differed considerably between cases and controls, with the control population having significantly fewer co‐morbidities than the HF‐SR patients. Thus, the increased risk of ischaemic stroke is, presumably, the result of a detrimental combination of HF, age, and co‐morbidity, and, in fact, very similar to that seen in patients with AF.
Conceivably, owing to worse haemodynamics, patients with low LVEF might have a higher risk of thromboembolic events and ischaemic stroke than patients with HF with preserved ejection fraction (HFpEF). However, our results indicated that patients with LVEF ≥ 40% had rates of ischaemic stroke that were comparable with those of patients with LVEF < 40%. Two additional analyses based on other LVEF categories [(i) LVEF < 30, 30–39, 40–49, and ≥50%; (ii) LVEF < 40, 40–49, and ≥50%] provided similar results (data not shown). A putative explanation for these findings would be that patients with LVEF ≥ 40% were older and had a higher co‐morbidity burden, which may have evened out the impact of reduced LVEF on stroke rates. To address this question, a subgroup analysis was performed in the 2368 HF‐SR patients without any reported co‐morbidity; age, but not LVEF or gender, was found to independently predict the risk of ischaemic stroke. Thus, in accordance with the findings by Abdul‐Rahim et al., 16 the study indicates that LVEF has no significant impact on the risk of IS. However, it might be that by excluding patients treated with anticoagulants, we discard from the analysis the patients with severely depressed systolic function who might have gotten anticoagulant treatment based on clinical suspicion, high probability of thrombosis, or diagnosed intracavitary thrombosis.
Similar to AF patients, age and co‐morbidity seem to play an essential role in determining the risk of ischaemic stroke even in patients with HF‐SR. In the analysis of pooled data from the CHARM‐Preserved and I‐Preserve trials 16 that included 4676 patients with HFpEF without AF, a risk score based on age, body mass index, NYHA class, history of stroke, and insulin‐treated diabetes identified patients at elevated risk of future stroke. This risk score was previously tested on HFrEF patients. 17 However, the definition of stroke did not discriminate between IS and HS; and moreover, about 6% of the patients were on anticoagulant therapy.
In this real‐world cohort, neuroimaging for diagnosing stroke was used throughout the course of the study, and accordingly distinguishing between IS and HS was not problematic. Surprisingly, even though the rate of HS showed a clear age‐related trend, the risk was similar among HF‐SR patients and their controls. Considering the co‐morbidity burden of the HF‐SR patients, these findings are intriguing, and any potential reasons remain unclear. Nevertheless, it is important to highlight that the rate of cerebral bleeding was very much lower than that of ischaemic stroke in the HF‐SR patients < 65 years, while, in terms of absolute risk, only 27.6% of the whole study cohort had an annual incidence of IS of ≤1%.
It has been suggested that in order to justify anticoagulation with warfarin, stroke rates in HF patients must be about 3–5% per year, 18 but with the use of newer, safer oral anticoagulants, stroke rates of ≥0.9% per year may be considered a reasonable limit. 9 Moreover, the recently published results from COMMANDER‐HF 11 showed that rivaroxaban reduced rates of ischaemic stroke compared to placebo, with the safety endpoint of fatal/critical bleeding occurring at a similar rate on rivaroxaban and placebo. In this context, a risk score is a valuable instrument in the selection of patients at high risk for IS, who may benefit from anticoagulant treatment.
Even though the risk score in our analysis only performed moderately well, its clinical relevance is supported by the fact that no difference was found between observed and predicted IS events, as shown in the ‘take‐home figure’ (Figure 2 ). Indeed, despite the fact that incidence of IS in HF‐SR is relatively low, it certainly cannot be ignored, in particular in the subgroup of patients with a high score, who carry a considerable risk; further studies investigating the benefit of anticoagulant therapy in this category of patients are warranted.
Melgaard et al. 19 tested the predictive value of CHA2DS2‐VASc score for ischaemic stroke, thrombo‐embolism, and death in a cohort of Danish HF patients with and without AF; and, similar to the present study, they found that the predictive accuracy for ischaemic stroke was modest (C‐statistic 0.67 for patients with AF and 0.64 for those without).
We chose not to use a predefined risk score but rather to develop one based on variables shown to significantly affect the probability of ischaemic stroke. However, our final selection of variables turned out to be very similar to the one included in the CHA2DS2‐VASc score, with the exception of sex category, which was not a predictor in our cohort; instead, kidney dysfunction and NYHA class were included in the score. The risk score predicted better than chance with a C‐statistic of 0.64, and this value remained unaffected in the sensitivity analysis. While the predictive accuracy is modest, it is still comparable with the one that the CHA2DS2‐VASc score has shown in patients with AF (C‐statistic 0.60), 20 notwithstanding the fact that the overall risk for IS in our study cohort is lower than in patients with HF and AF. Even if the proposed score needs to be further validated before it may be adopted for making clinical treatment decisions, it is expected to facilitate design for selection of HF‐SR patients for future trials on stroke prevention.
Our study has several strengths but also some evident limitations. It is based on a real‐world cohort, the sample size is large, and the definitions of diagnoses and the outcomes are well validated; the use of a matched control group enabled us to estimate absolute risk as well as the relative risk of IS and HS in patients with HF‐SR as compared with a background population; the analyses take into consideration the competing risk of death; patients receiving anticoagulants, as well as those with AF prior to HF diagnosis and/or during the follow‐up period, were excluded. We cannot rule out that some patients might have had silent AF that had escaped detection; however, this is a common difficulty in clinical praxis. Our study design is also, as other registry‐based studies, at risk of being subject to selection bias and confounding variables. Although Swede‐HF contains a large number of unique patients with extensive baseline variables, we cannot rule out inaccuracies in recorded data or unmeasured confounding variables. Another limitation is the variation of available treatments and routine praxis over the inclusion period of 10 years and the absence of standardised neuroimaging data. Still, a validation study of the diagnoses reported to the NPR shows a high degree of validity. 21 Some of the reported diagnoses for controls may be underestimated owing to the fact that some patients might only have had contact with the primary care where diagnoses are not reported to the NPR. Finally, the risk score that we propose has not been validated in any other patient cohort but still demonstrates a fair goodness of fit in this population.
One‐sentence summary
Patients with HF and sinus rhythm had an increased risk of ischaemic, but not haemorrhagic, stroke that cannot be ignored; a score compiling age and specific co‐morbidities identified patients with increased risk of IS, underlining the need of further investigation for stroke prevention in this specific group of patients.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by grants from the Swedish state under the agreement between the Swedish government and the County Councils Concerning Economic Support of Research and Education of Doctors (ALFGBG‐717211); the Swedish Heart and Lung Foundation (Hjärt‐Lungfonden; 2015‐0438 and 2018‐0366); and the Swedish Research Council (Vetenskapsrådet; 2013‐05187 and 2018‐02527).
Supporting information
Table S1. List of ICD 10‐codes and ATC‐codes used in the study
Hjalmarsson, C. , Fu, M. , Zverkova Sandström, T. , Schaufelberger, M. , Ljungman, C. , Andersson, B. , Bollano, E. , Dahlström, U. , and Rosengren, A. (2021) Risk of stroke in patients with heart failure and sinus rhythm: data from the Swedish Heart Failure Registry. ESC Heart Failure, 8: 85–94. 10.1002/ehf2.13091.
References
- 1. Borissoff JI, Spronk HM, Heeneman S, ten Cate H. Is thrombin a key player in the ‘coagulation‐atherogenesis’ maze? Cardiovasc Res 2009; 82: 392–403. [DOI] [PubMed] [Google Scholar]
- 2. Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow's triad revisited. J Am Coll Cardiol 1999; 33: 1424–1426. [DOI] [PubMed] [Google Scholar]
- 3. Alberts VP, Bos MJ, Koudstaal P, Hofman A, Witteman JC, Stricker B, Breteler M. Heart failure and the risk of stroke: the Rotterdam Study. Eur J Epidemiol 2010; 25: 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hopper I, Skiba M, Krum H. Updated meta‐analysis on antithrombotic therapy in patients with heart failure and sinus rhythm. Eur J Heart Fail 2013; 15: 69–78. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole‐Wilson PA. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J 2004; 148: 157–164. [DOI] [PubMed] [Google Scholar]
- 6. Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, Jafri SM, Krol WF, O'Connor CM, Schulman KA, Teo K, Warren SR, Investigators WT. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation 2009; 119: 1616–1624. [DOI] [PubMed] [Google Scholar]
- 7. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R, Investigators W. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012; 366: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK, Investigators H . Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail 2006; 8: 428–432. [DOI] [PubMed] [Google Scholar]
- 9. Ahrens I, Lip GY, Peter K. New oral anticoagulant drugs in cardiovascular disease. Thromb Haemost 2010; 104: 49–60. [DOI] [PubMed] [Google Scholar]
- 10. Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B, Investigators CH . Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018; 379: 1332–1342. [DOI] [PubMed] [Google Scholar]
- 11. Mehra MR, Vaduganathan M, Fu M, Ferreira JP, Anker SD, Cleland JGF, Lam CSP, van Veldhuisen DJ, Byra WM, Spiro TE, Deng H, Zannad F, Greenberg B. A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial. Eur Heart J 2019; 40: 3593–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferreira JP, Girerd N, Alshalash S, Konstam MA, Zannad F. Antithrombotic therapy in heart failure patients with and without atrial fibrillation: update and future challenges. Eur Heart J 2016; 37: 2455–2464. [DOI] [PubMed] [Google Scholar]
- 13. Jonsson A, Edner M, Alehagen U, Dahlstrom U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail 2010; 12: 25–31. [DOI] [PubMed] [Google Scholar]
- 14. http://www.ucr.uu.se/rikssvikt. 2020.
- 15. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology C . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 16. Abdul‐Rahim AH, Perez AC, MacIsaac RL, Jhund PS, Claggett BL, Carson PE, Komajda M, McKelvie RS, Zile MR, Swedberg K, Yusuf S, Pfeffer MA, Solomon SD, Lip GYH, Lees KR, McMurray JJV, Candesartan in Heart failure Assessment of Reduction in M, Morbidity P, the Irbesartan in Heart Failure with Preserved Systolic Function Steering C . Risk of stroke in chronic heart failure patients with preserved ejection fraction, but without atrial fibrillation: analysis of the CHARM‐Preserved and I‐Preserve trials. Eur Heart J 2017; 38: 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdul‐Rahim AH, Perez AC, Fulton RL, Jhund PS, Latini R, Tognoni G, Wikstrand J, Kjekshus J, Lip GY, Maggioni AP, Tavazzi L, Lees KR, McMurray JJ, Investigators of the Controlled Rosuvastatin Multinational Study in Heart F, Committees GI‐HF, Investigators . Risk of stroke in chronic heart failure patients without atrial fibrillation: analysis of the Controlled Rosuvastatin in Multinational Trial Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca‐Heart Failure (GISSI‐HF) Trials. Circulation 2015; 131: 1486–1494 discussion 1494. [DOI] [PubMed] [Google Scholar]
- 18. Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA 3rd, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Le Heuzey JY, Crijns HJ, Lowe JE, Curtis AB, Olsson SB, Ellenbogen KA, Prystowsky EN, Halperin JL, Tamargo JL, Kay GN, Wann LS, Jacobs AK, Anderson JL, Albert N, Hochman JS, Buller CE, Kushner FG, Creager MA, Ohman EM, Ettinger SM, Stevenson WG, Guyton RA, Tarkington LG, Halperin JL, Yancy CW, Accf/Aha/Hrs . 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 57: 223–242. [DOI] [PubMed] [Google Scholar]
- 19. Melgaard L, Gorst‐Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2‐VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 2015; 314: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 20. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 21. Ludvigsson J, Andersson E, Ekbom A, Feychting M, Kim J‐L, Reuterwall C, Heurgren M, Otterblad Olausson P. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of ICD 10‐codes and ATC‐codes used in the study


