Abstract
Aims
Little is known regarding acute heart failure (AHF) clinical characteristics and its hospital outcome in Latin America. This study sought to assess the prevalence of, and identify differences among, in‐hospital outcomes in patients hospitalized for AHF who were stratified by clinical phenotype at a hospital in Latin America.
Methods and results
This is a retrospective cohort study of patients with AHF who were hospitalized in the coronary care unit of a Latin American teaching hospital from January 2006 to December 2018. Cox regression analysis was used to identify predictors of mortality. Of 21 042 patients admitted, 7759 (36.6%) had AHF. Their median age was 62 years, and 35% were women. De novo heart failure was seen in 39.4% of patients. Most common was AHF‐associated acute coronary syndromes (ACS‐HF) in 43.0%, decompensated heart failure (DHF) in 33.7%, hypertensive heart failure (HT‐HF) in 11.8%, and cardiogenic shock (CS) in 5.2%. Pulmonary oedema (PO) (3.3%) and right heart failure (RHF) (3.0%) were least frequent. Coronary artery disease was the most frequent aetiology in 56.5% of patients, valvular heart disease in 22.4%, and cardiomyopathies in 12.3%. Other less frequent aetiology included adult congenital heart disease (2.5%), lung diseases (2.1%), acute aortic syndromes (1.4%), pericardial diseases (0.8%), and intracardiac tumours (0.3%). Aetiology could not be established in 1.6% of patients. Before admission, patients with worsening chronic heart failure and reduced ejection fraction were treated with renin–angiotensin system blockers (60.4%), beta‐blockers (42.5%), or spironolactone (34.4%). The percentages of patients given in‐hospital management with intravenous diuretics, vasodilators, inotropes, and vasopressors were 81.2%, 33.4%, 18.9%, and 20.4%, respectively. The overall in‐hospital mortality was 17.9% (71.3%, 43.9%, 23.8%, 14.9%, 13.6%, and 10.1% for CS, PO, RHF, DHF, ACS‐HF, and HT‐HF, respectively; P < 0.0001). Multivariate analysis revealed that PO (hazard ratio [HR] 2.68, 95% confidence interval [CI] 1.73–4.14, P < 0.0001) and CS (HR 3.37, 95% CI 2.12–5.35, P < 0.0001) were independent predictors of in‐hospital mortality. Use of intravenous diuretics was linked to reduction of in‐hospital mortality (HR 0.70, 95% CI 0.59–0.59, P < 0.0001). By contrast, increased in‐hospital mortality was associated with the use of intravenous inotrope or vasopressor (HR 1.49, 95% CI 1.27–1.76 and HR 2.91, 95% CI 2.41–3.51, P < 0.0001, respectively).
Conclusions
Real‐world evidence from a university hospital in Latin America shows that the high mortality among patients with AHF may depend, among other factors, on patients' AHF clinical phenotypes. The clinical characteristics and aetiologies of AHF appear to differ between these data from Mexico and those from European and US registries.
Keywords: Acute heart failure, Clinical phenotypes, Latin America, Mortality
Introduction
It is well known that in Europe and the USA, acute heart failure (AHF) is the leading cause of hospitalizations and that its high morbidity, mortality, and economic burden make it an important public health issue. 1 , 2 AHF is a heterogeneous and haemodynamically diverse syndrome that is frequently life‐threatening and requires urgent therapy. Accordingly, current recommendations emphasize the importance of immediate diagnosis and treatment of patients presenting with AHF. Several classification schemes have been proposed. 3 However, because clinical presentation at admission is highly heterogeneous, it may be more prudent to stratify patients with AHF based on their initial clinical presentation. In 2008, the European Society of Cardiology (ESC) proposed that patients be classified according to six clinical profiles at initial presentation: decompensated chronic HF (DHF), pulmonary oedema (PO), hypertensive HF (HT‐HF), cardiogenic shock (CS), right HF (RHF), and HF in the setting of acute coronary syndromes (ACS‐HF). 4
The primary data available on patients with AHF come from several large‐scale registries developed in Europe 5 , 6 , 7 and the USA, 8 although hospital‐based registries remain the primary source of real‐world data about AHF. 9 The roles of geographic differences and income inequality may be related to differences in patient characteristics, outcomes, and, most importantly, the effects of treatment observed in HF trials. 10 Because information on the prevalence of clinical phenotypes, management, and hospital outcomes of patients admitted for AHF in Latin America is scarce, we conducted a retrospective analysis to gain insight into the prevalence of different clinical presentation phenotypes as well as the aetiology, treatment, and hospital outcomes of AHF patients admitted to a contemporary teaching hospital in Mexico City.
Materials and methods
This was a retrospective cohort study using the database of the coronary care unit (CCU) of the National Institute of Cardiology in Mexico City. We analysed data from all consecutive patients admitted to the CCU between 1 January 2006 and 31 December 2018 with a diagnosis with AHF.
We gathered demographic characteristics, medical history, physiological parameters at admission (i.e. blood pressure and heart rate), biochemical findings, in‐hospital treatments, and in‐hospital mortality. In‐hospital mortality was defined as all‐cause mortality during hospitalization. Vital signs were determined at the initial medical presentation. Baseline creatinine clearance was estimated using the Cockcroft–Gault formula. We included patients with any type of AHF (i.e. including de novo or worsening chronic HF [WCHF]) at the time of presentation. De novo AHF was defined as AHF in patients with no prior history of HF. WCHF was defined as worsening of HF in patients with a previous diagnosis with, or hospitalization for, HF.
Because patients were not previously classified into one of the six AHF clinical phenotypes, we classified them at the time of admission based on the 2008 ESC guidelines 4 : (i) DHF—a history of progressive worsening of chronic HF with treatment and evidence of systemic and pulmonary congestion; does not fulfil criteria for CS, PO, or hypertensive crisis; (ii) HT‐HF—signs and symptoms of AHF are accompanied by high blood pressure (systolic blood pressure ≥ 140 mmHg) at admission; (iii) PO—symptoms of AHF are accompanied by severe respiratory distress, orthopnea, and crackles over the lungs; (iv) CS—we used the clinical definition of CS from the IABP‐SHOCK II study 11 with clinical criteria of systolic blood pressure < 90 mmHg for ≥30 min or catecholamines to maintain systolic blood pressure > 90 mmHg and clinical pulmonary congestion and lactate > 2.0 mmol/L; (v) RHF—evidence of right ventricular dysfunction and signs of systemic congestion; and (vi) ACS‐HF—signs and symptoms of HF in the presence of ACS, with the ACS diagnosis based on clinical characteristics, electrocardiographic changes, and biochemical markers of cardiac necrosis (creatinine kinase isoenzymes, creatinine phospho‐kinase, or troponin I above the upper normal limit).
The following primary underlying cardiopulmonary aetiologies were documented: (i) coronary artery disease (including ACS and ischaemic cardiomyopathy); (ii) valvular heart disease (aetiology organic including endocarditis and valvular prosthesis dysfunction); (iii) cardiomyopathies (including idiopathic dilated, hypertrophic, diabetic, hypertensive, peripartum, left ventricular non‐compaction, chagasic, restrictive, and Takotsubo cardiomyopathies, and myocarditis); (iv) lung diseases (e.g. pulmonary embolism, pre‐existing lung disease, and arterial pulmonary hypertension); (v) pericardial disease; (vi) intracardiac tumours; (vii) adult congenital heart disease; and (viii) acute aortic syndromes.
Additionally, depending on the left ventricular ejection fraction (LVEF) recorded by echocardiography during hospitalization, patients were categorized into LVEF subgroups, defined as <40%, 40–49%, and ≥50%, based on the 2016 ESC guidelines. 3
Statistical analyses
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test and are presented as medians and the 25th and 75th percentiles (interquartile ranges [IQRs]). Categorical variables are reported as values and percentages. Differences in baseline characteristics across clinical phenotype categories at study entry were assessed with either χ2 or Fisher's exact probability tests, for categorical variables, or the Kruskal–Wallis test for continuous variables.
The primary study outcome was all‐cause in‐hospital mortality. In‐hospital mortality rates were calculated for each clinical phenotype and expressed as a percentage; group differences were evaluated by χ2 tests. Using the cohort of patients admitted during the same period without HF as a reference, differences in mortality between the AHF phenotypes were investigated, survival was plotted with the Kaplan–Meier curve, the patients were censored at hospital discharge, and differences between groups were assessed by a log‐rank test. An age‐adjusted and gender‐adjusted Cox proportional hazards regression model was used to estimate the association between each clinical phenotype and their in‐hospital risks of death, compared with patients without HF. Univariate and multivariate Cox's proportional hazards regression models with backward selection were used to identify significant predictors of in‐hospital all‐cause mortality. Two multivariable analyses were performed using the two models. Model 1 included only variables available at the time of admission (demographic variables, medical history, clinical features on presentation, laboratory data—except N‐terminal pro‐brain natriuretic peptide [NT pro‐BNP] levels, which were missing for 30% of patients—and clinical phenotypes) that were associated (P < 0.05) with mortality in the univariate analysis.
Model 2 was used to assess the potential role of in‐hospital management in AHF patients. We repeated the multivariate model that included the use of intravenous diuretics, inotropes, vasopressors, intra‐aortic balloon pump (IABP), and mechanical ventilation. The hazard ratio (HR) with 95% confidence interval (CI) was calculated. All tests were two‐sided, and P < 0.05 was considered statistically significant. IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA) was used.
Results
Baseline characteristics of the study population
During the study period (January 2006 to December 2018), 21 042 consecutive patients were admitted to the CCU. Among these, overall AHF was documented in 7759 (36.6%). Of all patients with AHF, 3058 (39.4%) presented with de novo AHF, and WCHF was diagnosed in 4701 (60.6%).
The study sample was classified based on ESC clinical profile guidelines, and the prevalence rate of each was calculated. ACS‐HF, making up 3338 (43.0%) of all cases, was the most common clinical class at admission, followed by DHF in 2617 (33.7%), HT‐HF in 914 (11.8%), and CS in 404 (5.2%). Least frequent were PO, present in 255 (3.3%), and RHF, in 231 (3.0%) (Figure 1 ). Patient baseline characteristics in each clinical phenotype category are shown in Table 1 . Among the whole sample, the median age was 62 years (IQR 52–72 years), 35.0% were women, and there were high rates of a history of hypertension (53.5%), diabetes (37.0%), smoking (47.9), HF (60.6%), and previous myocardial infarction (23.2%). Patients with HT‐HF and ACS‐HF were older, while the proportion of women was highest in RHF, HT‐HF, and PO. Comorbidities such as diabetes were more frequent among patients with ACS‐HF or CS, hypertension was more frequent among those with HT‐HF and ACS‐HF, and a previous history of HF was more frequent among those with DHF, HT‐HF, or PO.
Figure 1.
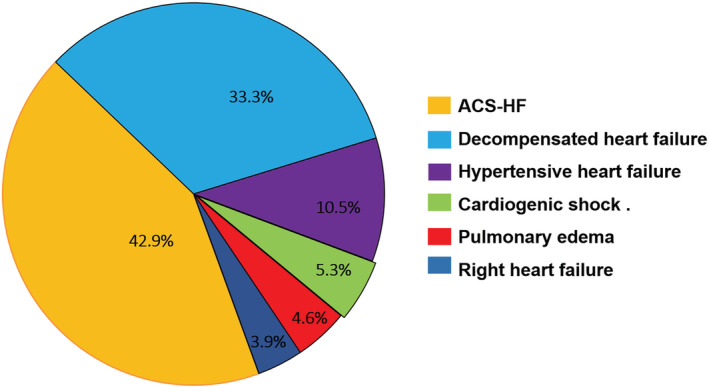
Classification of acute heart failure patients by clinical profile.
TABLE 1.
Comparison of baseline characteristics of patients according to the clinical phenotypes
| Overall (n = 7759) | ACS‐HF (n = 3338) | DHF (n = 2617) | HT‐HF (n = 914) | CS (n = 404) | PO (n = 255) | RHF (n = 231) | P value | |
|---|---|---|---|---|---|---|---|---|
| Age median (IQR) (years) | 62 (52–72) | 63 (56–72) | 60 (47–71) | 65 (54–75) | 62 (52–72) | 54 (41–66) | 49 (32–64) | <0.0001 |
| Female (%) | 35.0 | 24.5 | 41.3 | 47.4 | 35.4 | 40.4 | 59.3 | <0.0001 |
| Body mass index median (IQR) (kg/m2) | 26.1 (23.7–29.0) | 26.6 (24.2–29.2) | 25.3 (22.9–28.1) | 26.8 (24.0–30.4) | 26.1 (23.8–29.2) | 25.3 (22.8–27.6) | 26.4 (22.9–30.2) | <0.0001 |
| Medical history | ||||||||
| Current smoking (%) | 16.7 | 25.0 | 8.7 | 9.7 | 23.8 | 11.4 | 7.4 | <0.0001 |
| Previous smoking (%) | 31.2 | 34.2 | 30.5 | 28.6 | 25.2 | 28.6 | 20.3 | <0.0001 |
| Hypertension (%) | 53.5 | 60.6 | 41.7 | 74.6 | 49.3 | 32.9 | 31.2 | <0.0001 |
| Dyslipidaemia (%) | 27.0 | 37.3 | 17.6 | 25.3 | 25.0 | 11.8 | 10.0 | <0.0001 |
| Diabetes (%) | 37.0 | 51.1 | 22.7 | 37.6 | 42.1 | 12.9 | 11.7 | <0.0001 |
| Previous heart failure (%) | 60.6 | 28.5 | 100.0 | 73.0 | 33.4 | 75.7 | 59.7 | <0.0001 |
| Previous MI (%) | 23.2 | 32–0 | 16.9 | 19.7 | 18.3 | 11.4 | 3.5 | <0.0001 |
| Previous PCI (%) | 9.9 | 13.5 | 7.1 | 9.0 | 8.2 | 5.1 | 0.9 | <0.0001 |
| Previous CABG (%) | 4.1 | 4.7 | 4.2 | 3.9 | 2.2 | 2.7 | 1.0 | 0.01 |
| Previous valvular surgery (%) | 8.4 | 0.9 | 16.5 | 9.3 | 9.7 | 21.6 | 5.6 | <0.0001 |
| Previous stroke (%) | 5.2 | 4.0 | 6.5 | 5.4 | 6.9 | 8.6 | 1.7 | <0.0001 |
| Previous AF (%) | 16.2 | 3.7 | 28.6 | 21.7 | 16.8 | 30.2 | 18.6 | <0.0001 |
| Clinical presentation | ||||||||
| De novo AHF (%) | 39.4 | 71.5 | 27.0 | 66.6 | 24.3 | 40.3 | <0.0001 | |
| WCHF (%) | 60.6 | 28.5 | 100.0 | 73.0 | 33.4 | 75.7 | 59.7 | <0.0001 |
| SBP median (IQR) (mmHg) | 130 (104–139) | 125 (110–140) | 110 (100–121) | 155 (145–174) | 80 (70–85) | 105 (90–117) | 110 (100–120) | <0.0001 |
| HR median (IQR) (beats/min) | 90 (75–104) | 85 (74–100) | 90 (75–108) | 94 (78–110) | 100 (65–113) | 100 (80–120) | 96 (80–110) | <0.0001 |
| Peripheral oedema (%) | 39.6 | 15.7 | 59.7 | 60.0 | 31.7 | 68.2 | 56.7 | <0.0001 |
| Pulmonary congestion (%) | 81.4 | 84.9 | 79.4 | 80.2 | 84.2 | 89.4 | 42.9 | <0.0001 |
| Laboratory values | ||||||||
| Haemoglobin, median (IQR) (g/dL) (n = 7744) | 13.7 (11.8–15.2) | 14.0 (12.3–15.5) | 13.2 (11.3–14.9) | 13.45 (11.3–15.0) | 13.9 (11.9–15.8) | 12.9 (11.3–14.5) | 14.2 (11.7–16.1) | <0.0001 |
| Sodium, median (IQR) (mEq/L) (n = 7747) | 136 (133–139) | 137 (134–139) | 136 (132–138) | 137 (134–140) | 136 (132–139) | 134 (130–138) | 136 (133–139) | <0.0001 |
| Sodium < 136 mEq/L (%) (n = 7747) | 42.6 | 38.3 | 49.1 | 31.9 | 49.6 | 60.2 | 42.2 | <0.0001 |
| Albumin, median (IQR) (g/dL) (n = 7235 patients) | 3.5 (3.1–3.8) | 3.5 (3.2–3.8) | 3.4 (3.0–3.8) | 3.4 (3.0–3.8) | 3.2 (2.8–3.5) | 3.3 (3.0–3.6) | 3.2 (2.8–3.6) | <0.0001 |
| Albumin, <3.5 g/dL (n = 7235 patients) | 49.1 | 42.3 | 51.7 | 52.7 | 69.6 | 65.7 | 64.4 | <0.0001 |
| hs‐CRP, median (IQR) (mg/L) (n = 7099 patients) | 22.1 (8.0–73.6) | 25.0 (8.0–87.0) | 18.0 (7.0–57.0) | 16.6 (7.2–49.7) | 59.8 (23.5–146.5) | 38.5 (14.3–102.2) | 26.8 (10.2–79.2) | <0.0001 |
| NT‐proBNP (pg/mL), median (IQR) (n = 5657 patients) | 5540 (2213–15 506) | 4000 (1857–9043) | 7188 (2624–19 204) | 7107 (2340–21 257) | 15 112 (5251–25 000) | 16 450 (5115–25 000) | 4627 (1874–13 737) | <0.0001 |
| eGFR, median (IQR) (mL/min) (n = 7726) | 59.0 (36.2–85.1) | 65.4 (41.8–90.1) | 56.6 (35.0–81.8) | 52.5 (32.1–78.1) | 36.6 (25.2–56.6) | 52.9 (32.6–81.2) | 70.9 (45.3–100.5) | <0.0001 |
| eGFR, ≤30 mL/min (%) (n = 7726) | 18.2 | 14.0 | 19.9 | 22.7 | 34.5 | 20.2 | 11.8 | <0.0001 |
| ECHO available (n) | 7459 | 3225 | 2536 | 882 | 349 | 245 | 222 | |
| LVEF median (IQR) (%) | 40 (30–50) | 40 (32–46) | 40 (27–55) | 45 (32–58) | 30 (23–41) | 35 (25–55) | 59 (50.7–63) | <0.0001 |
| LVEF < 40% (%) | 46.1 | 44.9 | 49.3 | 38.0 | 70.2 | 58.4 | 7.2 | <0.0001 |
| LVEF 40–49% (%) | 24.8 | 34.7 | 18.3 | 20.9 | 11.7 | 9.8 | 7.2 | <0.0001 |
| LVEF ≥ 50% (%) | 29.1 | 20.3 | 32.4 | 41.2 | 18.1 | 31.8 | 85.6 | <0.0001 |
| Aetiology | ||||||||
| Coronary artery disease (%) | 56.5 | 100.0 | 19.6 | 23.6 | 69.1 | 12.9 | 3.5 | <0.0001 |
| Valvular heart disease (%) | 22.4 | 0 | 46.2 | 31.3 | 15.6 | 60.0 | 10.8 | <0.0001 |
| Cardiomyopathies (%) | 12.3 | 0 | 22.7 | 28.7 | 10.9 | 19.6 | 2.6 | <0.0001 |
| Adult congenital heart disease (%) | 2.5 | 0 | 3.2 | 2.2 | 1.0 | 3.1 | 33.3 | <0.0001 |
| Lung diseases (%) | 2.1 | 0 | 1.1 | 3.0 | 2.0 | 0 | 44.2 | <0.0001 |
| Acute aortic syndromes (%) | 1.4 | 0 | 2.6 | 3.7 | 0.2 | 1.2 | 0.9 | <0.0001 |
| Pericardial diseases (%) | 0.8 | 0 | 1.5 | 1.5 | 0.2 | 1.6 | 1.7 | <0.0001 |
| Intracardiac tumours (%) | 0.3 | 0 | 0.6 | 0.4 | 0.2 | 0 | 2.2 | <0.0001 |
| Aetiology not established (%) | 1.6 | 0 | 2.4 | 5.6 | 0.7 | 1.6 | 0.9 | <0.0001 |
ACS‐HF, acute heart failure and associated acute coronary syndromes; AF, atrial fibrillation; AHF, acute heart failure; CABG, coronary artery bypass grafting; CS, cardiogenic shock; DHF, decompensated heart failure; ECHO, echocardiography; eGFR, estimated glomerular filtration rate (according to the Cockroft–Gault formula); HR, heart rate; hs‐CRP, high‐sensitivity C‐reactive protein; HT‐HF, hypertensive heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PCI, percutaneous coronary intervention; PO, pulmonary oedema; RHF, right heart failure; SBP, systolic blood pressure; WCHF, worsening chronic heart failure.
Clinical presentation
De novo AHF was more frequent in patients with ACS‐HF, and WCHF was more frequent in those with DHF. At presentation, as expected, patients with CS were more likely to have high‐risk features, including lower systolic blood pressure, higher heart rate, lower LVEF, and lower estimated glomerular filtration rates (eGFRs) (P < 0.0001) (Table 1 ). Peripheral oedema and pulmonary congestion were found in 39.6% and 81.2% of patients, respectively, and were more frequently observed in patients admitted with PO or DHF.
Overall, sodium levels < 136 mEq/L and albumin < 3.5 g/dL were observed in 42.5% and 49.1% of patients, respectively. Hyponatraemia (sodium levels < 136 mEq/L) was more frequent in patients with PO, DHF, or CS, while hypoalbuminaemia (albumin < 3.5 g/dL) was more frequent in patients with CS, RHF, or PO. In contrast, levels of high‐sensitivity C‐reactive protein and NT pro‐BNP were significantly elevated in all clinical profiles, especially in patients with CS or PO.
The median estimated eGFR of all patients was 59.0 mL/min, with the best renal function in those with RHF or ACS‐HF. Among the 18.2% of patients with eGFR ≤ 30 mL/min, the CS group (33.9%) was primarily represented.
Most patients (96.1%) underwent echocardiography during hospitalization. Overall, their median LVEF was 40% (IQR 30–50). Left ventricular function also differed markedly among the clinical profiles, varying from 30% (IQR 23–41) in CS patients to 59% (IQR 50.7–63) in RHF patients. Reduced left ventricular function (LVEF < 40%) was present in 46.1% of the overall sample and was more common in CS patients (70.2%). In contrast, preserved left ventricular function (LVEF ≥ 50%) was present in 29.1% of the overall sample and was more common in RHF (85.6%) and HT‐HF (41.2%) patients.
Major acute heart failure aetiology
In our sample, coronary artery disease and valvular heart disease were the most frequent underlying aetiologies, observed in 4386 (56.5%) and 1736 (22.4%) of cases, respectively, followed by cardiomyopathies in 957 (12.3%), adult congenital heart disease in 192 (2.5%), lung diseases in 165 (2.1%), acute aortic syndromes in 109 (1.4%), pericardial diseases in 63 (0.8%), and intracardiac tumours in 27 (0.3%). Aetiology could not be established for 124 (1.6%) of the cases (Figure 2 ). The differences in aetiologies between patients with varying clinical phenotypes are shown in Table 1 . Of the 404 patients with CS, coronary artery disease (i.e. ACS) was the most frequent underlying disease, observed in 279 (69.1%) of patients; valvular heart disease was the main aetiology in patients with PO (60.0%), DHF (46.2%), and HT‐HF (31.3%).
Figure 2.
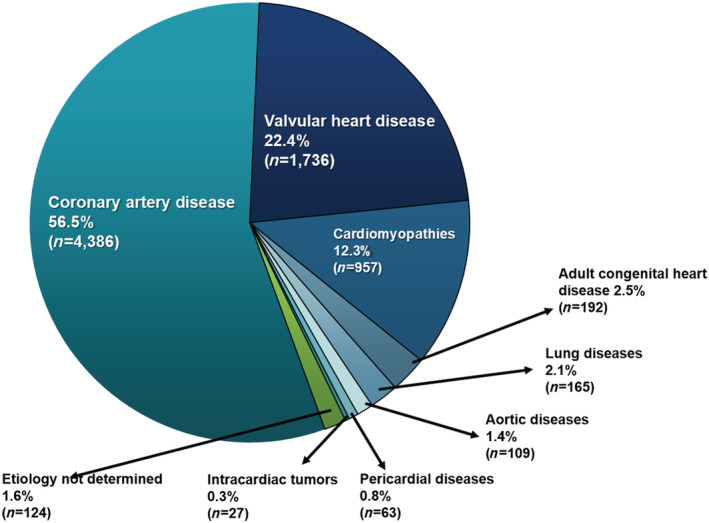
Aetiologies of acute heart failure. *Coronary artery disease included ACS (n = 3597) and chronic ischaemic cardiomyopathy (n = 789). **Cardiomyopathies included idiopathic dilated (n = 510), hypertensive (n = 237), chagasic (n = 56), hypertrophic (n = 37), restrictive (n = 27), peripartum (n = 17), left ventricular non‐compaction (n = 10), Takotsubo (n = 12), diabetic (n = 6), and myocarditis (n = 45).
Medical history prior to admission
Data about treatment prior to admission were available in 94.8% (7355/7759) of patients. Previous use of all analysed drugs was more frequent in patients with WCHF compared with those with de novo HF (Supporting Information, Table S1 ). In the whole sample with WCHF, before hospitalization, more than half received angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) (55.4%) and diuretics (54.2%); these were more frequently used by patients in the ACS‐HF, CS, or HT‐HF groups and by those in the DHF, PO, or CS groups, respectively. Previous use of beta‐blockers occurred among 38.4% of patients, and their use was more frequent in patients with CS or ACS‐HF. Digoxin and spironolactone were used by approximately one‐third of patients with WCHF.
Among the WCHF patients with LVEF < 40%, the ACEIs/ARBs, beta‐blockers, and spironolactone were used in 60.4%, 42.5%, and 34.4%, respectively. Surprisingly, only 32.8% of these patients were treated before admission with ACEIs or ARBs plus beta‐blockers. Up to 25% of patients did not previously receive ACEIs/ARBs, beta‐blockers, or mineralocorticoid receptor antagonists (Table 2 ).
TABLE 2.
Medications at hospital admission in worsening chronic heart failure according to left ventricular ejection fraction (available data in 4268 patients)
| Overall (n = 4268) | LVEF < 40% (n = 2254) | LVEF 40–49% (n = 864) | LVEF ≥ 50 (n = 1150) | P value | |
|---|---|---|---|---|---|
| ACEI (%) | 55.6 | 51.0 | 40.5 | 41.6 | <0.0001 |
| ARBs (%) | 13.5 | 13.2 | 14.0 | 10.7 | 0.83 |
| ACEI or ARBs (%) | 55.6 | 60.4 | 57.3 | 44.7 | <0.0001 |
| Beta‐blocker (%) | 38.5 | 42.5 | 37.4 | 31.3 | <0.0001 |
| Diuretics (%) | 54.4 | 58.0 | 50.9 | 49.9 | <0.0001 |
| Spironolactone (%) | 28.8 | 34.4 | 22.7 | 22.3 | <0.0001 |
| ACE/ARBs + beta‐blockers + spironolactone (%) | 11.4 | 16.0 | 7.9 | 5.0 | <0.0001 |
| ACE/ARBs + beta‐blockers (%) | 27.3 | 32.8 | 26.9 | 16.9 | <0.0001 |
| ACE/ARBs + spironolactone (%) | 19.9 | 25.4 | 14.0 | 13.6 | <0.0001 |
| None of the previous three (%) | 27.8 | 25.0 | 26.3 | 34.5 | <0.0001 |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; LVEF, left ventricular ejection fraction.
In‐hospital management
In‐hospital management is reported in Table 3 . Intravenous diuretics were used in most patients (81.2%), particularly those with HT‐HF, DHF, or PO. Overall, the use of inotropes and vasopressors in patients was 18.9% and 20.4%, respectively; as expected, their use in patients with CS was more frequent (inotropes 72.0%, vasopressors 96.3%), as were the use of IABP and mechanical ventilation. Among inotropes, dobutamine was used more frequently, while intravenous vasodilators were used in approximately one‐third of patients, primarily those with ACS‐HF, HT‐HF, or PO.
TABLE 3.
In‐hospital management according to the clinical phenotypes
| Overall (n = 7759) | ACS‐HF (n = 3338) | DHF (n = 2617) | HT‐HF (n = 914) | CS (n = 404) | PO (n = 255) | RHF (n = 231) | P value | |
|---|---|---|---|---|---|---|---|---|
| Intravenous diuretics (%) | 81.2 | 76.6 | 86.9 | 88.9 | 65.6 | 85.1 | 71.4 | <0.0001 |
| Intravenous vasodilators any (%) | 33.4 | 43.8 | 21.5 | 49.3 | 5.4 | 28.6 | 9.1 | <0.0001 |
| Nitroglycerine (%) | 30.9 | 43.4 | 17.7 | 43.4 | 5.4 | 20.0 | 8.7 | <0.0001 |
| Nitroprusside (%) | 3.1 | 0.9 | 4.3 | 7.7 | 0.5 | 9.4 | 0.4 | <0.0001 |
| Intravenous diuretic + vasodilators (%) | 29.0 | 35.4 | 20.6 | 45.8 | 5.0 | 28.2 | 7.8 | <0.0001 |
| Inotropes any (%) | 18.9 | 15.3 | 16.7 | 8.9 | 72.0 | 36.5 | 21.6 | <0.0001 |
| Dobutamine (%) | 15.6 | 13.5 | 12.5 | 5.8 | 64.9 | 31.0 | 18.6 | <0.0001 |
| Levosimendan (%) | 2.9 | 2.7 | 2.5 | 0.8 | 11.9 | 5.5 | 1.3 | <0.0001 |
| Dopamine (%) | 4.5 | 2.5 | 5.7 | 3.5 | 13.4 | 9.0 | 5.2 | <0.0001 |
| Vasopressors any (%) | 20.4 | 16.1 | 15.6 | 7.4 | 96.3 | 46.3 | 28.1 | <0.0001 |
| Norepinephrine (%) | 20.1 | 15.8 | 15.3 | 7.2 | 95.8 | 43.9 | 26.8 | <0.0001 |
| Vasopressin (%) | 10.1 | 7.6 | 5.6 | 3.5 | 63.1 | 24.7 | 13.4 | <0.0001 |
| Both vasopressors (%) | 9.7 | 7.3 | 5.3 | 3.3 | 62.6 | 22.4 | 12.1 | <0.0001 |
| ACEI (%) | 64.9 | 79.8 | 57.6 | 66.8 | 21.8 | 37.6 | 31.2 | <0.0001 |
| ARB (%) | 3.0 | 1.8 | 3.7 | 6.6 | 1.0 | 1.6 | 2.2 | <0.0001 |
| ACEI or ARB (%) | 66.9 | 80.8 | 60.3 | 70.7 | 22.8 | 38.8 | 32.9 | <0.0001 |
| Beta‐blocker (%) | 32.0 | 48.6 | 21.2 | 23.2 | 11.4 | 12.2 | 8.7 | <0.0001 |
| Spironolactone (%) | 19.1 | 17.7 | 24.2 | 15.8 | 10.6 | 17.3 | 13.0 | <0.0001 |
| Digoxin (%) | 19.8 | 7.9 | 31.8 | 25.5 | 15.6 | 34.9 | 22.5 | <0.0001 |
| Amioradone (%) | 12.0 | 10.4 | 13.3 | 10.1 | 21.8 | 12.9 | 10.0 | <0.0001 |
| IABP (%) | 5.1 | 8.0 | 0.8 | 0.3 | 25.7 | 2.0 | 0.0 | <0.0001 |
| Mechanical ventilation (%) | 14.7 | 12.0 | 8.9 | 10.0 | 66.8 | 39.6 | 19.5 | <0.0001 |
| Coronary angiography (%) | 43.2 | 75.2 | 13.3 | 17.8 | 46.5 | 13.7 | 3.0 | <0.0001 |
| Primary PCI (%) (n = 2035, STEMI patients) a | 24.9 | 24.7 | 26.0 | |||||
| Thrombolysis in our hospital (%) | 2.9 | 3.1 | 1.3 | |||||
| Thrombolysis outside our hospital (%) | 19.2 | 19.2 | 19.3 |
ACEIs, angiotensin‐converting enzyme inhibitors; ACS‐HF, acute coronary syndrome and HF; ARBs, angiotensin receptor blockers; CS, cardiogenic shock; DHF, decompensation heart failure; HT‐HF, hypertensive HF; IABP, intra‐aortic balloon pump; PCI, percutaneous coronary intervention; PO, pulmonary oedema; RHF, isolated right HF; STEMI, ST‐elevation myocardial infarction.
STEMI: ACS‐HF = n = 1812 and CS = n = 223.
Overall, ACEIs or ARBs were used in approximately two‐thirds of patients (66.9%) and beta‐blockers in one‐third (32.0%) and more frequently in the ACS‐HF patients. In contrast, the use of spironolactone and digoxin was low (approximately 20% of patients).
Coronary angiography was undertaken in 75.2% and 46.5% of patients with ACS‐HF or CS, respectively. However, of the 2035 patients with ST‐elevation myocardial infarction (STEMI) (ACS‐HF [n = 1812] and CS [n = 223]), only 448 (24.7%) and 58 (26.0%), respectively, received reperfusion therapy with primary percutaneous coronary intervention (Table 3 ).
Early (first 24 h) coronary care unit management
Overall, the use of intravenous diuretics during the first 24 h was 76.6%, predominantly in those with PO (83.5%), HT‐HF (86.4%), and DHF (83.1%). In fact, almost all patients with CS (95.3%) received vasopressors. In contrast, approximately half of the patients with ACS‐HF (41.1%) and HT‐HF (47.5%) received intravenous vasodilators. Unexpectedly, early use of ACEIs or ARBs occurred in only one‐half of patients, while beta‐blockers or spironolactone were administered to only one‐tenth (Table 4 ).
TABLE 4.
Early treatment within 24 h of admission according to the clinical phenotypes
| Overall (n = 7759) | ACS‐HF (n = 3338) | DHF (n = 2617) | HT‐HF (n = 914) | CS (n = 404) | PO (n = 255) | RHF (n = 231) | P value | |
|---|---|---|---|---|---|---|---|---|
| Intravenous diuretics (%) | 76.6 | 71.4 | 83.1 | 86.4 | 56.2 | 83.5 | 66.2 | <0.0001 |
| Inotropes any (%) | 13.0 | 8.7 | 11.3 | 6.2 | 66.1 | 25.9 | 15.6 | <0.0001 |
| Dobutamine (%) | 10.3 | 7.5 | 7.4 | 3.7 | 59.2 | 21.6 | 12.6 | <0.0001 |
| Levosimendan (%) | 0.8 | 0.7 | 0.6 | 0.2 | 4.5 | 0.8 | 0.0 | <0.0001 |
| Dopamine (%) | 3.5 | 1.6 | 4.3 | 3.0 | 12.4 | 7.5 | 4.3 | <0.0001 |
| Vasopressors any (%) | 14.8 | 9.8 | 9.8 | 3.6 | 95.3 | 37.6 | 20.8 | <0.0001 |
| Norepinephrine (%) | 14.5 | 9.6 | 9.6 | 3.5 | 94.6 | 36.5 | 19.9 | <0.0001 |
| Vasopressin (%) | 6.5 | 3.8 | 3.1 | 1.1 | 55.4 | 16.5 | 9.1 | <0.0001 |
| Both vasopressors (%) | 6.2 | 3.6 | 2.8 | 1.0 | 54.7 | 15.3 | 8.2 | <0.0001 |
| Intravenous vasodilators any (%) | 30.9 | 41.1 | 19.0 | 47.5 | 2.5 | 27.1 | 7.8 | <0.0001 |
| Nitroglycerine (%) | 28.9 | 40.8 | 16.3 | 41.7 | 2.2 | 18.4 | 7.4 | <0.0001 |
| Nitroprusside (%) | 2.3 | 0.4 | 2.8 | 6.9 | 0.2 | 9.0 | 0.4 | <0.0001 |
| ACEI (%) | 50.2 | 63.0 | 44.3 | 53.7 | 5.7 | 25.1 | 25.1 | <0.0001 |
| ARBs (%) | 1.7 | 0.8 | 2.6 | 3.3 | 0.0 | 0.4 | 1.7 | <0.0001 |
| ACEI or ARB (%) | 51.7 | 63.7 | 46.5 | 56.3 | 5.7 | 25.5 | 26.8 | <0.0001 |
| Beta‐blocker (%) | 11.1 | 14.7 | 10.0 | 9.7 | 1.5 | 3.5 | 3.0 | <0.0001 |
| Spironolactone (%) | 9.1 | 5.6 | 15.1 | 8.4 | 2.0 | 9.4 | 7.8 | <0.0001 |
| Digoxin (%) | 13.9 | 2.8 | 25.1 | 20.6 | 7.2 | 27.1 | 17.7 | <0.0001 |
| Amioradone (%) | 7.1 | 4.6 | 9.5 | 7.1 | 12.6 | 6.7 | 7.8 | <0.0001 |
ACEIs, angiotensin‐converting enzyme inhibitors; ACS‐HF, acute coronary syndrome and HF; ARBs, angiotensin receptor blockers; CS, cardiogenic shock; DHF, decompensation heart failure; HT‐HF, hypertensive HF; PO, pulmonary oedema; RHF, isolated right HF.
Outcomes during hospitalization
Overall, the median hospital stay was 9 days (IQR 5–17 days), while the length of stay in the CCU was 5 days (IQR 3–7 days). In‐hospital mortality occurred in 1390 (17.9%) of the 7759 patients with AHF included in analyses. For the 13 283 patients without HF admitted to the same CCU during the study period, the following numbers and pathologies were recorded: ACS 59.5% (n = 7901), valvular heart disease 8.6% (n = 1140), cardiomyopathy 8.9% (n = 1182), lung disease 2.5% (n = 332), cardiac arrhythmia and atrial‐ventricular block 9.7% (n = 1292), pericardial disease 1.1% (n = 142), cardiac tumour 0.7% (n = 93), acute aortic syndrome 3.1% (n = 410), hypertensive crisis 0.7% (n = 94), adult congenital heart disease 1.6% (n = 214), and critically ill patients without cardiovascular disease 3.6% (n = 483). All‐cause in‐hospital mortality was higher among patients with AHF compared with patients without AHF (17.9% vs. 5.0%; P < 0.0001).
Over the 13‐year period between 2006 and 2018, in‐hospital mortality decreased for patients with AHF (from 21.3% to 17.2%, P trend = 0.01) (Figure 3A ). The unadjusted in‐hospital mortality rates were significantly higher in patients with CS (71.3%), followed by PO (43.9%), RHF (23.8%), DHF (14.9%), ACS‐HF (13.6%), and HT‐HF (10.1%) (Figure 4 ). The mortality rates according to the year of presentation and the AHF phenotype are shown in Figure 3B . Only the group of patients with ACS‐HF exhibited a decline in in‐hospital mortality between 2006 and 2018 (19.0 to 10.4%, P trend < 0.0001). Using the patient group without HF as a reference in the age‐adjusted and gender‐adjusted Cox proportional hazards model, patients admitted with either CS or PO clinical phenotype showed a higher in‐hospital mortality risk (HR 16.6, 95% CI 14.48–19.19, P < 0.0001 and HR 7.4, 95% CI 6.05–9.08, P < 0.0001, respectively). At the other end of this spectrum, patients with HT‐HF or ADH clinical phenotypes showed a slightly higher risk of death compared with patients without HF (HR 1.4, 95% CI 1.18–1.83, P < 0.0001 and HR 1.42, 95% CI 1.30–1.56, P < 0.0001, respectively). Patients with AHF precipitated by ACS or RHF showed an intermediate risk of death compared with patients without HF (HR 2.3, 95% CI 2.09–2.66, P < 0.0001 and RHF HR 3.8, 95% CI 2.88–5.01, P < 0.0001, respectively) (Figure 5 ).
Figure 3.
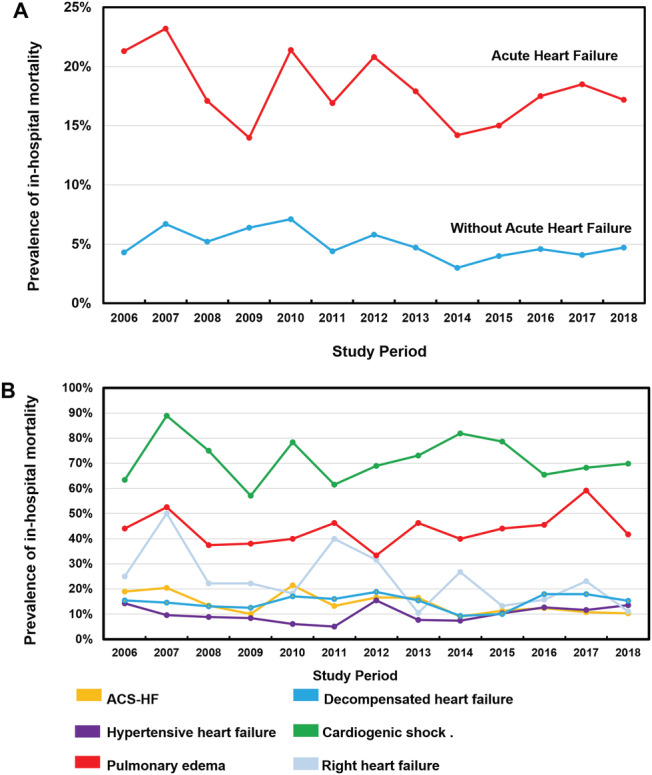
(A) Temporal trends from 2006 to 2018 in rates of all‐cause in‐hospital mortality in patients with and without acute heart failure hospitalized in the same coronary care unit during the same study period. (B) All‐cause in‐hospital mortality according to clinical phenotype of acute heart failure at admission. ACS‐HF, acute heart failure and associated acute coronary syndromes.
Figure 4.
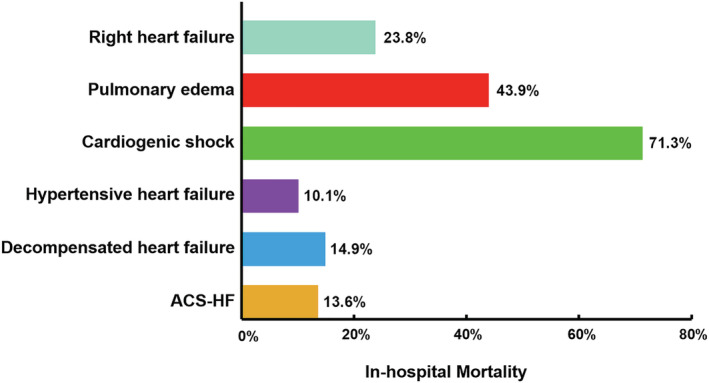
All‐cause in‐hospital mortality according to clinical phenotype of acute heart failure at admission. ACS‐HF, acute heart failure and associated acute coronary syndromes.
Figure 5.
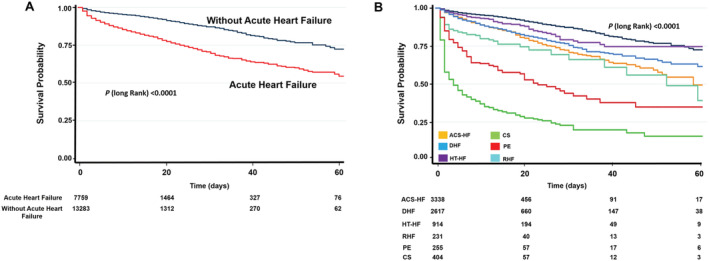
Kaplan–Meier curves for all‐cause in‐hospital mortality: (A) divided into hospitalized reference patients without heart failure (blue line) and patients with acute heart failure (red line); (B) using pairwise comparisons with reference patients without acute heart failure, there were differences in in‐hospital survival according to AHF clinical phenotype. ACS‐HF, acute heart failure and associated acute coronary syndromes; CS, cardiogenic shock; DHF, decompensated heart failure; HT‐HF, hypertensive heart failure; PO, pulmonary oedema; RHF, right heart failure.
Adjusted multivariate Cox proportional hazards regression models were generated with all significant univariate predictors of in‐hospital mortality listed in Supporting Information, Table S 2 . Using HT‐HF patients as a reference for the lowest in‐hospital mortality rate, the multivariate Cox proportional hazards analysis (Model 1) identified the clinical phenotypes of PO (HR 2.68, 95% CI 1.73–4.14, P < 0.0001) and CS (HR 3.37, 95% CI 2.12–5.35, P < 0.0001) as independent predictors of in‐hospital mortality among patients with AHF. Other factors independently associated with increased in‐hospital mortality were being female (P = 0.006), age per 10‐year group (P < 0.0001), previous valvular surgery (P = 0.01), de novo AHF (P = 0.005), sodium < 136 mEq/L (P = 0.001), hs‐CRP ≥ 10 mg/L (P < 0.0001), albumin < 3.5 g/dL (P = 0.03), eGFR ≤ 30 mL/min (P < 0.0001), and LVEF < 40% (P < 0.0001) (Table 5 ).
TABLE 5.
Multivariate analysis for the prediction of in‐hospital all‐cause mortality in patients with acute heart failure
| Model 1 | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Clinical phenotypes | |||
| HT‐HF | Reference group | ||
| ACS‐HF | 1.01 | 0.83 to 1.45 | 0.95 |
| DHF | 0.98 | 0.66 to 1.45 | 0.92 |
| RHF | 1.28 | 0.75 to 2.20 | 0.35 |
| PO | 2.68 | 1.73 to 4.14 | <0.0001 |
| CS | 3.37 | 2.12 to 5.35 | <0.0001 |
| Gender (female) | 1.22 | 1.05 to 1.41 | 0.006 |
| Age (per 10 years) | 1.11 | 1.06 to 1.16 | <0.0001 |
| Previous smoking | 0.80 | 0.69 to 0.93 | 0.005 |
| Previous valvular surgery | 1.31 | 1.05 to 1.62 | 0.01 |
| De novo AHF | 1.29 | 1.08 to 1.54 | 0.005 |
| SBP ≥ 140 (mmHg) (%) | Reference group | ||
| SBP 90–140 (mmHg) (%) | 1.47 | 1.10 to 1.97 | 0.008 |
| SBP < 90 (mmHg) (%) | 1.62 | 1.11 to 2.37 | 0.01 |
| Sodium < 136 mEq/L | 1.24 | 1.09 to 1.41 | 0.001 |
| hs‐CRP, ≥10 mg/L | 1.57 | 1.32 to 1.87 | <0.0001 |
| Albumin, <3.5 g/dL | 1.16 | 1.01 to 1.33 | 0.03 |
| eGFR, ≤30 mL/min | 1.59 | 1.36 to 1.85 | <0.0001 |
| LVEF ≥ 50% | Reference group | ||
| LVEF 40–49% | 0.86 | 0.70 to 1.04 | 0.17 |
| LVEF < 40% | 1.51 | 1.27 to 1.79 | <0.0001 |
| Model 2 | |||
| Clinical phenotypes | |||
| HT‐HF | Reference group | ||
| ACS‐HF | 1.15 | 0.87 to 1.53 | 0.30 |
| DHF | 1.13 | 0.86 to 1.48 | 0.38 |
| RHF | 1.31 | 0.82 to 2.07 | 0.24 |
| PO | 1.93 | 1.38 to 2.71 | <0.0001 |
| CS | 1.50 | 1.09 to 2.07 | 0.01 |
| Gender (female) | 1.17 | 1.01 to 1.36 | 0.02 |
| Age (per 10 years) | 1.13 | 1.08 to 1.19 | <0.0001 |
| Previous smoking | 0.82 | 0.70 to 0.95 | 0.01 |
| Previous valvular surgery | 1.26 | 1.01 to 1.57 | 0.03 |
| Sodium < 136 mEq/L | 1.26 | 1.11 to 1.43 | 0.001 |
| hs‐CRP, ≥10 mg/L | 1.30 | 1.10 to 1.55 | 0.003 |
| eGFR, ≤30 mL/min | 1.47 | 1.21 to 1.72 | <0.0001 |
| LVEF ≥ 50% | Reference group | ||
| LVEF 40–49% | 0.90 | 0.73 to 1.11 | 0.35 |
| LVEF < 40% | 1.24 | 1.04 to 1.47 | 0.01 |
| Intra‐aortic balloon pump | 1.32 | 1.08 to 1.62 | 0.006 |
| Mechanical ventilation* | 1.80 | 1.452to 2.14 | <0.0001 |
| Intravenous diuretics** | 0.70 | 0.59 to 0.84 | <0.0001 |
| Inotropes *** | 1.49 | 1.27 to 1.76 | <0.0001 |
| Vasopressors **** | 2.91 | 2.41 to 3.51 | <0.0001 |
ACS‐HF, acute heart failure and associated acute coronary syndromes; AHF, acute heart failure; CS, cardiogenic shock; DHF, decompensated heart failure; eGFR, estimated glomerular filtration rate (according to the Cockroft–Gault formula); hs‐CRP, high‐sensitivity C‐reactive protein; HT‐HF, hypertensive heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PO, pulmonary oedema; RHF, right heart failure; SBP, systolic blood pressure.
Interaction with cardiogenic shock (P value for interaction < 0.0001) and with PO (P value for interaction = 0.001).
Interaction with cardiogenic shock (P value for interaction < 0.0001), PO (P value for interaction < 0.0001), RHF (P value for interaction = 0.001), and with DHF (P value for interaction = 0.01).
Interaction with cardiogenic shock (P value for interaction < 0.0001).
Interaction with cardiogenic shock (P value for interaction < 0.0001) and with PO (P value for interaction = 0.04).
When the in‐hospital management was added to the multivariate model (Model 2), the use of inotropes (P < 0.0001), vasopressors (P < 0.0001), IABP (P < 0.0001), and mechanical ventilation (P < 0.0001) was independently associated with increased in‐hospital mortality. Conversely, the use of intravenous diuretics (P < 0.0001) was associated with a reduced risk of mortality. In Model 2, significant interactions were found between use of mechanical ventilation, inotropes, vasopressors, intravenous diuretics, and the clinical phenotypes of AHF, mainly PO and CS (Table 5 ).
Discussion
In this single‐centre cohort of patients with AHF admitted to a CCU in a centre specializing in cardiovascular diseases, we found a high mortality in patients with AHF, which may depend, among other factors, upon the AHF clinical phenotypes. We also found significant differences in clinical characteristics at presentation and in the aetiologies, compared with the large registries in Europe and the USA.
Reported prevalence rates of patients hospitalized with AHF have varied widely, both between and within countries (10–51%), 12 , 13 encompassing the prevalence of AHF in our sample (36.6%). Marked differences at hospital admission have been well documented across geographic regions, in terms of baseline demographics, clinical profiles, laboratory tests, comorbidity burden, and use of guideline‐recommended therapies. 14 , 15 To our knowledge, our study is the first to describe a large cohort of patients with AHF presenting to a hospital in Latin America who were classified by clinical phenotype. 16 In contrast to previous studies, 17 our sample's predominant clinical phenotype was AHF in an ACS context, while only one‐third had DHF. Chioncel et al. 18 recently analysed 6629 patients with AHF from 21 European and Mediterranean countries who were enrolled in the ESC Heart Failure Long‐Term (ESC‐HF‐LT) registry. The authors found that the most frequent clinical phenotype was DHF (61.1%), followed by ACS‐HF (14.4%) and PO (13.2%).
There are notable differences in the characteristics of our sample of patients with AHF compared with their counterparts in the large registries from the USA and Europe. 8 , 19 , 20 , 21 Our sample of patients with AHF was younger and had lower prevalence rates of female sex, hypertension, diabetes, and previous atrial fibrillation. Nevertheless, their prevalence of de novo AHF and WCHF were comparable with those reported previously (45.2% and 54.8%, respectively). 22 However, in our sample with WCHF, there was a striking underutilization of pre‐hospitalization evidence‐based medical therapies that improve outcomes and reduce the burden of hospitalization in patients with HF (i.e. diuretics, beta‐blockers, ACEIs/ARBs, and mineralocorticoid/aldosterone receptor antagonists). 3 The use of ACEI/ARB in addition to a beta‐blocker is recommended for patients with HF and reduced LVEF to reduce the risk of HF hospitalization and death. 3 This does not appear to be the case for the population with WCHF and reduced LVEF in our study because about one‐third were treated before hospitalization with the combination of ACEI/ARB and a beta‐blocker. These findings are surprising given that other research has shown that in patients with chronic HF with reduced LVEF, ACEIs/ARBs, beta‐blockers, and mineralocorticoid receptor antagonists were used in 92.2%, 92.7%, and 67.0% of patients, respectively. 23
Overall, in our study, 81.4% of patients had pulmonary congestion and 39.6% had peripheral oedema; these percentages are similar to those reported from a recent registry (74.6% and 55%, respectively). 24 Similarly, in our patients with RHF, the presence of pulmonary congestion (42.9%) was similar to that found in the study by Chioncel et al. (53.2%). 18 Ventricular interdependence, increased intravascular volume, and changes in pulmonary lymphatic drainage have been postulated as the potential mechanisms to explain pulmonary congestion in RHF. 25 , 26
It is important to note that appropriate therapy requires early identification of the patient's specific clinical AHF phenotype. In our study, the use of evidence‐based medicines was adequate based on expert consensus‐based recommendations. 27 Overall, the use of intravenous therapies such as diuretics (81%), vasodilators (31%), and inotropes (18.9%) was similar to that recently reported for hospitalized patients in the ESC‐HF‐LT registry. 18 In addition, the present study shows that 76.6% of these patients were treated with intravenous diuretics within 24 h of admission, predominantly in those with PO (83.5%), a strategy that has been associated with lower in‐hospital mortality. 28 Our study also found greater use of inotropes and vasopressors in patients with HT‐HF than in other studies (8.6% vs. 1.5%). 18 This may reflect the inappropriate application of aggressive therapies for some types of AHF, for example, the combination of intravenous diuretics and intravenous vasodilators, which were given to half of our patients with HT‐HF. 29 An outcome of this practice may have been iatrogenic hypotension and, as a consequence, the unjustified use of vasopressors and/or inotropes. Unfortunately, their use in patients with AHF has increased despite the evidence of their association with an increase in in‐hospital mortality. 30 , 31 , 32
Investigators have recently shown that natriuretic peptide‐guided therapy facilitates the optimization of therapy in AHF patients and reduces the in‐hospital mortality. However, there is insufficient information about whether natriuretic peptide‐guided therapy can be applied routinely to all AHF patients. 33 Unfortunately, in our study, it was not possible to evaluate the influence of NT pro‐BNP levels on in‐hospital treatment in each of the AHF phenotypes.
Coronary heart disease, valvular heart disease, and dilated cardiomyopathy are specific AHF‐associated cardiovascular conditions. 3 Our study emphasizes the heterogeneity of AHF patients; coronary heart disease and valvular heart disease were the most frequent underlying diseases, occurring in 78% of patients with AHF, followed by cardiomyopathies. Of note was the distribution of underlying disease within each AHF clinical phenotype, as well as the overlap of aetiologies among them. Specifically, we found that 10% of our study sample had an aetiology that has been infrequently reported in previous studies, including congenital heart disease in adults, acute aortic syndromes, intracardiac tumours, and pericardial diseases.
Latin America‐based data are mainly from South America (usually Brazil and Argentina). The aetiology of AHF reported in this region is like other regions (i.e. coronary heart disease, valvular heart disease, and cardiomyopathy), with special interest in chagasic cardiomyopathy. 16
Our patients' median hospital stay was 9 days, which is similar to that reported from European registries but longer than that reported in US registries. 19 , 22 Yet the most striking finding from the present study is the substantial rate of in‐hospital mortality (17.9%), which is in stark contrast to rates reported from European registries (6.4–7.3%) and in the USA (4%), 9 , 19 although it is similar to the overall mortality rate reported from intensive care units in a survey of 666 hospitals in nine countries (17.8%). 21 However, other studies from Latin America have also reported high in‐hospital mortality (11.7%), with a higher rate among patients with a reduced ejection fraction, ischaemic heart disease, or Chagas disease. 34
In our study population, after adjusting for all variables at admission as well as for treatment procedures, we found that the clinical phenotypes of PO and CS were independent predictors of in‐hospital mortality. This finding is consistent with that of Oliva et al., who showed that the phenotypes of CS and PO are independent predictors of in‐hospital mortality. 22 Consistent with the literature, our study found that other factors associated with hospital mortality were renal dysfunction, older age, low systolic blood pressure, hyponatraemia, and low LVEF. 19 , 22 , 35 An interesting finding is that the use of intravenous diuretics was associated with lower in‐hospital mortality rate, as has been shown in recent studies. 28 By contrast, our analysis showed that intravenous inotrope and/or vasopressor use was an independent predictor of a detrimental outcome, which is consistent with the finding of previous studies. 30 , 31 , 32
Several factors may explain the high rate of mortality in our sample, among which the predominant ACS clinical phenotype stands out. Previous data have shown that ACS complicated by AHF carries a particularly high risk of adverse outcomes, including the highest risk of short‐term death (around 13%). 36 , 37 Furthermore, the frequency of CS in our sample was higher compared with previous studies (5.2% vs. 3%) with a significantly higher in‐hospital mortality compared with that reported in the literature. 38 In our population of patients with CS, ACS was the most common cause in most patients (69.1%), and other aetiologies were associated with the remaining 30%. This finding is consistent with that of Harjola et al., 39 who reported that 81% of the CS patients had ACS.
One interesting finding of our study was the high rate of in‐hospital mortality among patients with CS (69%), which contrasts with the published rate of 40%, depending on the underlying aetiology. 38 It is now well established from a variety of studies that the prognosis of patients with acute myocardial infarction (AMI) complicated by CS has improved over the past decade mainly thanks to early revascularization. 40 , 41 Primary percutaneous coronary intervention was only performed in a quarter of the STEMI patients with CS, suggesting that the majority of patients with STEMI delayed their hospital attendance.
On the other hand, in our analysis, the IABP was used only on 25.7% of all patients with CS. Data from several studies suggest that IABP is now less and less often used, and on the contrary, the application of other mechanical circulatory support has increased both in Europe and in the USA. 42 , 43 , 44 In our CCU, IABP is the most widely used mechanical circulatory support device because other advanced forms of mechanical circulatory support are not available for us yet.
The Heart Failure Association of the ESC has suggested that, despite advances in therapy, CS remains the most common cause of in‐hospital death after AMI and is a major cause of death in young patients with other potentially reversible underlying cardiac pathologies. According to the Heart Failure Association, CS management should consider appropriate organization of the health‐care services, and therapies must be given to appropriately selected patients in a timely manner, while avoiding iatrogenic harm. This association also suggested that further research is needed on this topic. 45
Alternatively, in our sample of patients with WCHF, HF therapies that modify the disease (e.g. ACEIs, ARBs, beta‐blockers, and mineralocorticoid/aldosterone receptor antagonists) were underutilized. 3 Finally, patients with endocarditis and prosthetic dysfunction were included in the group with valvular heart disease; both of these in the presence of AHF are associated with a high rate of in‐hospital mortality. 46 , 47
Analysis of HF patients from lower‐income to middle‐income and high‐inequality countries has showed higher mortality rates than those patients from high‐income and low‐inequality countries. 48 In developing countries such as Mexico, unfavourable social circumstances, along with inadequate and inefficient public spending on health care, can present considerable barriers to improving outcomes in patients with AHF. 49 There is a need to develop more practical strategies to improve adherence to guidelines. Such strategies should be based on multidisciplinary models involving HF teams, structured referral schemes, telemedicine, synchronized education of patients and health‐care providers, care standardization, and quality control and auditing. The development of centres of excellence, such as those recently described for the treatment of advanced HF, may contribute to this goal. 50
Study limitations
Our study has several limitations. First, our retrospective data reflect the experiences of a single tertiary university centre specialized in cardiovascular diseases. Therefore, we cannot be certain that these cases represent the overall AHF patient population in Mexico. Second, these patients were not classified according to the 2008 ESC guidelines at hospital admission; rather, we applied this classification retrospectively for the purposes of these analyses and may potentially have incomplete or inaccurate results. Third, the absence of criteria for the degree of severity in pulmonary congestion may turn out to be an erroneous classification that overlaps between patients with PO and HT‐HF. Finally, our study included only patients who were hospitalized in the CCU, rather than including those hospitalized in internal wards as was the case in other reports.
Conclusions
These data, from real‐world AHF patients admitted to a CCU of a university hospital in a developing Latin American country, show significant differences in both clinical characteristics at presentation and aetiologies compared with large European and US registries. The present study highlights a high in‐hospital mortality rate that may reflect a high‐risk patient cohort and/or inefficient public spending on health care for patients with chronic HF. Regardless, this profile represents a significant public health challenge.
Conflict of interest
None declared.
Supporting information
Table S1. Medications at hospital admission according to the clinical phenotypes.
Table S2. Univariate analysis for the prediction of in‐hospital all‐cause mortality in patients with acute heart failure.
González‐Pacheco, H. , Álvarez‐Sangabriel, A. , Martínez‐Sánchez, C. , Briseño‐Cruz, J. L. , Altamirano‐Castillo, A. , Mendoza‐García, S. , Manzur‐Sandoval, D. , Amezcua‐Guerra, L. M. , Sandoval, J. , Bojalil, R. , Araiza‐Garaygordobil, D. , Sierra‐Lara, D. , Guiza‐Sánchez, C. A. , Gopar‐Nieto, R. , Cruz‐Rodríguez, C. , Valdivia‐Nuño, J. J. , Salas‐Teles, B. , and Arias‐Mendoza, A. (2021) Clinical phenotypes, aetiologies, management, and mortality in acute heart failure: a single‐institution study in Latin‐America. ESC Heart Failure, 8: 423–437. 10.1002/ehf2.13092.
References
- 1. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure‐associated hospitalizations in the United States. J Am Coll Cardiol 2013; 6112: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zannad F, Agrinier N, Alla F. Heart failure burden and therapy. Europace 2009; 11: v1–v9. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008 . the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 5. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006; 27: 2725–2736. [DOI] [PubMed] [Google Scholar]
- 6. Tavazzi L, Maggioni AP, Lucci D, Cacciatore G, Ansalone G, Oliva F, Porcu M, Italian survey on Acute Heart Failure Investigators . Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J 2006; 27: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 7. Zannad F, Mebazaa A, Juillière Y, Cohen‐Solal A, Guize L, Alla F, Rougé P, Blin P, Barlet MH, Paolozzi L, Vincent C, Desnos M, Samii K, EFICA Investigators . Clinical profile, contemporary management and one‐year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail 2006; 8: 697–705. [DOI] [PubMed] [Google Scholar]
- 8. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 9. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 10. Ferreira JP, Girerd N, Rossignol P, Zannad F. Geographic differences in heart failure trials. Eur J Heart Fail 2015; 17: 893–905. [DOI] [PubMed] [Google Scholar]
- 11. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K, IABP‐SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 12. Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, Lagu T, Krumholz HM. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation 2013; 127: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Diepen S, Bakal JA, Lin M, Kaul P, McAlister FA, Ezekowitz JA. Variation in critical care unit admission rates and outcomes for patients with acute coronary syndromes or heart failure among high‐ and low‐volume cardiac hospitals. J Am Heart Assoc 2015; 4: e001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M, EVEREST Investigators . Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol 2008; 52: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 15. Metra M, Felker GM, Zacà V, Bugatti S, Lombardi C, Bettari L, Voors AA, Gheorghiade M, Dei CL. Acute heart failure: multiple clinical profiles and mechanisms require tailored therapy. Int J Cardiol 2010; 144: 175–179. [DOI] [PubMed] [Google Scholar]
- 16. Bocchi EA, Arias A, Verdejo H, Diez M, Gómez E, Castro P, Interamerican Society of Cardiology . The reality of heart failure in Latin America. J Am Coll Cardiol 2013; 62: 949–958. [DOI] [PubMed] [Google Scholar]
- 17. Logeart D, Isnard R, Resche‐Rigon M, Seronde MF, de Groote P, Jondeau G, Galinier M, Mulak G, Donal E, Delahaye F, Juilliere Y, Damy T, Jourdain P, Bauer F, Eicher JC, Neuder Y, Trochu JN, Heart Failure of the French Society of Cardiology . Current aspects of the spectrum of acute heart failure syndromes in a real‐life setting: the OFICA study. Eur J Heart Fail 2013; 15: 465–476. [DOI] [PubMed] [Google Scholar]
- 18. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, ESC Heart Failure Long‐Term Registry . Investigators Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 19. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF Investigators and Coordinators . Predictors of in‐hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). J Am Coll Cardiol 2008; 52: 347–356. [DOI] [PubMed] [Google Scholar]
- 20. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L, EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology . EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006; 27: 2725–2736. [DOI] [PubMed] [Google Scholar]
- 21. Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, Burrows N, McLean A, Vilas‐Boas F, Mebazaa A. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM‐HF). Intensive Care Med 2011; 37: 619–626. [DOI] [PubMed] [Google Scholar]
- 22. Oliva F, Mortara A, Cacciatore G, Chinaglia A, Di Lenarda A, Gorini M, Metra M, Senni M, Maggioni AP, Tavazzi L, IN‐HF Outcome Investigators . Acute heart failure patient profiles, management and in hospital outcome: results of the Italian Registry on Heart Failure Outcome. Eur J Heart Fail 2012; 14: 1208–1217. [DOI] [PubMed] [Google Scholar]
- 23. Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L, Heart Failure Association of the ESC . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 24. Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, Piepoli MF, Crespo‐Leiro MG, Lainscak M, Ponikowski P, Filippatos G, Ruschitzka F, Seferovic P, Coats AJS, Lund LH, ESC‐EORP‐HFA Heart Failure Long‐Term Registry Investigators . Acute heart failure congestion and perfusion status—impact of the clinical classification on in‐hospital and long‐term outcomes; insights from the ESC‐EORP HFA Heart Failure Long‐Term Registry. Eur J Heart Fail 2019; 21: 1338–1352. [DOI] [PubMed] [Google Scholar]
- 25. Schrier RW, Bansal S. Pulmonary hypertension, right ventricular failure, and kidney: different from left ventricular failure? Clin J Am Soc Nephrol 2008; 3: 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase‐5 inhibition in a 1‐year study. Circulation 2011; 124: 164–174. [DOI] [PubMed] [Google Scholar]
- 27. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, de Filippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail 2015; 17: 544–558. [DOI] [PubMed] [Google Scholar]
- 28. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. [DOI] [PubMed] [Google Scholar]
- 29. Collins S, Martindale J. Optimizing hypertensive acute heart failure management with afterload reduction. Curr Hypertens Rep 2018; 20: 9. [DOI] [PubMed] [Google Scholar]
- 30. Kang J, Cho HJ, Lee HY, Lee S, Park SK, Lee SE, Kim JJ, Jeon ES, Chae SC, Baek SH, Kang SM, Choi DJ, Yoo BS, Kim KH, Cho MC, Oh BH. Effects of widespread inotrope use in acute heart failure patients. J Clin Med 2018; 7: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalogeropoulos AP, Marti CN, Georgiopoulou VV, Butler J. Inotrope use and outcomes among patients hospitalized for heart failure: impact of systolic blood pressure, cardiac index, and etiology. J Card Fail 2014; 20: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mebazaa A, Motiejunaite J, Gayat E, Crespo‐Leiro MG, Lund LH, Maggioni AP, Chioncel O, Akiyama E, Harjola VP, Seferovic P, Laroche C, Julve MS, Roig E, Ruschitzka F, Filippatos G, ESC Heart Failure Long‐Term Registry Investigators . Long‐term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail 2018; 20: 332–341. [DOI] [PubMed] [Google Scholar]
- 33. Chioncel O, Collins SP, Greene SJ, Ambrosy AP, Vaduganathan M, Macarie C, Butler J, Gheorghiade M. Natriuretic peptide‐guided management in heart failure. J Cardiovasc Med (Hagerstown) 2016; 17: 556–568. [DOI] [PubMed] [Google Scholar]
- 34. Ciapponi A, Alcaraz A, Calderón M, Matta MG, Chaparro M, Soto N, Bardach A. Burden of heart failure in Latin America: a systematic review and meta‐analysis. Rev Esp Cardiol (Engl Ed) 2016; 69: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 35. Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003; 290: 2581–2587. [DOI] [PubMed] [Google Scholar]
- 36. Bahit MC, Kochar A, Granger CB. Post‐myocardial infarction heart failure. JACC Heart Fail 2018; 6: 179–186. [DOI] [PubMed] [Google Scholar]
- 37. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi DJ, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen‐Solal A, Iwashyna TJ, Mebazaa A, GREAT Network . Precipitating factors and 90‐day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail 2017; 19: 201–208. [DOI] [PubMed] [Google Scholar]
- 38. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019; 12: e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, Di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A, CardShock Study Investigators; GREAT network . Clinical picture and risk prediction of short‐term mortality in cardiogenic shock [published correction appears in Eur J Heart Fail. 2015 Sep;17(9):984]. Eur J Heart Fail 2015; 17: 501–509. [DOI] [PubMed] [Google Scholar]
- 40. Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefèvre T, Durand E, Blanchard D, Simon T, Cambou JP, Danchin N. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST‐MI French nationwide registries. Eur Heart J 2012; 33: 2535–2543. [DOI] [PubMed] [Google Scholar]
- 41. De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, Casella G, Chiarella F, Boccanelli A, Di Pasquale G, De Servi S. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eur J Heart Fail 2015; 17: 1124–1132. [DOI] [PubMed] [Google Scholar]
- 42. Helgestad OKL, Josiassen J, Hassager C, Jensen LO, Holmvang L, Sørensen A, Frydland M, Lassen AT, Udesen NLJ, Schmidt H, Ravn HB, Møller JE. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur J Heart Fail 2019; 21: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 43. Rathod KS, Koganti S, Iqbal MB, Jain AK, Kalra SS, Astroulakis Z, Lim P, Rakhit R, Dalby MC, Lockie T, Malik IS, Knight CJ, Whitbread M, Mathur A, Redwood S, MacCarthy PA, Sirker A, O'Mahony C, Wragg A, Jones DA. Contemporary trends in cardiogenic shock: incidence, intra‐aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care 2018; 7: 16–27. [DOI] [PubMed] [Google Scholar]
- 44. Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, Jorde UP. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non‐infarction related cardiogenic shock in the United States. Clin Res Cardiol 2018; 107: 287–303. [DOI] [PubMed] [Google Scholar]
- 45. Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, Harjola VP, Antohi EL, Arrigo M, Gal TB, Celutkiene J, Collins SP, DeBacker D, Iliescu VA, Jankowska E, Jaarsma T, Keramida K, Lainscak M, Lund LH, Lyon AR, Masip J, Metra M, Miro O, Mortara A, Mueller C, Mullens W, Nikolaou M, Piepoli M, Price S, Rosano G, Vieillard‐Baron A, Weinstein JM, Anker SD, Filippatos G, Ruschitzka F, Coats AJS, Seferovic P. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1315–1341. [DOI] [PubMed] [Google Scholar]
- 46. Kiefer T, Park L, Tribouilloy C, Cortes C, Casillo R, Chu V, Delahaye F, Durante‐Mangoni E, Edathodu J, Falces C, Logar M, Miró JM, Naber C, Tripodi MF, Murdoch DR, Moreillon P, Utili R, Wang A. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA 2011; 306: 2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dürrleman N, Pellerin M, Bouchard D, Hébert Y, Cartier R, Perrault LP, Basmadjian A, Carrier M. Prosthetic valve thrombosis: twenty‐year experience at the Montreal Heart Institute. J Thorac Cardiovasc Surg 2004; 127: 1388–1392. [DOI] [PubMed] [Google Scholar]
- 48. Ferreira JP, Rossignol P, Dewan P, Lamiral Z, White WB, Pitt B, McMurray JJV, Zannad F. Income level and inequality as complement to geographical differences in cardiovascular trials. Am Heart J 2019; 218: 66–74. [DOI] [PubMed] [Google Scholar]
- 49. Dewan P, Rørth R, Jhund PS, Ferreira JP, Zannad F, Shen L, Køber L, Abraham WT, Desai AS, Dickstein K, Packer M, Rouleau JL, Solomonm SD, Swedberg K, Zile MR, McMurray JJV, PARADIGM‐HF and ATMOSPHERE Investigators . Income inequality and outcomes in heart failure: a global between‐country analysis. JACC Heart Fail 2019; 7: 336–346. [DOI] [PubMed] [Google Scholar]
- 50. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Medications at hospital admission according to the clinical phenotypes.
Table S2. Univariate analysis for the prediction of in‐hospital all‐cause mortality in patients with acute heart failure.


