Abstract
Aims
We sought to investigate the time course of cardiac disorders after catheter ablation for atrial fibrillation (AF) in patients with coexisting heart failure (HF) during long‐term follow‐up.
Methods and results
We analysed consecutive 280 patients undergoing first‐time catheter ablation for AF who had coexisting HF, which was defined as prior HF hospitalization, estimated right ventricular systolic pressure ≥45 mmHg, or B‐type natriuretic peptide (BNP) ≥200 pg/dL before the procedure. The primary endpoints were improvements in left ventricular ejection fraction (LVEF), E/e′, BNP, left atrial dimension (LAD), and mitral regurgitation (MR) at 1 year. The secondary endpoints were serial changes of LVEF, E/e′, BNP, LAD, and MR at 6 months, 1 year, and 5 years and cumulative incidence of HF hospitalization. During the mean follow‐up of 5.1 ± 3.0 years, 70.7% of patients were free from recurrent AF. Among patients with LVEF < 50%, E/e′ ≥ 15, BNP ≥ 200 pg/dL, LAD ≥ 40 mm, and moderate‐to‐severe MR, changes in those parameters from baseline to 1 year were 34.5 ± 9.9% to 43.2 ± 14.4% (P < 0.001), 19.7 ± 3.9 to 12.5 ± 6.6 (P < 0.001), 290 to 85 pg/dL (P < 0.001), and 100% to 37.8% (P < 0.001), respectively. The improvements in the cardiac disorders were maintained up to 5 years except for E/e′. In patients with LVEF < 40%, significant delayed improvement of LVEF beyond 1 year was observed (ΔLVEF = 10.5 ± 18.5, P = 0.001), but not in patients with LVEF of 40–49%. The cumulative incidence of HF hospitalization was 12.6% at 5 years. Baseline diastolic dysfunction was the only independent predictor for subsequent HF hospitalization.
Conclusions
In patients undergoing AF ablation with coexisting HF, all cardiac disorders significantly improved after the procedure, which was mostly maintained during 5 year follow‐up.
Keywords: Atrial fibrillation, Catheter ablation, Heart failure, Systolic dysfunction, Diastolic dysfunction
Introduction
Both atrial fibrillation (AF) and heart failure (HF) are common disease increasing with age and each disease predisposing to the other. 1 Atrial kick during a diastolic phase and atrioventricular synchronization disappear during AF rhythm. In addition, high‐rate ventricular contraction induces systolic and diastolic dysfunction. Furthermore, high‐rate atrial contraction leads to left atrial (LA) dilation, which causes secondary mitral regurgitation (MR). Conversely, HF also increases the incidence of AF, especially at the time of acute exacerbation and dehydration. Thus, AF begets HF and vice versa, leading to increased mortality and morbidities. 1 , 2 , 3
Catheter ablation for AF has become increasingly popular as non‐pharmacological rhythm control therapy. 4 , 5 In AF patients with coexisting HF and systolic dysfunction, several studies have reported the superiority of catheter ablation to conventional therapy in improvement of systolic dysfunction. 6 , 7 , 8 , 9 Recently, a randomized controlled trial in AF patients with coexisting HF (CASTLE‐AF study) firstly demonstrated significantly lower rate of death or worsening HF and higher rate of improvement in systolic dysfunction after catheter ablation for AF as compared with medical therapy. 10 However, long‐term change in cardiac disorders after catheter ablation for AF in patients with coexisting HF has not been fully evaluated. In addition, predictors of the improvement in cardiac function have been rarely evaluated. Therefore, the recommendation level of catheter ablation for AF in HF patients is still Class IIb even in recent guidelines. 4 , 5 The aim of the current study is to investigate long‐term impact of catheter ablation for AF on cardiac disorders in patients with coexisting HF.
Methods
Study population
Among 1206 consecutive patients undergoing radiofrequency catheter ablation for AF in Kyoto University Hospital between February 2004 and March 2015, 280 patients with coexisting HF were included in the current study. HF was defined as prior HF hospitalization, lung congestion on echocardiography (estimated right ventricular systolic pressure ≥45 mmHg), or biological cardiac overload [B‐type natriuretic peptide (BNP) ≥200 pg/dL] at the time of the procedure. 11 Written informed consent was obtained from all patients. A 12‐lead electrocardiogram was routinely measured at each clinical visit, and 24 h Holter monitoring was recommended at 3, 6, and 12 months and yearly thereafter. Follow‐up information was obtained by review of hospital chart and/or contact with the patient, relatives, and/or referring physicians. The study protocol was approved by the institutional review board of Kyoto University Hospital.
Ablation procedure and post‐procedural management
Extensive encircling pulmonary vein isolation and tricuspid valve isthmus ablation were routinely performed. Superior vena cava isolation, LA linear ablations, and additional complex fractionated atrial electrogram ablation were performed if necessary. The detail of ablation procedure was described in our previous report. 12 After the first procedure, oral anticoagulant was continued for at least 3 months. Thereafter, discontinuation of oral anticoagulant in patients without arrhythmia recurrence was left to the discretion of the attending physician. Antiarrhythmic drugs were discontinued before the ablation procedure and were restarted only when recurrent atrial tachyarrhythmias were detected. The second procedure was recommended to the patients with recurrent atrial tachyarrhythmias after the blanking period of 3 months.
Definitions and outcome measures
Atrial fibrillation was classified into paroxysmal (lasting <7 days) and persistent (lasting ≥7 days) AF. ‘C’ as a component of CHADS2 and CHA2DS2‐VASC scores included hospitalization for exacerbation of HF within 100 days before the index ablation procedure and/or left ventricular (LV) ejection fraction (LVEF) of <40%. Other components of the scores were described elsewhere. 13 , 14 Stroke was defined as neurological deficit requiring hospitalization with symptoms lasting for >24 h. Recurrent AF after procedure was defined as documented AF and/or atrial tachycardia lasting for >30 s or those requiring repeat ablation procedures with a blanking period of 90 days after procedure. 15 Maintained sinus rhythm was defined as free from recurrent AF without antiarrhythmic drugs.
Systolic dysfunction was defined as LVEF < 50% and was further subclassified into two groups [HF with reduced LVEF (HFrEF): LVEF < 40%; HF with mid‐range LVEF (HFmrEF): LVEF 40–49%]. 16 HF with LVEF ≥ 50% was considered as HF with preserved LVEF (HFpEF). Diastolic dysfunction, LA dilation, and significant MR were defined as E/e′ ≥ 15, LA dimension (LAD) ≥40 mm, and moderate‐to‐severe MR, respectively.
The primary outcome measures were improvements of cardiac functions at 1 year, including LV systolic function (∆LVEF ≥ 10% or normalization of LVEF to ≥50%), LV diastolic function (normalization of E/e′ to <15), BNP level (reduction to half of the baseline level or normalization to <200 pg/dL), LAD (∆LAD ≥ 10% or normalization to <40 mm), and MR (reduction to none or mild grade). 9 The secondary outcome measures were serial changes of all those parameters at 6 months, 1 year, and 5 years, as well as the cumulative incidence rates of all‐cause death and HF hospitalization during follow‐up.
Statistical analysis
Categorical variables were presented as number and percentage and were compared with χ 2 test or Fisher's exact test. Continuous variables were presented as mean with standard deviation or median with inter‐quartile range and were compared using Student's t‐test or the Wilcoxon rank‐sum test based on their distributions. The cumulative incidence and the event‐free rates were estimated by the Kaplan–Meier method, and the differences were assessed by the log‐rank test.
Logistic regression or Cox proportional hazard analysis with clinically relevant variables was conducted to identify independent risk factors for recurrent atrial tachyarrhythmia, the improvement of cardiac disorders, all‐cause death, and HF hospitalization after the ablation procedure. Because of the limited number of events, only variables with P < 0.10 or <0.30 on univariate analysis were included in the multivariable model. Statistical analyses were performed using JMP Pro 14 (SAS Institute Inc., Cary, NC) software. All analyses were two‐tailed, and P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the 280 study patients were summarized in Table 1 . Mean age was 66.8 ± 8.5 years, and 34.6% were female. Mean CHADS2 and CHA2DS2‐VASc scores were 1.6 ± 1.1 and 2.7 ± 1.5, respectively. Majority of patients had a previous history of hospitalization for HF exacerbation before the ablation procedure. Median BNP level was 232 (132–349) pg/dL, and 64.1% of patients had BNP level of ≥200 pg/dL. Regarding baseline echocardiographic parameters before the index procedure, LV diastolic dimension (LVDd), LVEF, LAD, and E/e′ were 58.0 ± 8.1 mm, 53.3 ± 17.7%, 44.3 ± 6.5 mm, and 13.4 ± 6.2, respectively. The prevalence of baseline systolic dysfunction, diastolic dysfunction, LA dilation, and moderate‐to‐severe MR was 40.4%, 30.0%, 78.9%, and 19.3%, respectively. About half of patients received angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, and beta‐blockers. Most baseline characteristics were comparable between patients with and without diastolic dysfunction, except for higher age, higher CHADS2 score, larger LAD, and higher prevalence of paroxysmal AF in patients with diastolic dysfunction (Supporting Information, Table S1 ).
Table 1.
Baseline characteristics
| Baseline characteristics | |
|---|---|
| Age (years) | 66.8 ± 8.5 |
| ≥75 years | 52 (18.6%) |
| AF duration (years) | 1.8 [0.5–5.5] |
| Paroxysmal AF | 147 (52.5%) |
| Female | 97 (34.6%) |
| Previous heart failure hospitalization | 152 (54.3%) |
| NYHA class | 1.8 [0.5–5.5] |
| ≥II | 60 (21.4%) |
| Hypertension | 185 (66.1%) |
| Diabetes | 61 (21.8%) |
| Ischaemic stroke | 30 (10.7%) |
| CHADS2 score | 1.6 ± 1.1 |
| CHA2DS2‐VASc score | 2.7 ± 1.5 |
| ≥2 | 216 (77.1%) |
| Echocardiography data | |
| Left ventricular diastolic dimension (mm) | 58.0 ± 8.1 |
| ≥55 mm | 41 (14.6%) |
| Left ventricular ejection fraction (%) | 53.3 ± 17.7 |
| 40–49% | 28 (10.0%) |
| <40% | 85 (30.4%) |
| Left atrial diameter (mm) | 44.3 ± 6.5 |
| ≥40 mm | 221 (78.9%) |
| E/e′ | 13.4 ± 6.2 |
| ≥15 | 51 (30.0%) |
| Moderate‐to‐severe mitral regurgitation | 54 (19.3%) |
| Laboratory data | |
| Cre (mg/dL) | 0.9 (0.8–1.1) |
| eGFR (mL/min/1.73 m2) | 57.4 ± 20.3 |
| BNP (pg/dL) | 232 (132–349) |
| ≥200 pg/dL | 177 (64.1%) |
| Medications at discharge | |
| Oral anticoagulant | 280 (100%) |
| Antiplatelet | 81 (28.9%) |
| Statin | 73 (26.1%) |
| ACE‐I/ARB | 141 (50.4%) |
| Beta‐blockers | 143 (51.1%) |
| Verapamil/diltiazem | 44 (15.7%) |
| Other Ca channel blockers | 52 (18.6%) |
| Digitalis | 46 (16.4%) |
| Furosemide | 50 (17.9%) |
| Mineralocorticoid receptor antagonist | 46 (16.4%) |
ACE‐I, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; Ca, calcium; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association.
Categorical variables are presented as number (percentage). Continuous variables are presented as mean ± standard deviation or median and inter‐quartile range.
Recurrent atrial fibrillation after ablation procedure
Mean follow‐up duration was 5.1 ± 3.0 years. The event‐free survival from recurrent atrial tachyarrhythmias after the first procedure was 42.1% at 5 years (Supporting Information, Figure S1 ). During follow‐up period, the prevalence of multiple procedures was 42.2% (Supporting Information, Figure S2 ). The event‐free survival from recurrent atrial tachyarrhythmias after multiple procedures was 91.3% at 6 months, 87.0% at 1 year, 77.0% at 3 years, and 70.7% at 5 years, respectively. In the multivariable analysis, the independent predictors for arrhythmia recurrence were LVDd ≥ 55 mm [hazard ratio (HR) 2.10, 95% confidence interval (CI) 1.19–3.55, P = 0.01] and LAD ≥ 40 mm (HR 2.50, 95% CI 1.25–5.72, P = 0.008) (Supporting Information, Table S2 ).
Improvements in cardiac disorders at 1 year
The primary outcome measures of the current study were improvements in cardiac disorders at 1 year after ablation. Among patients with systolic dysfunction (LVEF < 50%), diastolic dysfunction (E/e′ ≥ 15), high BNP level (BNP ≥ 200 pg/dL), dilated LA (LAD ≥ 40 mm), and moderate‐to‐severe MR, changes in those parameters from baseline to 1 year were 34.5 ± 9.9% to 43.2 ± 14.4% (P < 0.001), 19.7 ± 3.9 to 12.5 ± 6.6 (P < 0.001), 290 to 85 pg/dL (P < 0.001), and 100% to 37.8% (P < 0.001), respectively (Figure 1 A ). The prevalence of significant improvements in those parameters at 1 year was 64.4%, 50.0%, 81.1%, 51.2%, and 68.9%, respectively (Figure 1 B ). In the multivariable analysis, LV dilation (LVDd ≥ 55 mm) was an independent negative predictor for the improvement of LVEF at 1 year (HR 0.18, 95% CI 0.05–0.59, P = 0.004). Other independent predictors of improved LVEF were persistent AF (HR 4.20, 95% CI 1.52–12.8, P = 0.005) and moderate‐to‐severe MR (HR 5.52, 95% CI 1.49–25.3, P = 0.009) (Table 2 A ). The independent negative predictors for reduction in BNP level were baseline dilated LV (HR 0.27, 95% CI 0.09–0.88, P = 0.03) and diastolic dysfunction (HR 0.33, 95% CI 0.12–0.88, P = 0.03) (Table 2 B ). The independent predictors for reverse remodelling of the LA were baseline moderate‐to‐severe MR and maintained sinus rhythm (Table 2 C ). There were no independent predictors for improvements in LV diastolic function and MR (Supporting Information, Tables S3 and S4 ).
Figure 1.
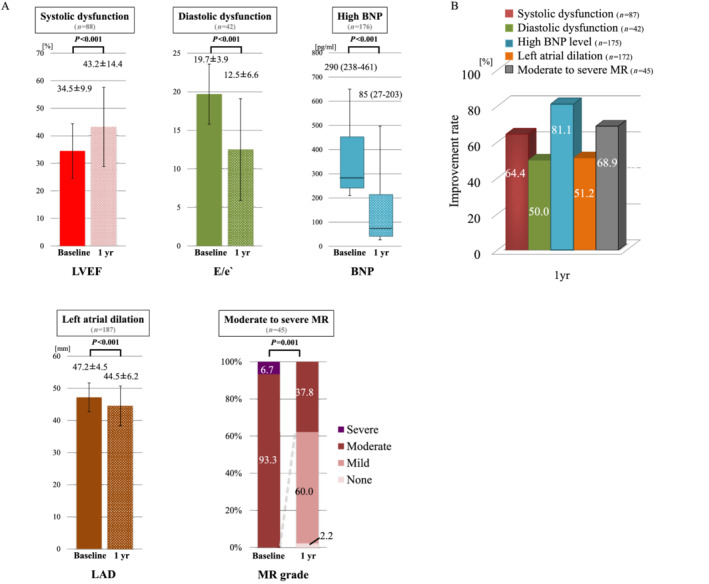
Improvements in cardiac disorders at 1 year after procedure. (A) Serial changes of cardiac parameters in patients with cardiac disorder. (B) The prevalence of significant improvements in cardiac disorders at 1 year. BNP, B‐type natriuretic peptide; LAD, left atrial dimension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
Table 2.
Independent predictors of improvements in cardiac disorders at 1 year
| Variables | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| (A) Improvement in systolic dysfunction (∆LVEF ≥ 10% or normalization to LVEF ≥ 50%) | ||||||
| Age ≥75 years | 0.81 | 0.21–3.40 | 0.76 | |||
| Female | 0.78 | 0.28–2.25 | 0.64 | |||
| Persistent AF | 3.25 | 1.31–8.45 | 0.01 | 4.20 | 1.52–12.8 | 0.005 |
| Diabetes | 2.04 | 0.64–7.83 | 0.23 | |||
| LVDd ≥ 55 mm | 0.38 | 0.15–0.93 | 0.03 | 0.18 | 0.05–0.59 | 0.004 |
| LVEF < 40% (HFrEF) | 1.19 | 0.45–3.06 | 0.72 | |||
| E/e′ ≥ 15 | 0.39 | 0.10–1.47 | 0.16 | |||
| BNP ≥ 200 pg/dL | 0.78 | 0.31–1.92 | 0.58 | |||
| LAD ≥ 40 mm | 0.98 | 0.31–2.91 | 0.97 | |||
| Moderate‐to‐severe MR | 2.67 | 0.94–8.87 | 0.07 | 5.52 | 1.49–25.3 | 0.009 |
| Recurrent AF after procedure | 0.44 | 0.17–1.12 | 0.08 | 0.63 | 0.21–1.88 | 0.40 |
| (B) Reduction in BNP level (reduction to half of the baseline level or normalization to <200 pg/mL) | ||||||
| Age ≥75 years | 0.78 | 0.34–1.91 | 0.57 | |||
| Female | 0.71 | 0.33–1.53 | 0.38 | |||
| Persistent AF | 0.88 | 0.41–1.88 | 0.75 | |||
| Diabetes | 0.60 | 0.25–1.49 | 0.26 | |||
| LVDd ≥ 55 mm | 0.18 | 0.07–0.47 | <0.001 | 0.27 | 0.09–0.88 | 0.03 |
| LVEF < 50% | 0.53 | 0.24–1.21 | 0.13 | |||
| E/e′ ≥ 15 | 0.31 | 0.12–0.79 | 0.01 | 0.33 | 0.12–0.88 | 0.03 |
| LAD ≥ 40 mm | 0.59 | 0.16–1.65 | 0.33 | |||
| Moderate‐to‐severe MR | 0.99 | 0.41–2.68 | 0.99 | |||
| Recurrent AF after procedure | 0.42 | 0.19–0.93 | 0.03 | 0.71 | 0.25–2.16 | 0.53 |
| (C) Improvement of left atrial dilation (∆LAD ≥ 10% or normalization to LAD < 40 mm) | ||||||
| Age ≥75 years | 0.75 | 0.34–1.63 | 0.46 | |||
| Female | 1.42 | 0.75–2.69 | 0.28 | |||
| Persistent AF | 0.82 | 0.45–1.50 | 0.53 | |||
| Diabetes | 0.66 | 0.31–1.38 | 0.27 | |||
| LVDd ≥ 55 mm | 0.94 | 0.45–1.97 | 0.88 | |||
| LVEF < 50% | 1.12 | 0.61–2.07 | 0.71 | |||
| E/e′ ≥ 15 | 0.87 | 0.41–1.87 | 0.72 | |||
| BNP ≥ 200 pg/dL | 1.20 | 0.62–2.31 | 0.59 | |||
| LAD ≥ 50 mm | 0.63 | 0.32–1.22 | 0.17 | |||
| Moderate‐to‐severe MR | 2.94 | 1.38–6.63 | 0.005 | 2.14 | 1.06–4.51 | 0.03 |
| Recurrent AF after procedure | 0.33 | 0.17–0.64 | <0.001 | 0.35 | 0.19–0.65 | <0.001 |
AF, atrial fibrillation; BNP, B‐type natriuretic peptide; CI, confidence interval; HFrEF, heart failure with reduced left ventricular ejection fraction; LAD, left atrial dimension; LVDd, left ventricular diastolic dimension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; OR, odds ratio.
Time course of changes in cardiac disorders
The improvements in most cardiac disorders were maintained up to 5 years after ablation, although E/e′ at 5 years was not significantly different from E/e′ at baseline (P = 0.08) (Figure 2 ). In both HFmrEF and HFrEF patients, LVEF significantly increased at 6 months. Delayed improvement in LVEF beyond 1 year after procedure was observed in HFrEF patients (ΔLVEF = 10.5 ± 18.5, P = 0.001), but not in HFmrEF patients (ΔLVEF = 1.0 ± 8.9, P = 0.64) (Supporting Information, Figure S3 ). The prevalence of significant improvements in patients with systolic dysfunction, diastolic dysfunction, high BNP level, dilated LA, and moderate‐to‐severe MR at 5 years was 86.2%, 66.7%, 85.4%, 62.0%, and 73.7%, respectively (Supporting Information, Figure S4 ).
Figure 2.
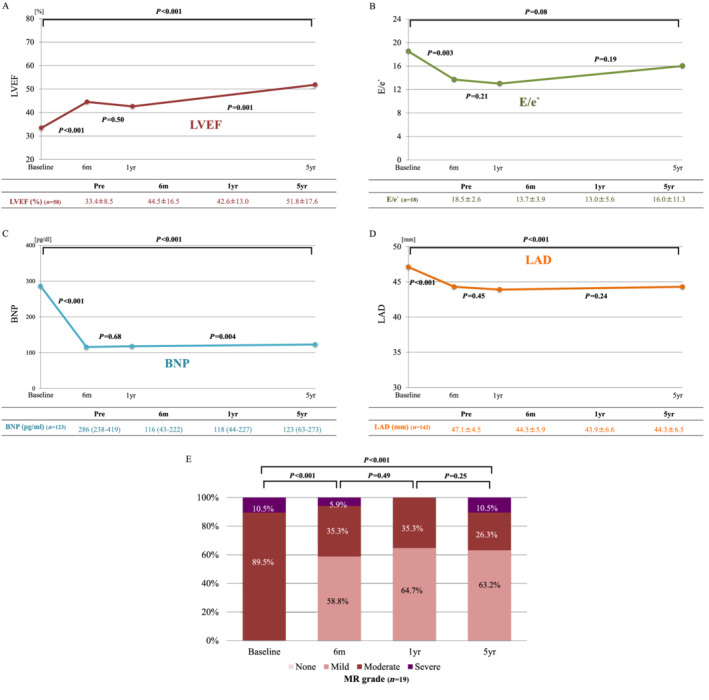
(A–E) Long‐term time course of cardiac parameters in patients with cardiac disorder. BNP, B‐type natriuretic peptide; LAD, left atrial dimension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
Impact of cardiac disorders and co‐morbidities on clinical outcomes after atrial fibrillation ablation
The cumulative all‐cause mortality was 2.1% at 1 year and 11.7% at 5 years (Supporting Information, Figures S5 and S6 ). The cumulative incidence of HF hospitalization was 3.6% at 1 year and 12.6% at 5 years. Both risks were much higher in patients with baseline diastolic dysfunction than those without (40.4% vs. 4.0%, P < 0.001 for all‐cause death, and 20.3% vs. 8.1%, P = 0.03, for HF hospitalization) (Figure 3 ), whereas there were modest or no significant differences between patients with and without other cardiac disorders (Supporting Information, Figures S5 and S6 ). After adjustment of baseline differences by the multivariable Cox regression model, diastolic dysfunction remained independent risk factor for both all‐cause death (HR 6.81, 95% CI 2.47–21.8, P < 0.001) and HF hospitalization (HR 2.95, 95% CI 1.03–8.46, P = 0.04) (Table 3 ).
Figure 3.
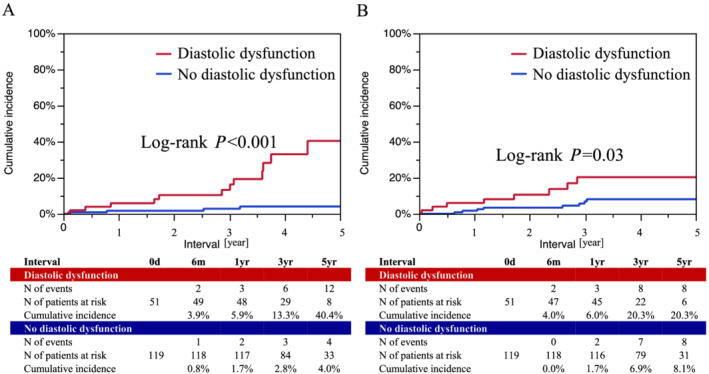
Cumulative incidence of all‐cause death and heart failure hospitalization according to diastolic dysfunction. (A) All‐cause death. (B) Heart failure hospitalization.
Table 3.
Independent predictors for clinical outcomes after AF ablation
| Variables | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| (A) All‐cause death | ||||||
| Age ≥75 years | 1.01 | 0.34–2.42 | 0.99 | |||
| Female | 0.68 | 0.29–1.45 | 0.33 | |||
| Persistent AF | 0.95 | 0.46–1.93 | 0.89 | |||
| Diabetes | 2.96 | 1.45–5.94 | 0.004 | 3.10 | 1.09–8.30 | 0.03 |
| LVDd ≥ 55 mm | 3.65 | 1.68–7.49 | 0.002 | 1.64 | 0.49–4.78 | 0.40 |
| LVEF < 50% | 1.52 | 0.73–3.21 | 0.26 | |||
| E/e′ ≥ 15 | 7.27 | 2.68–23.0 | <0.001 | 6.81 | 2.47–21.8 | <0.001 |
| BNP ≥ 200 pg/dL | 2.05 | 0.96–4.78 | 0.06 | 2.65 | 0.67–17.6 | 0.18 |
| LAD ≥ 40 mm | 2.33 | 0.91–7.88 | 0.08 | 2.31 | 0.49–18.3 | 0.31 |
| Moderate‐to‐severe MR | 2.12 | 0.96–4.36 | 0.06 | 1.68 | 0.49–5.00 | 0.39 |
| Recurrent AF after procedure | 1.46 | 0.69–2.96 | 0.31 | |||
| (B) Heart failure hospitalization | ||||||
| Age ≥75 years | 2.65 | 1.22–5.43 | 0.02 | 0.74 | 0.16–2.43 | 0.65 |
| Female | 0.80 | 0.35–1.69 | 0.58 | |||
| Persistent AF | 1.28 | 0.63–2.63 | 0.49 | |||
| Diabetes | 2.56 | 1.21–5.21 | 0.02 | 2.55 | 0.85–7.02 | 0.09 |
| LVDd ≥ 55 mm | 4.29 | 2.02–8.76 | <0.001 | 2.44 | 0.80–6.91 | 0.11 |
| LVEF < 50% | 1.27 | 0.62–2.59 | 0.51 | |||
| E/e′ ≥ 15 | 2.85 | 1.05–7.78 | 0.04 | 2.95 | 1.03–8.46 | 0.04 |
| BNP ≥ 200 pg/dL | 2.25 | 1.02–5.66 | 0.04 | 2.00 | 0.61–9.17 | 0.27 |
| LAD ≥ 40 mm | 2.78 | 0.99–11.7 | 0.054 | 1.80 | 0.33–33.3 | 0.55 |
| Moderate‐to‐severe MR | 1.83 | 0.80–3.85 | 0.15 | |||
| Recurrent AF after procedure | 3.11 | 1.53–6.42 | 0.002 | 1.93 | 0.66–5.38 | 0.22 |
AF, atrial fibrillation; BNP, B‐type natriuretic peptide; CI, confidence interval; HR, hazard ratio; LAD, left atrial dimension; LVDd, left ventricular diastolic dimension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
Discussion
The main findings of the current study enrolling patients undergoing AF ablation who had coexisting HF were as follows: (i) all cardiac disorders including LV systolic and diastolic dysfunctions, high BNP level, dilated LA, and moderate‐to‐severe MR were significantly improved at 1 year after the procedure; (ii) most of those improvements were maintained up to 5 years after the procedure, except diastolic dysfunction; (iii) significant delayed improvement in LVEF beyond 1 year was observed in HFrEF patients with LVEF of <40%, but not in patients with HFmrEF patients with LVEF of 40–49%; (iv) normal LVDd, persistent AF, and moderate‐to‐severe MR were independent predictors for the improvement of LVEF; and (v) baseline diastolic dysfunction was an independent predictor for all‐cause death and HF hospitalization.
The development of AF has harmful effect on HF patients regardless of the presence of systolic dysfunction. 1 , 2 In 2004, two studies reported that restoration of sinus rhythm by catheter ablation for AF improved LVEF in HFrEF patients. 6 , 7 Thereafter, several randomized clinical studies demonstrated the superiority of AF ablation over medical therapy in HFrEF patients. 8 , 9 , 10 Recently reported CASTLE‐AF trial investigated whether catheter ablation for AF decreases the risks for HF hospitalization and mortality as compared with medical therapy in HFrEF patients. 10 Catheter ablation was associated with significantly lower rates of all‐cause death and HF hospitalization by reducing AF burden. However, there have been few data regarding the time course of improvement in LVEF as well as that in E/e, BNP, LAD, and MR during long‐term follow‐up. In the current study, all those parameters of cardiac function significantly improved at 1 year and were maintained throughout the follow‐up period of 5 years except for diastolic function.
In the current study, ∆LVEF at 5 years after ablation in patients with LVEF < 40% was 18.4%, which was much higher than ∆LVEF of 7.3% at 5 years in the CASTLE‐AF study. This is mainly due to the difference in patient selection between the current study and CASTLE‐AF trial. In the current study, the population was consecutive patients undergoing AF ablation who had coexisting HF, and AF was considered the main cause of reduced LVEF in majority of patients. On the other hand, in the CASTLE‐AF trial, the study population consisted of patients with New York Heart Association (NYHA) Class II–IV HF, LVEF ≤ 35%, and prior implantation of a cardioverter defibrillator or a cardiac resynchronization therapy defibrillator, suggesting that underlying cardiomyopathy rather than AF was the main cause of reduced LVEF in most patients. Notably, in the current study, significant delayed improvement in LVEF was observed beyond 1 year after the ablation procedure. The delayed improvement was significant only in HFrEF patients with LVEF of <40%, but not in HFmrEF patients with LVEF of 40–49%. Normal LV size, persistent AF, and moderate‐to‐severe MR were the independent predictors for the improvement in LVEF at 1 year. Thus, patients with those baseline parameters are considered good candidates for AF ablation with coexisting HF.
Diastolic dysfunction is considered the most important cause of HF in HFpEF patients, 17 , 18 and no medical treatment has been reported to improve their prognosis. 19 , 20 Regarding AF ablation for HFpEF patients, few studies focused on the improvement of diastolic dysfunction. Machino‐Ohtsuka et al. reported that sinus rhythm restoration by AF ablation improved diastolic dysfunction in 74 patients with HFpEF during 34 month follow‐up period. 21 In the current study, diastolic dysfunction significantly improved at 1 year after procedure in 50% of patients, and mean E/e′ decreased from 19.7 at baseline to 12.5 at 1 year (P < 0.001). However, E/e′ at 5 years was not significantly different from E/e′ at baseline, although delayed increase in E/e′ from 1 to 5 years was not significant. Given the fact that diastolic dysfunction was the strong independent predictor for both all‐cause death and HF hospitalization in the current study, whether diastolic dysfunction is likely to relapse beyond 1 year after AF ablation should be evaluated in future larger studies.
B‐type natriuretic peptide level markedly and promptly reduced after AF ablation, which was maintained throughout the follow‐up period. Regarding negative remodelling of the LA, reduction of LAD was modest but significant, which was in accordance with previous studies, 22 , 23 and was maintained up to 5 years after the ablation procedure. Sinus rhythm maintenance was an independent predictor for negative remodelling of the LA.
The current study has several limitations. First, the current study was a retrospective observational study with inherent biases. Second, complete serial echocardiographic data up to 5 years after procedure were available only in relatively small number of patients, precluding us from drawing any definitive conclusions. Third, we had no control group of AF patients with coexisting HF not undergoing AF ablation and could not assess the relative utility of ablation on cardiac function and clinical outcomes. Fourth, we had no information regarding NYHA class during follow‐up and could not assess the impact of AF ablation on NYHA class. Also, data regarding 6 min walk test and quality‐of‐life scores were not available. Finally, the multivariable analyses might have not adequately eliminated the influence of unmeasured confounders on determining the independent predictors of improvements in cardiac disorders and clinical outcomes.
In conclusion, among patients undergoing AF ablation who had coexisting HF, LV systolic and diastolic dysfunctions as well as high BNP level, dilated LA, and moderate‐to‐severe MR improved in majority of cases at 1 year, which was maintained up to 5 years after the procedure except for LV diastolic function. Significant delayed improvement in LVEF beyond 1 year after the procedure was observed in patients with reduced LVEF of <40%. Normal LV size, persistent AF, and moderate‐to‐severe MR were the independent predictors for improvement in LVEF. Therefore, patients with those baseline parameters are considered good candidates for AF ablation with coexisting HF. Although LV diastolic dysfunction was the strong independent predictor of all‐cause death and HF hospitalization, the impact of AF ablation on long‐term diastolic function should be evaluated in future larger studies.
Conflict of interest
None declared.
Funding
None.
Data availability statement
All relevant data are within the manuscript.
Supporting information
Table S1. Baseline characteristics between patients with and without diastolic dysfunction
Table S2. Independent risk factors for improvement of recurrent atrial tachyarrhythmias after multiple procedures
Table S3. Independent risk factors for improvement of diastolic dysfunction at 1‐year (normalization to E/e′ < 15)
Table S4. Independent risk factors for improvement of significant mitral regurgitation at 1‐year (reduction to none or mild grade)
Figure S1. Event free survival from recurrent atrial tachyarrhythmias with a blanking period of 90 days after procedure
Figure S2. Repeat ablation procedures
Figure S3. Long‐term time course of left ventricular ejection fraction in patients with systolic dysfunction
Figure S4. The prevalence of significant improvements in cardiac disorders at 5‐year
Figure S5. Cumulative incidence of all‐cause death according to cardiac disorders
(A) Overall, (B) systolic dysfunction, (C) high BNP level, (D) left atrial dilation, (E) moderate to severe MR
Figure S6. Cumulative incidence of heart failure hospitalization according to cardiac disorders
(A) Overall, (B) systolic dysfunction, (C) high BNP level, (D) left atrial dilation, (E) moderate to severe MR
Acknowledgements
We appreciate all the members of the cardiac catheterization laboratory in Kyoto University Hospital for their contribution to this study.
Kawaji, T. , Shizuta, S. , Aizawa, T. , Yamagami, S. , Kato, M. , Yokomatsu, T. , Miki, S. , Ono, K. , and Kimura, T. (2021) Impact of catheter ablation for atrial fibrillation on cardiac disorders in patients with coexisting heart failure. ESC Heart Failure, 8: 670–679. 10.1002/ehf2.13160.
References
- 1. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016; 133: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rivero‐Ayerza M, Scholte Op Reimer W, Lenzen M, Theuns DA, Jordaens L, Komajda M, Follath F, Swedberg K, Cleland JG. New‐onset atrial fibrillation is an independent predictor of in‐hospital mortality in hospitalized heart failure patients: results of the EuroHeart Failure Survey. Eur Heart J 2008; 29: 1618–1624. [DOI] [PubMed] [Google Scholar]
- 3. Bajaj NS, Bhatia V, Sanam K, Ather S, Hashim T, Morgan C, Fonarow GC, Nanda NC, Prabhu SD, Adamopoulos C, Kheirbek R, Aronow WS, Fletcher RD, Anker SD, Ahmed A, Deedwania P. Impact of atrial fibrillation and heart failure, independent of each other and in combination, on mortality in community‐dwelling older adults. Am J Cardiol 2014; 114: 909–913. [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Cosedis Nielsen J, Curtis AB, Davies DW, Day JD, d'Avila A, Natasja de Groot NMS, di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018; 20: e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019; 140: e125–e151. [DOI] [PubMed] [Google Scholar]
- 6. Chen MS, Marrouche NF, Khaykin Y, Gillinov AM, Wazni O, Martin DO, Rossillo A, Verma A, Cummings J, Erciyes D, Saad E, Bhargava M, Bash D, Schweikert R, Burkhardt D, Williams‐Andrews M, Perez‐Lugones A, Abdul‐Karim A, Saliba W, Natale A. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol 2004; 43: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 7. Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 2004; 351: 2373–2383. [DOI] [PubMed] [Google Scholar]
- 8. Khan MN, Jais P, Cummings J, di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al‐Ahmad A, Beheiry S, Santarelli P, Starling RC, dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haissaguerre M, Natale A. Pulmonary‐vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008; 359: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 9. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA‐MRI study. J Am Coll Cardiol 2017; 70: 1949–1961. [DOI] [PubMed] [Google Scholar]
- 10. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bansch D, Investigators C‐A. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 11. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 12. Kawaji T, Shizuta S, Morimoto T, Aizawa T, Yamagami S, Yoshizawa T, Ota C, Onishi N, Sasaki Y, Yahata M, Nakai K, Hayano M, Nakao T, Hanazawa K, Goto K, Doi T, Ono K, Kimura T. Very long‐term clinical outcomes after radiofrequency catheter ablation for atrial fibrillation: a large single‐center experience. Int J Cardiol 2017; 249: 204–213. [DOI] [PubMed] [Google Scholar]
- 13. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 14. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 15. Kuck K‐H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque J‐P, Tondo C. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245. [DOI] [PubMed] [Google Scholar]
- 16. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 17. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011; 306: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, Jaber WA. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012; 125: 782–788. [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 20. Lund LH, Benson L, Dahlstrom U, Edner M, Friberg L. Association between use of beta‐blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014; 312: 2008–2018. [DOI] [PubMed] [Google Scholar]
- 21. Machino‐Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2013; 62: 1857–1865. [DOI] [PubMed] [Google Scholar]
- 22. Machino‐Ohtsuka T, Seo Y, Ishizu T, Yanaka S, Nakajima H, Atsumi A, Yamamoto M, Kawamura R, Koshino Y, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Tada H, Aonuma K. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circulation Journal: Official Journal of the Japanese Circulation Society 2013; 77: 1695–1704. [DOI] [PubMed] [Google Scholar]
- 23. Xiong B, Li D, Wang J, Gyawali L, Jing J, Su L. The effect of catheter ablation on left atrial size and function for patients with atrial fibrillation: an updated meta‐analysis. PLoS ONE 2015; 10: e0129274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics between patients with and without diastolic dysfunction
Table S2. Independent risk factors for improvement of recurrent atrial tachyarrhythmias after multiple procedures
Table S3. Independent risk factors for improvement of diastolic dysfunction at 1‐year (normalization to E/e′ < 15)
Table S4. Independent risk factors for improvement of significant mitral regurgitation at 1‐year (reduction to none or mild grade)
Figure S1. Event free survival from recurrent atrial tachyarrhythmias with a blanking period of 90 days after procedure
Figure S2. Repeat ablation procedures
Figure S3. Long‐term time course of left ventricular ejection fraction in patients with systolic dysfunction
Figure S4. The prevalence of significant improvements in cardiac disorders at 5‐year
Figure S5. Cumulative incidence of all‐cause death according to cardiac disorders
(A) Overall, (B) systolic dysfunction, (C) high BNP level, (D) left atrial dilation, (E) moderate to severe MR
Figure S6. Cumulative incidence of heart failure hospitalization according to cardiac disorders
(A) Overall, (B) systolic dysfunction, (C) high BNP level, (D) left atrial dilation, (E) moderate to severe MR
Data Availability Statement
All relevant data are within the manuscript.


