Abstract
Aims
This study sought to determine whether clinical clusters exist in takotsubo cardiomyopathy. Takotsubo cardiomyopathy (TCM) is a heterogeneous disorder with a complex, poorly understood pathogenesis. To better understand the heterogeneity of TCM, we identified different clinical phenotypes in a large sample of TCM patients by using latent class analysis (LCA).
Methods and results
Using the National Inpatient Sample (NIS) database, we identified 3139 patients admitted to hospitals in 2016–2017 with a primary diagnosis of TCM. We performed LCA based on several patient demographics and comorbidities: age, sex, hypertension, hyperlipidaemia, diabetes mellitus, obesity, current smoking, asthma, chronic obstructive pulmonary disease (COPD), and anxiety and depressive disorders. We then repeated LCA separately with the NIS 2016 and 2017 data sets and performed a robust test to validate our results. We also compared in‐hospital outcomes among the different clusters identified by LCA. Four patient clusters were identified. C1 (n = 1228, 39.4%) had the highest prevalence of hyperlipidaemia (93.4%), hypertension (61.6%), and diabetes (34.3%). In C2 (n = 440, 14.0%), all patients had COPD, and many were smokers (45.8%). C3 (n = 376, 11.8%) largely comprised patients with anxiety disorders (98.4%) and depressive disorders (80.1%). C4 (n = 1097, 34.8%) comprised patients with isolated TCM and few comorbidities. Among all clusters, C1 had the lowest in‐hospital mortality (1.0%) and the shortest length of stay (3.2 ± 3.1 days), whereas C2 had the highest in‐hospital mortality (3.4%).
Conclusions
Using LCA, we identified four clinical phenotypes of TCM. These may reflect different pathophysiological processes in TCM. Our findings may help identify treatment targets and select patients for future clinical trials.
Keywords: Latent class analysis, Phenotype, Takotsubo cardiomyopathy
Introduction
Since it was first described in 1990, takotsubo cardiomyopathy (TCM), also known as stress‐induced cardiomyopathy, has been increasingly reported worldwide. 1 This disorder is characterized by a wall motion abnormality that extends beyond the territory of a single coronary artery, with no angiographic evidence of acute plaque rupture. 2 Although it is considered a reversible myocardial injury, TCM has in‐hospital mortality rates similar to those of acute myocardial infarction and acute coronary syndrome. 3 Additionally, the frequency of hospital admissions for TCM has been increasing recently. 4
The pathogenesis of TCM is not well understood. Its widely accepted putative pathophysiological mechanisms include endothelial dysfunction, 5 coronary artery spasm, 6 myocardial stunning resulting from excessive catecholamine release, 7 reperfusion injury, and abnormalities in cardiac fatty acid metabolism. 8 Despite increasing recognition of the clinical and biological heterogeneity within TCM, TCM has been mainly managed with supportive therapy only, with no specific treatment developed so far. Traditional cardiovascular medications such as β‐blockers, angiotensin‐converting enzyme inhibitors (ACEIs), and statins have been studied as treatments for TCM in both retrospective and meta‐analysis studies. These studies yielded results that were either conflicting or showed no benefit of treatment, which might reflect the incomplete understanding of the pathophysiology of TCM and again indicate the heterogeneity of this disease. 3 , 9 , 10 , 11
Dividing TCM into subtypes to compensate for this heterogeneity is crucial and could potentially inform the development of novel treatment regimens for TCM. Some investigators have classified TCM into different subtypes on the basis of the inciting event 12 or anatomic variants. 13 Although those classifications have been used in clinical settings, they do not provide specific guidance on treatment planning for different groups of patients, which limits their clinical utility for TCM management.
Multiple clinical risk factors for TCM have been reported and proposed to be involved in the underlying pathophysiology of TCM. These include conventional cardiovascular risk factors [smoking, obesity, 14 hypertension, and diabetes mellitus (DM) 15 ], psychiatric disorders (i.e. depressive and anxiety disorders 16 ), and pulmonary diseases [e.g. chronic obstructive pulmonary disease (COPD) 17 and asthma 18 ]. These risk factors may reflect the heterogeneity of TCM and should be incorporated into clinical parameters for identifying the phenotypes of TCM.
Latent class analysis (LCA), a validated statistical method of mixture modelling for finding the best‐fit model for a dataset, has been used to identify phenotypes based on clinical risk factors in diseases such as acute respiratory distress syndrome and gout, and these findings have significant pathophysiological and clinical implications. 19 , 20 In our study, we capitalized on the wealth of clinical data available from the National Inpatient Sample (NIS) database, using LCA to identify and validate novel phenotypes of TCM and testing their association with clinical outcomes.
Methods
Data source and study population
We identified all patients with a primary diagnosis of TCM from the 2016 and 2017 NIS database by the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes (Supporting Information, Table S1 ). Patients with no recorded age or sex were excluded. The NIS database represents a stratified sample of 20% of all inpatient hospitalizations in the United States and contains the following data: primary and secondary diagnoses, patient demographic characteristics, hospital characteristics, total charges, expected payer, discharge status, length of stay (LOS), and comorbidity measures. Because the NIS is a deidentified, publicly available database, using the NIS does not require Institutional Review Board approval. The process of patient selection in this study is shown in Figure 1 .
Figure 1.
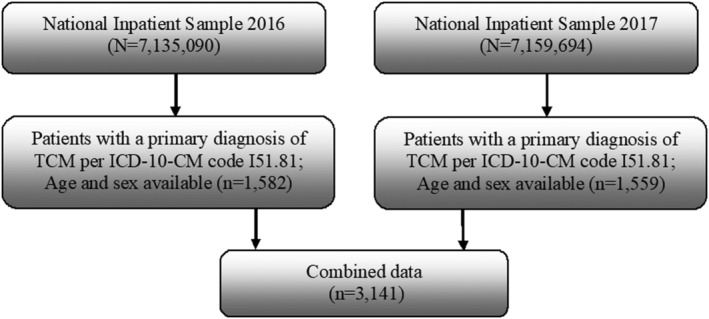
Patient selection. Flowchart showing the process by which patients were selected for this study from the 2016 and 2017 National Inpatient Sample databases. Patients with takotsubo cardiomyopathy were identified by ICD‐10‐CM code I51.81. ICD‐10‐CM, International Classification of Diseases, Tenth Revision, Clinical Modification; TCM, takotsubo cardiomyopathy.
Assignment of clinical phenotype and statistical analysis
Latent class analysis is a statistical method that uses responses to a set of observed categorical variables to identify potential discrete, mutually exclusive latent classes of objects. 21 As a type of model‐based clustering approach, LCA has the advantage of not relying on traditional modelling assumptions such as linearity, normality, and homogeneity. Additionally, LCA can determine the number of clusters that best fits the data. This technique calculates estimates of item response probabilities (IRP) conditional upon class membership (i.e. the probability for an individual in a given cluster to provide a certain response to a specific item) and each individual's class membership probabilities (i.e. an individual's probability of membership in each class). Thereby, LCA assigns each individual to the class for which that individual has the largest membership probability.
We calculated the IRP in the data sets to define four phenotypes' characteristics. The greater the IRP, the more likely the individual is to be assigned to the corresponding subgroup. For example, a COPD IRP of 0.8 corresponds to an 80% chance for the individual in the cluster to have COPD.
In an effort to classify the TCM patients, we selected multiple known risk factors for TCM, including older age, female sex, current smoking, hypertension, DM, hyperlipidaemia, obesity, anxiety, depression, COPD, and asthma. We examined the 2016 and 2017 NIS databases, starting with a model with two classes and increasing the number of classes to determine whether the set of available model diagnostics suggested a more appropriate number of classes. By examining the number of parameters, log‐likelihood, Bayesian information criterion (BIC), sample‐size‐adjusted BIC, Akaike's information criterion, Pearson χ 2 goodness of fit, and the likelihood ratio χ 2 (G 2) statistic, we found that four classes presented the most parsimonious solution in considering goodness‐of‐fit measures and the interpretability of the model. That is, four classes were the minimum number from which the solution could be interpreted meaningfully, according to the BIC model's recommendation.
In addition, we repeated the LCA test on NIS 2016 and 2017 separately, using the same method, to test the replicability of our results. Finally, we tested the association between subgroups and outcomes in all patients with TCM. The primary outcomes measured were in‐hospital mortality, LOS, and the total charges of hospitalization. The secondary outcomes included cardiac arrest, cardiogenic shock, acute kidney injury, and acute respiratory failure (ARF). All the variables and outcomes in this study were identified by International Classification of Diseases, Tenth Revision, Clinical Modification codes or were directly extracted from the NIS.
To further examine the reliability of the clustering results, we conducted a robust LCA test by excluding from the cohort those patients who did not undergo diagnostic angiography or who had percutaneous coronary intervention or surgical revascularization during the hospitalization according to their ICD‐10 Procedure Coding System codes (Table S1 ). The criteria for TCM patients' identification in the robust‐test cohort were consistent with those used in the previous study. 22
Performing LCA requires categorical variables, so we treated all baseline variables accordingly. Age was separated into categories of <50 and ≥50 years, as studies show that 50 is the average age at which women reach menopause 23 and that postmenopausal women are at higher risk of TCM 2 and have worse outcomes 24 than premenopausal women. All categorical variables are represented by numbers and percentages and were compared by using the χ 2 test. Continuous variables are presented as mean and standard deviation and were tested with analysis of variance. All statistical analyses were performed by the R statistics software. A P value < 0.05 was considered statistically significant.
Results
Population characteristics
In the NIS data, we identified 3141 patients with a primary diagnosis of TCM: 1582 from the 2016 database and 1559 from the 2017 database. Similar to prior studies, 90.4% of the patients were women with an average age of 66.7 years. 3 Conventional cardiovascular risk factors included hypertension (49.0%), hyperlipidaemia (48.8%), DM (19.5%), current smoking (18.7%), and obesity (12.7%); respiratory diseases, including COPD (21.8%) and asthma (9.5%); and psychiatric disorders such as anxiety (25.2%) and depression (17.6%). The risk factor distributions were similar in the 2016 and 2017 data (Table 1 ).
Table 1.
Baseline characteristics of patients with takotsubo cardiomyopathy in three data sets
| Overall | 2016 | 2017 | |
|---|---|---|---|
| Characteristic | N = 3141 | N = 1582 | N = 1559 |
| Age (years) | 66.7 ± 12.7 | 66.8 ± 12.9 | 66.5 ± 12.4 |
| Age ≥ 50 years | 2878 (91.6) | 1455 (92.0) | 1423 (91.3) |
| Female | 2841 (90.4) | 1422 (89.9) | 1419 (91) |
| Hypertension | 611 (19.5) | 311 (19.7) | 300 (19.2) |
| Diabetes mellitus | 1532 (48.8) | 766 (48.4) | 766 (49.1) |
| Hyperlipidaemia | 399 (12.7) | 189 (11.9) | 210 (13.5) |
| Obesity | 793 (25.2) | 375 (23.7) | 418 (26.8) |
| Anxiety disorder | 554 (17.6) | 269 (17.0) | 285 (18.3) |
| Depressive disorder | 299 (9.5) | 159 (10.1) | 140 (9.0) |
| Current smoking | 586 (18.7) | 272 (17.2) | 314 (20.1) |
| Asthma | 684 (21.8) | 324 (20.5) | 360 (23.1) |
| Chronic obstructive pulmonary disease | 2878 (91.6) | 1455 (92.0) | 1423 (91.3) |
Values are mean ± SD or n (%).
Latent class analysis
In each cohort, analysis of latent class models suggested that a four‐class model provided the lowest BIC (Tables 2 , S2 , S3 , and S4 ) and, therefore, the best model performance. Table 3 shows the distribution of risk factors among the four subgroups.
Table 2.
Fit statistics for latent class models from two to five classes for the full cohort
| Model | Npar | LL | BIC | aBIC | AIC | χ 2 | G 2 | Entropy |
|---|---|---|---|---|---|---|---|---|
| Two classes | 23 | −16 172.33 | 32 529.85 | 32 456.77 | 32 390.65 | 1700.94 | 3255.44 | 0.50 |
| Three classes | 35 | −16 016.33 | 32 314.50 | 32 203.26 | 32 102.67 | 1388.42 | 2789.70 | 0.50 |
| Four classes | 47 | −15 919.95 | 32 218.35 | 32 068.99 | 31 933.89 | 1194.58 | 2624.13 | 0.63 |
| Five classes | 59 | −15 874.22 | 32 223.52 | 32 036.02 | 31 866.43 | 1101.87 | 2565.15 | 0.62 |
aBIC, sample‐size‐adjusted Bayesian information criterion; AIC, Akaike's information criterion; BIC, Bayesian information criterion; LL, log‐likelihood; Npar, number of parameters.
Table 3.
Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the full cohort
| Overall | C1 | C2 | C3 | C4 | P value | |
|---|---|---|---|---|---|---|
| Characteristic | N = 3141 | n = 1228 | n = 440 | n = 376 | n = 1097 | |
| Age (years) | 66.7 ± 12.7 | 70.0 ± 10.4 | 67.5 ± 10.6 | 64.0 ± 11.6 | 63.6 ± 15.0 | <0.001 |
| Age ≥ 50 years | 2878 (91.6) | 1217 (99.1) | 431 (98.0) | 328 (87.2) | 902 (82.2) | <0.001 |
| Female | 2841 (90.4) | 1106 (90.1) | 386 (87.7) | 370 (98.9) | 979 (89.2) | <0.001 |
| Hypertension | 1538 (49.0) | 752 (61.2) | 170 (38.6) | 203 (54.0) | 413 (37.6) | <0.001 |
| Diabetes mellitus | 611 (19.5) | 417 (34.0) | 55 (12.5) | 73 (19.4) | 66 (6.0) | <0.001 |
| Hyperlipidaemia | 1532 (48.8) | 1154 (94.0) | 131 (29.8) | 218 (58.0) | 29 (2.6) | <0.001 |
| Obesity | 399 (12.7) | 212 (17.3) | 21 (4.8) | 94 (25.0) | 72 (6.6) | <0.001 |
| Anxiety disorder | 793 (25.2) | 166 (13.5) | 86 (19.5) | 359 (95.5) | 182 (16.6) | <0.001 |
| Depressive disorder | 554 (17.6) | 129 (10.5) | 49 (11.1) | 309 (82.2) | 67 (6.1) | <0.001 |
| Asthma | 299 (9.5) | 117 (9.5) | 46 (10.5) | 57 (15.2) | 79 (7.2) | <0.001 |
| Current smoking | 586 (18.7) | 83 (6.8) | 211 (48.0) | 125 (33.2) | 167 (15.2) | <0.001 |
| Chronic obstructive pulmonary disease | 684 (21.8) | 128 (10.4) | 440 (100.0) | 116 (30.9) | 0 (0.0) | <0.001 |
Values are mean ± SD or n (%).
The latent class models were applied again independently to the 2016 data (Table S5 ), the 2017 data (Table S6 ), and the robust‐test cohort (Table S7 ). We also examined the association between the phenotypes and clinical outcomes in all TCM and robust‐test patients (Tables 4 and S8 , respectively). The IRPs of the four phenotypes are shown for each of the four data sets in Figures 2 , 3 , 4 , 5 and S1 .
Table 4.
Inpatient outcomes in the four clusters of takotsubo cardiomyopathy patients in the full cohort
| Overall | C1 | C2 | C3 | C4 | P value | |
|---|---|---|---|---|---|---|
| Outcomes | N = 3141 | n = 1228 | n = 440 | n = 376 | n = 1097 | |
| Death | 52 (1.7) | 12 (1.0) | 15 (3.4) | 4 (1.1) | 21 (1.9) | 0.005 |
| Length of stay, days | 3.4 ± 3.3 | 3.2 ± 3.1 | 4.0 ± 2.9 | 3.9 ± 3.8 | 3.4 ± 3.5 | <0.001 |
| Total charges (USD) | 49 805 ± 47 946 | 46 825 ± 40 164 | 55 580 ± 47 343 | 49 666 ± 44 240 | 50 861 ± 56 464 | 0.009 |
| Cardiac arrest | 56 (1.8) | 18 (1.5) | 8 (1.8) | 1 (0.3) | 29 (2.6) | 0.016 |
| Cardiogenic shock | 145 (4.6) | 48 (3.9) | 29 (6.6) | 14 (3.7) | 54 (4.9) | 0.102 |
| Acute kidney injury | 287 (9.1) | 115 (9.4) | 45 (10.2) | 30 (8.0) | 97 (8.9) | 0.699 |
| Acute respiratory failure | 342 (10.9) | 91 (7.4) | 103 (23.4) | 50 (13.3) | 99 (9.1) | <0.001 |
Values are mean ± SD or n (%).
Figure 2.
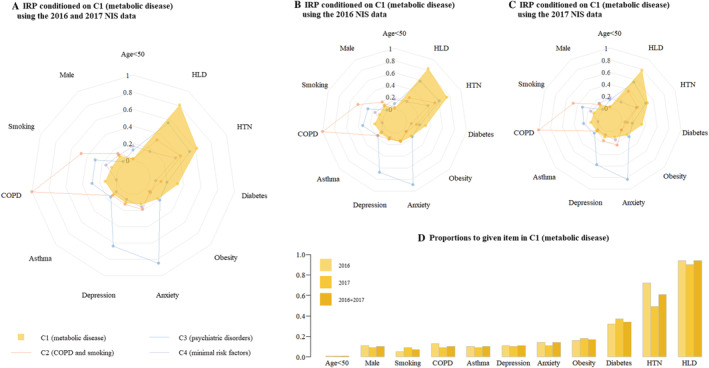
Risk factors in Cluster 1 (metabolic disease). (A) Combined 2016 and 2017 NIS data. (B) 2016 NIS data. (C) 2017 NIS data. (D) Distribution of variables. COPD, chronic obstructive pulmonary disease; HLD, hyperlipidaemia; HTN, hypertension; IRP, item response probabilities; NIS, National Inpatient Sample.
Figure 3.
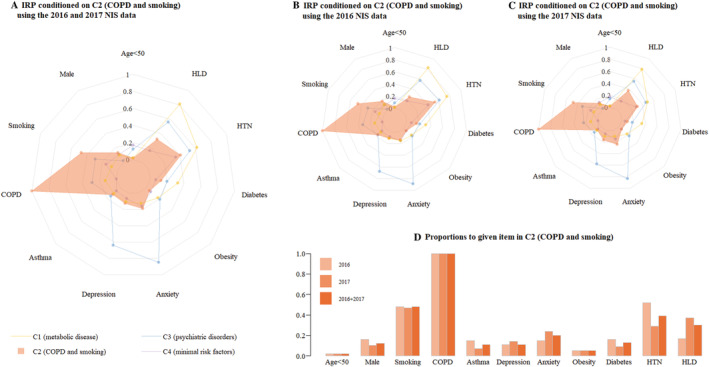
Risk factors in Cluster 2 (COPD and smoking). (A) Combined 2016 and 2017 NIS data. (B) 2016 NIS data. (C) 2017 NIS data. (D) Distribution of variables. COPD, chronic obstructive pulmonary disease; HLD, hyperlipidaemia; HTN, hypertension; IRP, item response probabilities; NIS, National Inpatient Sample.
Figure 4.
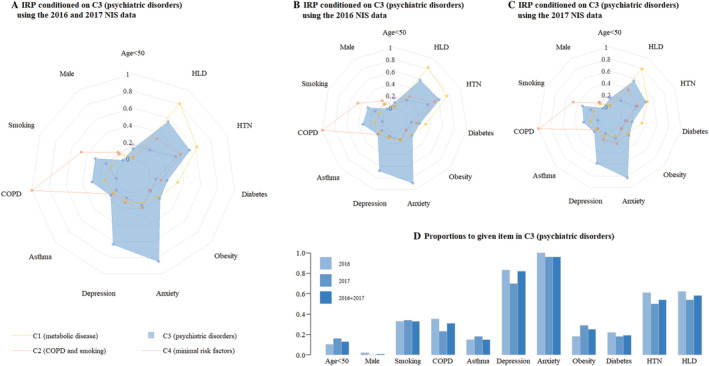
Risk factors in Cluster 3 (psychiatric disorders). (A) Combined 2016 and 2017 NIS data. (B) 2016 NIS data. (C) 2017 NIS data. (D) Distribution of variables. COPD, chronic obstructive pulmonary disease; HLD, hyperlipidaemia; HTN, hypertension; IRP, item response probabilities; NIS, National Inpatient Sample.
Figure 5.
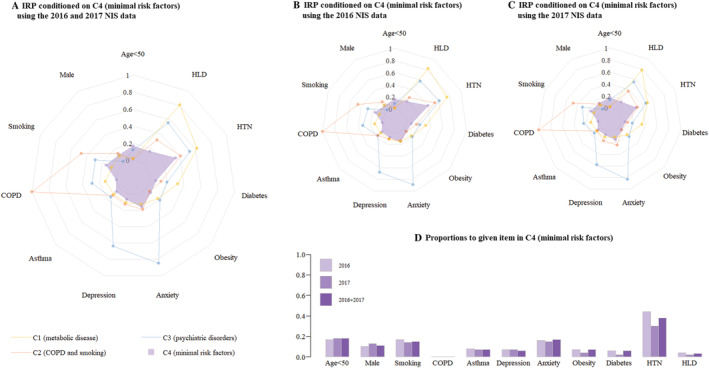
Risk factors in Cluster 4 (minimal risk factors). (A) Combined 2016 and 2017 NIS data. (B) 2016 NIS data. (C) 2017 NIS data. (D). Distribution of variables. COPD, chronic obstructive pulmonary disease; HLD, hyperlipidaemia; HTN, hypertension; IRP, item response probabilities; NIS, National Inpatient Sample.
Phenotypes identified by clustering
The LCA identified four clusters of patients with common characteristics. For each cluster, the LCAs run for the separated 2016, 2017, and robust‐test data sets produced similar results to those of the LCA for the entire cohort. For the association between the phenotypes and clinical outcomes, the robust‐test cohort yielded similar results to those of the entire cohort.
C1: Metabolic disease cluster
In Cluster 1 (n = 1228), 99.1% of patients were more than 50 years old. Metabolic diseases, such as hyperlipidaemia (94.0%), hypertension (61.2%), and diabetes (34.0%), were most prevalent among patients in this cluster. Interestingly, patients in Cluster 1 had the lowest inpatient mortality (1.0%), shortest LOS (3.2 ± 3.1 days), and lowest incidence of ARF (7.4%) among the four clusters.
C2: Chronic obstructive pulmonary disease and smoking cluster
Cluster 2 (n = 440) had the highest prevalence of COPD (100.0%) and smoking (48.0%). This cluster of patients had the poorest overall outcomes, having the highest in‐hospital mortality (3.4%), the longest LOS (4.0 ± 2.9 days), and the highest incidence of ARF (23.4%).
C3: Psychiatric disorders cluster
Cluster 3 (n = 376) was characterized by significant psychiatric disorders. In this cluster, 95.5% of patients had a diagnosis of anxiety disorder, and 82.2% had a diagnosis of major depression disorder. Notably, C3 was noted to have the lowest incidence of cardiac arrest (0.3%). In addition, C3 had an in‐hospital mortality of 1.1% and an LOS of 3.9 ± 3.8 days.
C4: Minimal risk factors cluster
Overall, Cluster 4 (n = 1097) had the fewest risk factors among the four clusters. Individuals in this cluster tended to be younger (mean age 63.6 years) and had the fewest comorbidities, having low rates of hypertension (37.6%), DM (6.0%), hyperlipidaemia (2.6%), asthma (7.2%), and depression (6.1%). There were no patients with COPD in this cluster.
While C4 had the fewest risk factors and the lowest hospitalization cost ($50 861 ± $56 464), it also had the highest incidence of cardiac arrest (2.6%). This group had an in‐hospital mortality of 1.9% and a LOS of 3.4 ± 3.5 days.
Discussion
Efforts have been made to classify TCM by distribution of regional wall motion abnormalities or by precipitating factor (emotional vs. physical triggering event). One study found no significant difference in cardiogenic shock and death rates between patients with typical and atypical TCM. 25 A study by Sobue et al. 12 associated TCM caused by a physical trigger with a higher in‐hospital death rate than TCM caused by a non‐physical trigger, although many TCM cases do not have an identifiable trigger. Those classifications did not prove useful in guiding treatment or predicting outcome.
In this study, we sought to identify clinical phenotypes of primary TCM. Whereas TCM is described as secondary when it occurs in individuals already hospitalized for other medical, surgical, anaesthetic, obstetric, or psychiatric conditions and is considered a complication of the patient's primary medical condition, TCM is considered primary when the specific symptoms described are the chief reason for the patient's acute presentation. These patients include those with or without clearly identifiable stress triggers or any coexisting medical conditions that could be risk factors for TCM. 26
By using the NIS database, we used risk factors to classify TCM patients into four different clusters and tested for outcome differences among them. Surprisingly, Cluster 1, which had significant metabolic disease, had the best performance in terms of inpatient mortality, LOS, and ARF rate. In contrast, Cluster 2, which was characterized by significant COPD and smoking, had the worst primary and secondary outcomes. To our knowledge, this study is the first to use LCA to identify different TCM phenotypes according to risk factors.
C1: Metabolic disease
This cluster had the most patients more than 50 years old, and the patients were the most likely to have metabolic diseases such as hyperlipidaemia, hypertension, and DM. Nonetheless, these patients tended to have lowest in‐hospital mortality and the shortest LOS. Because of their known cardiovascular risk factors, these individuals might have been more likely to be receiving cardiac‐protective medications such as β‐blockers, calcium channel blockers, ACEIs, angiotensin receptor blockers, and statins, which now are widely used to treat TCM in its acute and chronic phases. Previous studies found that β‐blockers can relieve the ventricular discordance that typifies TCM by antagonizing the activation of sympathetic nerves in patients with a significant intraventricular pressure gradient. 27 The ACEIs, angiotensin receptor blockers, and statins, serving as direct and indirect antioxidants, can suppress nitrate tolerance and endothelial dysfunction to relieve coronary artery spasm, especially in patients with the aldehyde dehydrogenase 2‐deficient genotype. 28
There is conflicting evidence regarding the benefit of using those medications for TCM. One meta‐analysis showed that early receipt of β‐blockers after TCM had no significant association with inpatient mortality. 9 However, another study associated the increasing percentage of patients taking long‐term cardiac medications after TCM with a decline in the incidence of complications from TCM. 29 The mixed therapeutic effectiveness of traditional cardiac medications may illustrate the heterogeneity of TCM patients and suggest that some of them may benefit from these medications. Taking cardiac‐protective medications could be protective for these patients, potentially explaining why they tended to have a better outcome than patients in the other clusters. However, further study is needed to confirm this hypothesis.
C2: Chronic obstructive pulmonary disease and smoking
Patients in C2, characterized by a high prevalence of COPD and smoking, had the highest mortality rate, highest incidence of ARF, and longest LOS. Smoking and COPD share the pathophysiological mechanisms of endothelial dysfunction, arterial stiffness, and inflammation, which can cause adverse cardiovascular events. 30 , 31 Smoking can also disrupt the balance of the autonomic nervous system and increase plasma catecholamine levels. 32 Additionally, the use of β2‐adrenergic agonists for bronchospasm may mimic the actions of the endogenous catecholamines epinephrine and norepinephrine in COPD patients. Studies have suggested that sympathetic nervous system activation and higher plasma catecholamine levels are associated with poor prognosis in TCM patients. 33
In addition, TCM has been noted to occur during severe dyspnoea in COPD. As a result, a specific phenotype called ‘bronchogenic TCM’ was proposed because of its correlation with COPD, as well as its atypical presentation. 34 Exacerbation of COPD can mask the symptoms of bronchogenic TCM, causing delay of TCM treatment and resulting in poor prognosis.
Patients with COPD are more likely to develop ARF than those without COPD, which, in combination with severe physical diseases such as TCM, worsens inpatient outcomes. In addition, ARF that necessitates mechanical ventilation is a risk factor for TCM 35 and makes treating it more difficult. One previous study has shown that TCM patients with cardiopulmonary failure have greater in‐hospital mortality than those without. 36 More attention should be paid to the respiratory diseases of such patients to prevent ARF and reduce their need for mechanical ventilation.
C3: Psychiatric disorders
Cluster 3 had the lowest incidence of cardiac arrest and a moderate inpatient mortality rate and LOS compared with the other clusters. Because anxiety and depression adversely affect autonomic nervous system activity and haemodynamics, these disorders are associated with poor outcomes in patients with cardiovascular disease. 37 Although catecholamine release induced by emotional or physical stress is the most widely accepted theory of TCM pathophysiology. 6 , 8 neither depression nor anxiety disorder was correlated with greater mortality in our study. This result is consistent with a prior study that found no correlation between depression and anxiety disorders and TCM‐related mortality, 38 although some research found that psychiatric disorders had predictive value for the reoccurrence of TCM. 39 Another hypothesis is that patients with psychiatric disorders, especially anxiety disorders, may be more likely to seek medical attention; thus, their TCM is diagnosed at a relatively early stage, which may reduce in‐hospital mortality risk.
C4: Minimal risk factors
Patients in Cluster 4 had the fewest risk factors. Although this group had the lowest total costs, it had the highest incidence of cardiac arrest and the second‐highest in‐hospital mortality rate among the four clusters. It is possible that this group of patients is the least likely to seek medical attention, and that when they do, acute coronary syndrome and TCM are less likely to be considered as potential diagnoses because these patients are generally at lower risk. Likewise, patients in this cluster might be less likely to be prescribed cardiac medications after risk stratification. Nevertheless, further research is required to understand the aetiology of this cluster's poor prognosis and its unique underlying pathology.
To our knowledge, this study is the first to use LCA to identify different phenotypes of patients with TCM by their clinical characteristics and to study these phenotypes' correlation with outcomes. Our study used a large, well‐characterized sample of TCM patients. We also repeated LCA independently in two subsets of our data to confirm our findings from the entire cohort.
Identification of takotsubo cardiomyopathy
In this study, we formed our main cohort by using the ICD‐10 code for TCM to identify patients with the primary diagnosis of TCM. Previous studies have showed that the discharge diagnosis of TCM has a high accuracy and positive predictive value. Basic et al. 40 conducted a study to validate the hospital discharge diagnosis of cardiomyopathy at three hospitals in western Sweden. The authors found that ‘other cardiomyopathy’ (defined as restrictive, arrhythmogenic right ventricular, left ventricular noncompaction, takotsubo, and peripartum cardiomyopathies) had a diagnostic accuracy rate of 100%. Another validation study in a similar inpatient database, the Danish National Patient Registry, found that the positive predictive value of the ICD‐10 code for a discharge diagnosis of TCM (which was the same ICD code we used in our study) was 100% in all age and sex groups. 41 Another study designed to validate the in‐hospital invasive cardiac procedure codes in an administrative health database showed that the negative predictive values of PCI and angiography codes were 87.9% and 79.9%, respectively, which means there is a chance that patients without discharge codes for PCI and angiography nonetheless underwent these procedures. 42 Thus, we only used the discharge code for TMC to identify TMC patients. However, we did not find any research articles on the validity of using the ICD‐10 code for TCM in the NIS database specifically.
To examine the reliability of our primary results, we conducted a robust analysis by using the code for TCM together with the code for angiography without PCI/CABG to identify TCM, as described in a previous study. 22 The results of the robust analysis are in accordance with our primary results. The LCA runs for the robust‐test cohort produced similar results to those of the LCA for the entire cohort.
Study limitations
This is a retrospective study with no follow‐up data. In addition, not all potentially relevant variables were available in the NIS database. These variables included the medications each patient was taking before admission (e.g. cardiac medications and inhalational beta agonists); echocardiographic, physiological, and laboratory data; anatomic type of TCM (apical, reverse, mid‐ventricular, and right ventricular involvement); the specific trigger for the patient's TCM; and the severity of each risk factor, all of which may also reflect the heterogeneity of TCM and could have enhanced phenotype identification. Furthermore, because the NIS is a deidentified public database, we were unable to identify any patient or access their original medical records. Thus, duplication due to patient transfer could not be eliminated. However, by comparing patients' characteristics between transferred TCM patients and the rest, we found that the maximum rate of duplication due to patient transfer being recorded as two separated admissions was low: 0.64%. On the other hand, patients with TCM are often readmitted with recurrent TCM, which could also lead to data duplication (the frequency of which we cannot estimate).
Conclusions
By using the LCA method, our study identified four clusters of TCM patients based on differences in their risk factors for TCM. We also found statistically significant differences in in‐hospital outcomes among these clusters. Our findings support the clinical and pathophysiological heterogeneity of TCM, which could be the key to optimizing TCM treatment and predicting outcomes for individual patients. Our study suggests that future efforts should aim to further characterize these phenotypes with comprehensive clinical and biological data. In addition, our study has the potential to directly inform future randomized controlled trials of novel treatments for TCM.
Conflict of interest
None declared.
Supporting information
Table S1. International Classification of Diseases, Version 10, Clinical Modification (ICD‐10‐CM) and Procedure Coding System (ICD‐10‐PCS) codes used to identify risk factors, outcomes, and procedures.
Table S2. Fit statistics for latent class models from two to five classes for the 2016 dataset.
Table S3. Fit statistics for latent class models from two to five classes for the 2017 dataset.
Table S4. Fit statistics for latent class models from two to five classes for the robust‐test cohort.
Table S5. Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the 2016 dataset.
Table S6. Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the 2017 dataset.
Table S7. Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the robust‐test cohort.
Table S8. Inpatient outcomes in the four clusters of TCM patients in the robust‐test cohort.
Figure S1. Risk factors of the four clusters in the robust‐test cohort.
Acknowledgements
Stephen N. Palmer, PhD, ELS, contributed to the editing of the manuscript.
Li, P. , Dai, Q. , Cai, P. , Teng, C. , Pan, S. , Dixon, R. A. F. , and Liu, Q. (2021) Identifying different phenotypes in takotsubo cardiomyopathy by latent class analysis. ESC Heart Failure, 8: 555–565. 10.1002/ehf2.13117.
References
- 1. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006; 27: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 2. Medina de Chazal H, Del Buono MG, Keyser‐Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress cardiomyopathy diagnosis and treatment: JACC state‐of‐the‐art review. J Am Coll Cardiol 2018; 72: 1955–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015; 373: 929–938. [DOI] [PubMed] [Google Scholar]
- 4. Redfors B, Vedad R, Angerås O, Råmunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, Libungan B, Shao Y, Albertsson P, Stone GW, Omerovic E. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int J Cardiol 2015; 185: 282–289. [DOI] [PubMed] [Google Scholar]
- 5. Naegele M, Flammer AJ, Enseleit F, Roas S, Frank M, Hirt A, Kaiser P, Cantatore S, Templin C, Fröhlich G, Romanens M, Lüscher TF, Ruschitzka F, Noll G, Sudano I. Endothelial function and sympathetic nervous system activity in patients with takotsubo syndrome. Int J Cardiol 2016; 224: 226–230. [DOI] [PubMed] [Google Scholar]
- 6. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol 1991; 21: 203–214. [PubMed] [Google Scholar]
- 7. Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539–548. [DOI] [PubMed] [Google Scholar]
- 8. Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Umemura T, Nakamura S, Yoshida M, Sato H. Myocardial perfusion and fatty acid metabolism in patients with tako‐tsubo‐like left ventricular dysfunction. J Am Coll Cardiol 2003; 41: 743–748. [DOI] [PubMed] [Google Scholar]
- 9. Isogai T, Matsui H, Tanaka H, Fushimi K, Yasunaga H. Early β‐blocker use and in‐hospital mortality in patients with takotsubo cardiomyopathy. Heart 2016; 102: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 10. Santoro F, Ieva R, Musaico F, Ferraretti A, Triggiani G, Tarantino N, Di Biase M, Brunetti ND. Lack of efficacy of drug therapy in preventing takotsubo cardiomyopathy recurrence: a meta‐analysis. Clin Cardiol 2014; 37: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunetti ND, Santoro F, De Gennaro L, Correale M, Gaglione A, Di Biase M, Madias JE. Combined therapy with beta‐blockers and ACE‐inhibitors/angiotensin receptor blockers and recurrence of takotsubo (stress) cardiomyopathy: a meta‐regression study. Int J Cardiol 2017; 230: 281–283. [DOI] [PubMed] [Google Scholar]
- 12. Sobue Y, Watanabe E, Ichikawa T, Koshikawa M, Yamamoto M, Harada M, Ozaki Y. Physically triggered takotsubo cardiomyopathy has a higher in‐hospital mortality rate. Int J Cardiol 2017; 235: 87–93. [DOI] [PubMed] [Google Scholar]
- 13. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018; 39: 2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelliccia F, Parodi G, Greco C, Antoniucci D, Brenner R, Bossone E, Cacciotti L, Capucci A, Citro R, Delmas C, Guerra F, Ionescu CN, Lairez O, Larrauri‐Reyes M, Lee PH, Mansencal N, Marazzi G, Mihos CG, Morel O, Nef HM, Nunez Gil IJ, Passaseo I, Pineda AM, Rosano G, Santana O, Schneck F, Song BG, Song J‐K, Teh AW, Ungprasert P, Valbusa A, Wahl A, Yoshida T, Gaudio C, Kaski JC. Comorbidities frequency in takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med 2015; 128: 654.e611–654.e619. [DOI] [PubMed] [Google Scholar]
- 15. Stiermaier T, Santoro F, El‐Battrawy I, Möller C, Graf T, Novo G, Santangelo A, Mariano E, Romeo F, Caldarola P, Fanelli M, Thiele H, Brunetti ND, Akin I, Eitel I. Prevalence and prognostic impact of diabetes in takotsubo syndrome: insights from the international, multicenter GEIST registry. Diabetes Care 2018; 41: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 16. Nayeri A, Rafla‐Yuan E, Krishnan S, Ziaeian B, Cadeiras M, McPherson JA, Wells QS. Psychiatric illness in takotsubo (stress) cardiomyopathy: a review. Psychosomatics 2018; 59: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landefeld K, Saleh Q, Sander GE. Stress cardiomyopathy in the setting of COPD exacerbation. J Investig Med High Impact Case Rep 2015; 3 2324709615612847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costantini M, Gelonesi F, Tritto C, De Jaco G, Sicuro S. Bronchial asthma as a trigger of tako‐tsubo‐like cardiomyopathy in elderly women. J Cardiovasc Med (Hagerstown) 2016; 17: e247–e248. [DOI] [PubMed] [Google Scholar]
- 19. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richette P, Clerson P, Périssin L, Flipo R‐M, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis 2015; 74: 142–147. [DOI] [PubMed] [Google Scholar]
- 21. Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling 2007; 14: 671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vallabhajosyula S, Dunlay SM, Murphree DH Jr, Barsness GW, Sandhu GS, Lerman A, Prasad A. Cardiogenic shock in takotsubo cardiomyopathy versus acute myocardial infarction: an 8‐year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail 2019; 7: 469–476. [DOI] [PubMed] [Google Scholar]
- 23. Muka T, Asllanaj E, Avazverdi N, Jaspers L, Stringa N, Milic J, Ligthart S, Ikram MA, Laven JSE, Kavousi M, Dehghan A, Franco OH. Age at natural menopause and risk of type 2 diabetes: a prospective cohort study. Diabetologia 2017; 60: 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dias A, Franco E, Figueredo VM, Hebert K. Can previous oophorectomy worsen the clinical course of takotsubo cardiomyopathy females? Age and gender‐related outcome analysis. Int J Cardiol 2014; 177: 1134–1136. [DOI] [PubMed] [Google Scholar]
- 25. Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Lüscher TF, Templin C. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol 2016; 1: 335–340. [DOI] [PubMed] [Google Scholar]
- 26. Sheppard MN. Takotsubo syndrome—stress‐induced heart failure syndrome. Eur Cardiol 2015; 10: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kyuma M, Tsuchihashi K, Shinshi Y, Hase M, Nakata T, Ooiwa H, Abiru M, Hikita N, Adachi T, Shoji T, Fujise Y, Shimamoto K. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): three cases. Circ J 2002; 66: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 28. Yasue H, Mizuno Y, Harada E. Coronary artery spasm—clinical features, pathogenesis and treatment. Proc Jpn Acad Ser B Phys Biol Sci 2019; 95: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalighi K, Farooq MU, Aung TT, Oo S. Takotsubo cardiomyopathy: a long term follow‐up shows benefit with risk factor reduction. J Cardiovasc Dev Dis 2015; 2: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004; 43: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 31. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis 2018; 12 1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol 2014; 64: 1740–1750. [DOI] [PubMed] [Google Scholar]
- 33. Matsuura T, Ueno M, Iwanaga Y, Miyazaki S. Importance of sympathetic nervous system activity during left ventricular functional recovery and its association with in‐hospital complications in takotsubo syndrome. Heart Vessels 2019; 34: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 34. Rajwani A, Adam Z, Hall JA. Bronchogenic stress cardiomyopathy: a case series. Cardiology 2015; 130: 106–111. [DOI] [PubMed] [Google Scholar]
- 35. Franco E, Dias A, Figueredo VM, Hebert K. Is acute respiratory failure requiring mechanical ventilation associated with development of takotsubo cardiomyopathy in the critical care setting? Int J Cardiol 2014; 176: 1273–1274. [DOI] [PubMed] [Google Scholar]
- 36. El‐Battrawy I, Lang S, Ansari U, Sattler K, Behnes M, Schramm K, Fastner C, Tülümen E, Zhou X, Hoffmann U, Borggrefe M, Akin I. Incidence and prognostic relevance of cardiopulmonary failure in takotsubo cardiomyopathy. Sci Rep 2017; 7: 14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015; 28: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim H, Senecal C, Lewis B, Prasad A, Rajiv G, Lerman LO, Lerman A. Natural history and predictors of mortality of patients with takotsubo syndrome. Int J Cardiol 2018; 267: 22–27. [DOI] [PubMed] [Google Scholar]
- 39. Nayeri A, Rafla‐Yuan E, Farber‐Eger E, Blair M, Ziaeian B, Cadeiras M, McPherson JA, Wells QS. Pre‐existing psychiatric illness is associated with increased risk of recurrent takotsubo cardiomyopathy. Psychosomatics 2017; 58: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basic C, Rosengren A, Lindström S, Schaufelberger M. High validity of cardiomyopathy diagnoses in western Sweden (1989‐2009). ESC Heart Fail 2018; 5: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016; 6: e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Youngson E, Welsh RC, Kaul P, McAlister F, Quan H, Bakal J. Defining and validating comorbidities and procedures in ICD‐10 health data in ST‐elevation myocardial infarction patients. Medicine (Baltimore) 2016; 95: e4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Version 10, Clinical Modification (ICD‐10‐CM) and Procedure Coding System (ICD‐10‐PCS) codes used to identify risk factors, outcomes, and procedures.
Table S2. Fit statistics for latent class models from two to five classes for the 2016 dataset.
Table S3. Fit statistics for latent class models from two to five classes for the 2017 dataset.
Table S4. Fit statistics for latent class models from two to five classes for the robust‐test cohort.
Table S5. Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the 2016 dataset.
Table S6. Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the 2017 dataset.
Table S7. Characteristics of patients with takotsubo cardiomyopathy after clustering on risk factors in the robust‐test cohort.
Table S8. Inpatient outcomes in the four clusters of TCM patients in the robust‐test cohort.
Figure S1. Risk factors of the four clusters in the robust‐test cohort.


