Abstract
Aims
Dilated cardiomyopathy (DCM) is a complex disease where genetics interplay with extrinsic factors. This study aims to compare the phenotype, management, and outcome of familial DCM (FDCM) and non‐familial (sporadic) DCM (SDCM) across Europe.
Methods and results
Patients with DCM that were enrolled in the prospective ESC EORP Cardiomyopathy & Myocarditis Registry were included. Baseline characteristics, genetic testing, genetic yield, and outcome were analysed comparing FDCM and SDCM; 1260 adult patients were studied (238 FDCM, 707 SDCM, and 315 not disclosed). Patients with FDCM were younger (P < 0.01), had less severe disease phenotype at presentation (P < 0.02), more favourable baseline cardiovascular risk profiles (P ≤ 0.007), and less medication use (P ≤ 0.042). Outcome at 1 year was similar and predicted by NYHA class (HR 0.45; 95% CI [0.25–0.81]) and LVEF per % decrease (HR 1.05; 95% CI [1.02–1.08]. Throughout Europe, patients with FDCM received more genetic testing (47% vs. 8%, P < 0.01) and had higher genetic yield (55% vs. 22%, P < 0.01).
Conclusions
We observed that FDCM and SDCM have significant differences at baseline but similar short‐term prognosis. Whether modification of associated cardiovascular risk factors provide opportunities for treatment remains to be investigated. Our results also show a prevalent role of genetics in FDCM and a non‐marginal yield in SDCM although genetic testing is largely neglected in SDCM. Limited genetic testing and heterogeneity in panels provides a scaffold for improvement of guideline adherence.
Keywords: Dilated cardiomyopathy, Sporadic, Familial, Genetic, Prognosis, Europe
Introduction
Dilated cardiomyopathy (DCM), defined as left ventricular dilation and systolic left ventricular (LV) impairment unexplained by coronary artery disease or abnormal loading conditions, is a leading cause of heart failure and heart transplantation with an estimated prevalence of ~36 cases per 100 000 in Europe. 1
Prior studies on clinical characteristics of patients with non‐familial (sporadic) forms of DCM (SDCM) and familial DCM (FDCM) have reported that FDCM presents at earlier age but presented conflicting evidence regarding phenotype severity. Some studies found more favourable clinical profiles in FDCM compared with SDCM, whereas others report similar baseline phenotypes without any distinctive features. 2 , 3 , 4 , 5 Due to genotype–phenotype associations, such as frequent ventricular arrhythmias in LMNA and PLN mutation carriers, heterogeneity in prognosis however may be expected between FDCM and SDCM. 6 Others postulated that FDCM presents as SDCM and that proper active family screening is needed to distinguish true SDCM from undetected FDCM. This screening has been shown to effectively identify DCM and improve prognosis. 3
Many studies have shown that DCM can be inherited as a genetic trait 1 , 7 , 8 , 9 , 10 , 11 and that structural or functional LV abnormalities are present in 20% of asymptomatic relatives of patients with DCM. 12 , 13 Genetic mutations are reported in more than a third of index patients with FDCM and in 8–25% of SDCM. 8 , 14 The most common disease causing variants are found in genes coding for sarcomere proteins such as TTN, MYH7, and FLNC, and the nuclear envelope gene LMNA. 15 , 16 , 17 Evidence also suggests that genetic predisposition in DCM may interact with extrinsic disease triggers such as toxin exposure (ethanol, chemotherapy, and cocaine), viral infection, and pregnancy. 1 , 18 , 19 Nonetheless, toxin exposure may also be the sole putative trigger of DCM, for instance in alcoholic cardiomyopathy. 18
The ESC EORP Cardiomyopathy & Myocarditis Registry is a prospective observational multinational survey of consecutive patients with cardiomyopathies. 20 To investigate the complexity in clinical characteristics and genetic yield, the current analysis of this registry aims to (i) study clinical cardiovascular differences in adult FDCM and SDCM, (ii) report the frequency of genetic testing across Europe, and (iii) report differences in genetic yield between familial and sporadic DCM.
Methods
The general policy as well as baseline results of the new EURObservational Research Programme (EORP) cardiomyopathy registry of the ESC have been previously published. In short, the EORP cardiomyopathy registry is a multicentre registry where participating centres were asked to enter baseline, follow‐up, and genetic data of about 40 consecutive patients with cardiomyopathy per centre. 20 Patients were included from 1 December 2012 until 30 December 2016.
Inclusion/exclusion criteria
Adult patients with DCM defined by ESC consensus criteria were studied. Specifically, (i) left ventricular ejection fraction <45% (>2 SD) and/or fractional shortening <25% (>2 SD), as ascertained by echocardiography, radionuclide scanning, or cardiac magnetic resonance imaging; and (ii) left ventricular end‐diastolic diameter >117% of the predicted value corrected for age and body surface area (Henry's formula), which corresponds to 2 SD of the predicted normal limit +5%. Patients with heart failure attributable to coronary artery disease and clinically suspected or biopsy‐proven myocarditis were excluded. 20 , 21
Definitions
Familial dilated cardiomyopathy (FDCM) was defined by the presence of two or more affected individuals in a single family or the presence of an index patient with DCM and a first degree relative with documented unexplained sudden cardiac death at <35 years of age. Patients that did not meet these criteria were deemed sporadic DCM (SDCM). Patients with missing data concerning familial status (n = 315) were compared with both FDCM and SDCM for clinical differences to account for bias. Primary outcome was defined as a composite of cardiovascular death, implantation of a ventricular assist device, or heart transplantation. Secondary endpoint was hospitalization for urgent cardiac reason. Genetic testing and variant classification were planned and performed according to clinician's judgement. Genetic variants and their classifications were reported by individual researchers representing their centres. Because lab techniques and genetic coordinates were not recorded, centralized variant classification was not possible. The definitions of included variables have been listed in prior EORP publications and are included in the Supporting Information, Data S1 . 20 , 22
Statistical analysis
Univariable analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean ± standard deviation and/or as median and interquartile range (IQR) when appropriate. Among‐group comparisons were made using a non‐parametric test (Kruskal–Wallis). Categorical variables were reported as counts and percentages. Among‐group comparisons were made using a χ 2 test or a Fisher's exact test if any expected cell count was less than 5. Plots of Kaplan–Meier curves for primary outcome were performed. Cox proportional hazards model was used for survival estimates reporting hazard ratios (HRs) and 95% confidence intervals (95% CIs) in univariable and multivariable analysis. As the goal was to report covariates and their association to outcome rather than a clinical risk calculator, both multivariable results and variable selection (P < 0.05) were reported. To compare with external datasets, comparison of proportions was calculated using the “N‐1” χ 2 test. A two‐sided P‐value of <0.05 was considered as statistically significant. For sensitivity analyses, probands of FDCM were compared with SDCM. Analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
The cohort comprised 1260 patients, of whom 238 had FDCM, 707 SDCM, and 315 were unclassified (unknown). Patients with unknown status were compared with FDCM and SDCM. The analysis revealed the ‘unknown’ group to be similar to SDCM. These results and head to head group comparisons are available in the Supporting Information, Data S2 .
The characteristics and treatment of patients with FDCM and SDCM are reported in Table 1 . Compared with SDCM, patients with FDCM were younger (44 years [IQR 31–52]) vs. 51 years [IQR 41–58], P < 0.001), had lower NYHA class (P < 0.001) and BNP and NT‐proBNP levels (P < 0.028), less frequent left bundle branch block (9% vs. 22%, P < 0.001), smaller left ventricular end diastolic diameter (P < 0.001), and higher left ventricular ejection fraction (LVEF) (37% vs. 31%, P < 0.001). Sixty per cent of FDCM patients were index cases compared with 99% of SDCM patients (P < 0.001). Patients with SDCM had a higher burden of cardiovascular risk factors (hypertension, dyslipidaemia, diabetes, smoking, alcohol intake, and high BMI than FDCM (all P ≤ 0.007). A larger proportion of patients with SDCM received beta‐blockers, diuretics, mineralocorticoid receptor antagonists, and other anti‐arrhythmic agents (P ≤ 0.042) compared with FDCM. A sensitivity analysis comparing solely FDCM index patients with SDCM showed that all characteristics except BMI remained significantly different between the groups (Supporting Information, Data S2 , Table 1.1.A).
Table 1.
Baseline table of patient characteristics, pharmacotherapy and outcome
| All (N = 1260) | FDCM (n = 238) | SDCM (n = 707) | P‐value a | |
|---|---|---|---|---|
| Age at diagnosis (years), median (IQR) | 49 (40–58) | 44 (31–52) | 51 (41–58) | <0.01 |
| Male | <0.05 | |||
| NYHA class | <0.01 | |||
| I | 935 (74%) | 165 (69%) | 536 (76%) | |
| II | 198 (19%) | 65 (35%) | 84 (14%) | |
| III | 448 (43%) | 79 (42%) | 261 (44%) | |
| IV | 316 (30%) | 37 (20%) | 187 (31%) | |
| Family history of SCD | 87 (8%) | 6 (3%) | 65 (11%) | <0.01 |
| Hypertension | 479 (38%) | 54 (23%) | 288 (41%) | <0.01 |
| Dyslipidaemia | 472 (38%) | 62 (26%) | 274 (39%) | <0.01 |
| Diabetes Mellitus | 211 (17%) | 25 (11%) | 127 (18%) | <0.01 |
| Alcohol use ≥ 1 units/day | 174 (16%) | 20 (10%) | 124 (20%) | <0.01 |
| Smoking (current and former) | 507 (42%) | 71 (31%) | 323 (47%) | <0.01 |
| Renal impairment | 172 (14%) | 24 (10%) | 94 (13%) | 0.20 |
| History of Atrial Fibrillation | 356 (28%) | 63 (27%) | 202 (29%) | 0.53 |
| History of Stroke | 87 (7%) | 12 (5%) | 47 (7%) | 0.38 |
| History of resuscitation | 61 (5%) | 15 (6%) | 32 (5%) | 0.28 |
| Atrioventricular block | 0.02 | |||
| 1st | 108 (9%) | 20 (9%) | 57 (8%) | |
| 2nd | 6 (1%) | 3 (1%) | 2 (0%) | |
| 3rd | 14 (1%) | 6 (3%) | 4 (1%) | |
| QRS Duration (ms), median, IQR | 105 (92–130) | 100 (90–112) | 104 (90–130) | 0.02 |
| LBBB | 219 (21%) | 18 (9%) | 133 (22%) | <0.01 |
| RBBB | 41 (4%) | 6 (3%) | 24 (4%) | 0.53 |
| LVEF (%), median (IQR) | 31 (25–40) | 37 (29–45) | 31 (24–40) | <0.01 |
| LVEDD (mm), median (IQR) | 64 (58–70) | 60 (54–67) | 64 (59–70) | <0.01 |
| Diastolic dysfunction (grades) | <0.01 | |||
| Normal | 63 (38%) | 97 (20%) | ||
| I (impaired relaxation) | 57 (35%) | 169 (35%) | ||
| II (pseudonormal) | 28 (17%) | 108 (22%) | ||
| III/IV (restrictive) | 16 (9%) | 109 (22%) | ||
| Haemoglobin (g/dL), median (IQR) | 14 (13–15) | 14 (13–15) | 14 (13–15) | 0.23 |
| BNP (pg/mL), median (IQR) | 297 (72–717) | 108 (36–555) | 404 (106–718) | 0.03 |
| NT‐proBNP (pg/mL), median (IQR) | 1102 (312–3341) | 589 (156–1663) | 1276 (452–3527) | <0.01 |
| Performed | 259 (21%) | 66 (28%) | 148 (21%) | 0.04 |
| Abnormal | 237 (19%) | 57 (24%) | 136 (19%) | 0.04 |
| Late gadolinium enhancement | 153 (64%) | 41 (65%) | 92 (67%) | 0.83 |
| β ‐blockers | 1130 (90%) | 203 (85%) | 644 (91%) | 0.01 |
| Diuretics | 895 (72%) | 123 (54%) | 540 (77%) | <0.01 |
| ACE‐inhibitors or ATII‐receptor blockers | 1121 (89%) | 208 (88%) | 634 (89%) | 0.329 |
| Mineralocorticoid receptor antagonists | 795 (63%) | 119 (50%) | 470 (67%) | <0.01 |
| Other antiarrhythmics | 361 (29%) | 56 (24%) | 215 (30%) | 0.04 |
BMI, body mass index; LBBB, left bundle branch block; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RBBB, right bundle branch block; SCD, sudden cardiac death.
Data are presented as number and percentages of valid. Patients with missing family status were not included in the subgroup SDCM in this table because their status was unknown. They have been included in the Supporting Information, Data S2 , Table 1 both a separate group and combined with SDCM. Continuous data are presented as medians. Median presented with first and third interquartiles (IQR). Mean presented with ±standard deviation (SD).
FDCM versus SDCM.
Follow‐up data were available in 1105 (88%) cases (median follow‐up duration: 372 days; interquartile range [IQR] 363–428). There were no differences observed in primary and secondary outcomes or all‐cause mortality when comparing FDCM (n = 209) to SDCM (n = 611) (Supporting Information, Data S2 , Table 3B). Age at first primary event was also similar for FDCM and SDCM (Figure 1 ).
Figure 1.
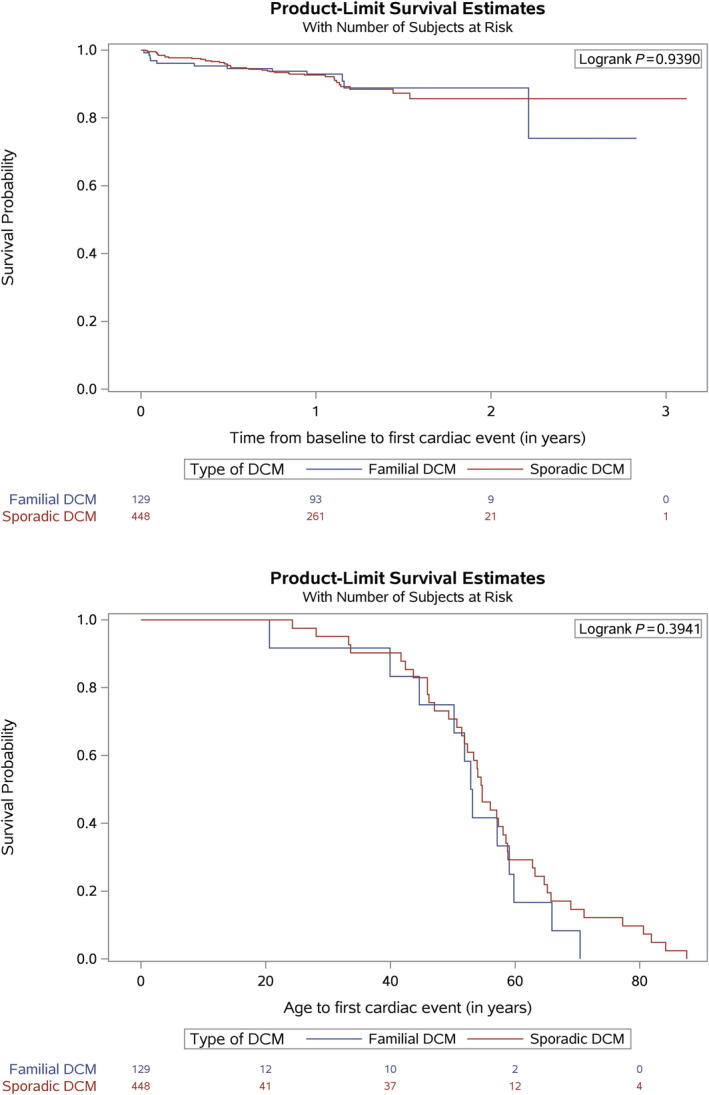
Survival probability curves for primary outcome plotted over both time and age comparing SDCM to FDCM.
In multivariable analysis, BMI per unit decrease (HR 1.11; 95% CI [1.02–1.22]) and LVEF per % decrease (HR 1.08; 95% CI [1.03–1.11]) were associated with primary and secondary outcomes. After stepwise selection, NYHA class I/II versus III/IV (HR 0.45; 95% CI [0.25–0.81]) and LVEF per % decrease (HR 1.05; 95% CI [1.02–1.08] were predictive (Table 2 ). There were no significant associations for all‐cause mortality. Results of all analyses are available in the Supporting Information, Data S2 , Table 10.
Table 2.
Multivariable analysis for primary outcome
| Variable | Pval hazard ratio | Hazard ratio (95% CI) |
|---|---|---|
| Age at diagnosis per year increase | 0.040 (S) | 0.973 (0.949; 0.999) |
| LBBB | 0.635 (NS) | 0.804 (0.327; 1.977) |
| NYHA class I or II versus III or IV | 0.061 (NS) | 0.483 (0.226; 1.035) |
| Alcohol intake 1 unit per day or more | 0.618 (NS) | 1.224 (0.553; 2.705) |
| Body mass index (kg/m2) per unit increase | 0.012 (S) | 0.896 (0.823; 0.977) |
| Diabetes mellitus | 0.029 (S) | 2.815 (1.111; 7.131) |
| Hypertension | 0.859 (NS) | 1.070 (0.509; 2.251) |
| Smoking current of former | 0.921 (NS) | 0.964 (0.472; 1.970) |
| LVEF per % decrease | <0.001 (S) | 1.073 (1.031; 1.117) |
LBBB, left bundle branch block; LV, left ventricular ejection fraction; NS, not significant; NYHA, New York Heart Association; S, significant.
Primary endpoint was defined as a composite of cardiovascular death, implantation of a ventricular assist device, or heart transplantation. Cox proportional hazards model is presented (hazard ratio's and 95% confidence intervals).
Genetic testing
In total, 214 out of the 1260 (17%) cases were genetically tested (58 out of 707 [8%] SDCM and 114 out of 238 [48%] of FDCM). In 110 cases, the information on genetic testing was missing. Genetic testing was less frequently performed in Eastern Europe and North Africa (respectively 4% and 0%), whereas North, South, and West Europe performed genetic testing in 21–27% of their population (Table 3 ).
Table 3.
Regional differences for genetic testing in DCM
| All (N = 1260) | FDCM (N = 238) | SDCM (N = 707) | P‐value a | |
|---|---|---|---|---|
| North Europe (n = 179) | 37 (21%) | 19 (56%) | 11 (16%) | <0.001 |
| South Europe (n = 425) | 116 (27%) | 74 (59%) | 23 (14%) | <0.001 |
| West Europe (n = 206) | 48 (23%) | 15 (56%) | 24 (16%) | <0.001 |
| East Europe (n = 302) | 13 (4%) | 6 (18%) | 0 (0%) | <0.001 |
| North Africa (n = 38) | 0 (0%) | 0 (0%) | 0 (0%) | NC |
| Total (1150) | 214 (17%) | 114 (48%) | 58 (8%) | <0.001 |
NC, not calculable.
In 110 cases, genetic testing was unknown.
FDCM versus SDCM.
In 63 out of the 114 (55%) tested cases of FDCM, at least one disease causing variant was reported compared with 13 out of 58 (22%) in SDCM. These variants were most prevalent in sarcomere (n = 42) and nuclear genes (n = 27), with most variants being discovered in LMNA (16% yield in FDCM and 3% yield in SDCM) and MYH7 (14% yield in FDCM and 0% in SDCM) (Supporting Information, Data S2 , Table 7).
Discussion
The three main findings of this study are (i) patients with FDCM present at younger age with a less severe phenotype and lower burden of cardiovascular risk factors but similar short‐term prognosis to SDCM; (ii) patients with FDCM are genetically tested more frequently and with higher diagnostic yield than SDCM and; (iii) there are important differences in the use of genetic testing across European centres enrolled in the ESC cardiomyopathy registry. For a central illustration, these findings are summarized in Figure 2 .
Figure 2.
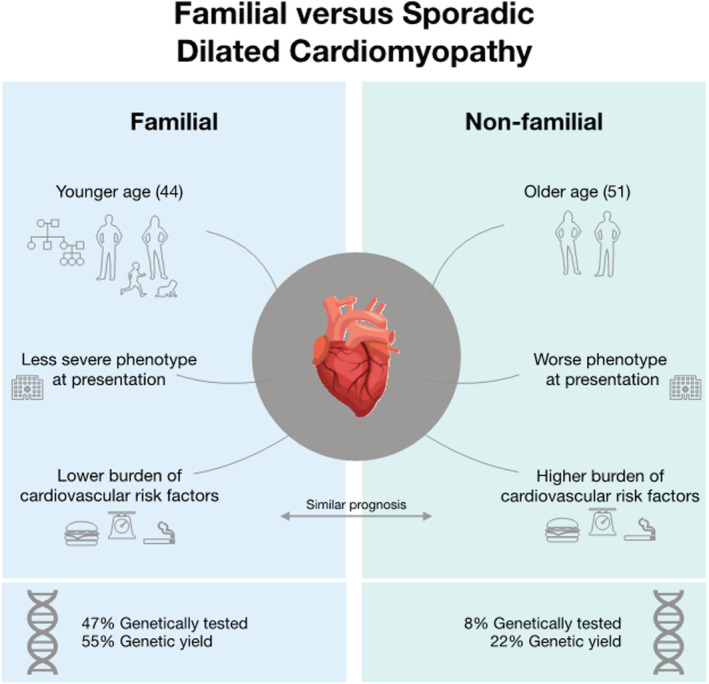
Summary of the main findings of this study.
Disease burden
Overall, DCM patients included in this cohort had similar demographic and clinical characteristics compared with DCM populations described in the literature. 2 , 4 , 5 , 12 , 23 However, in contrast to some studies, we found that patients with FDCM were diagnosed at a younger age and have a less severe phenotype than patients with SDCM but have similar cardiovascular prognosis. 2 , 5 This was also seen in the prescribed medication, where we observed that patients with FDCM received less beta‐blockers, diuretics and mineralocorticoid receptor antagonists. These findings may reflect of cascade family screening in patients with suspected FDCM. However, when comparing FDCM index patients with SDCM patients, the clinical differences remained significant. Because family screening has shown to effectively identify DCM patients at earlier stages of disease, potentially benefiting prognosis, genetic counselling remains an important pillar of care in patients with DCM and their relatives. 3 Prognosis of DCM has been improving for the endpoint of cardiovascular death of heart transplantation/assist device implantation, arguably because of better cardiovascular care and the early identification and classification of disease. 24 Our observations however are more in line with earlier reports and less favourable outcome, which may be caused because of a selection bias towards more expert/tertiary centres. 23
A relevant finding of this study was that patients with SDCM had a higher burden of cardiovascular risk factors, such as hypertension, hypercholesterolaemia, and smoking. Because it is well known that these risk factors are more prevalent with increasing age, and our patients with SDCM were significantly older than FDCM, this result might be expected. 25 However, the age difference between SDCM and FDCM does not fully account for the observed increase in risk factors. When comparing our patients with SDCM with external cohorts from the same age group, we still observe a significantly increased burden of cardiovascular factors (P < 0.01) in our patients with SDCM. 25 , 26 In addition, the presence of common cardiovascular risk factors is also associated with worse outcome in SDCM. Smoking, for instance, independently increased sudden cardiac death in SDCM 27 and diabetes confers a two‐fold to five‐fold added risk for heart failure development, even after adjustment for other traditional risk factors such as coronary heart disease. 22 Alcohol is also known to be the both sole cause of cardiomyopathy and may negatively affect cardiac function in mutation carriers. In a recent report including 716 DCM cases, excessive alcohol consumption resulted in an absolute LVEF reduction of ±9% in patients with a truncating TTN variant. 18 These results support the hypothesis that cardiovascular risk factors and external toxic substances interplay with genetic frailty, (auto)immunity, or endocrinological disorders in the DCM phenotype. 1 Whether modification of cardiovascular risk factors provide opportunities for treatment remains to be investigated.
Differences in prognosis between FDCM and SDCM remain controversial with most follow‐up studies of DCM not dividing populations in familial and non‐familial categories. 3 , 28 , 29 Conflicting results of prior studies may be due to the absence of reliable clinical or morphological parameters to differentiate between FDCM and SDCM. 1 , 3 , 28 , 29 Genotype–phenotype associations affecting prognosis in DCM have been reported: mutations in LMNA and RBM20 predispose for more/younger heart transplantations. 6 Mutations in FLNC, LMNA, and PLN have been associated with sustained ventricular arrhythmias in DCM. 30 Nonetheless, our analysis did not yield a significant difference in outcome between FDCM and SDCM.
Genetic mutations in dilated cardiomyopathy
Mutations in over 40 genes are causally related to DCM and explain up to 61% of cases in FDCM and up to 25% in SDCM. 1 , 8 , 14 , 16 , 31 Our data confirm the higher yield in FDCM. 8 At present, genetic screening is advised in patients with family history of DCM or a personal history of atypical features such as conduction/rhythm‐disturbances, 7 , 8 , 11 , 31 and yet less than 50% of all cases in the registry were tested. Moreover, the fact that heterogeneous screening strategies were used may have impacted on the yield of testing. 20 For instance, mutations in Titin (TTN) have been implicated in up to 22% of SDCM, but this gene was only tested in 19% of our patients with SDCM. 32 , 33 The selective nature of genetic testing may explain the high prevalence of mutations in genes coding for sarcomere genes and LMNA.
Genetic testing in Europe
We observed differences in genetic testing between European centres. Genetic testing was most prevalent in Northern, Southern, and Western Europe (around 20% of the cohort) and least common in Eastern Europe and North Africa. Importantly, Southern Europe included a majority (54%) of the patients with FDCM in this cohort, which could have led to sampling bias. 34 , 35 These shortcomings should be accounted for in terms of external validity of this study. Furthermore, the regional differences in our study as well as differences described by prior EORP studies provide opportunities to improve guideline adherence in Europe. 20 , 35 , 36 Even though guideline adherence is highest in secondary and tertiary centres and higher for cardiologists than other specialities, care may be fragmented in highly complex diseases such as inherited cardiomyopathies. 37 This fragmentation can cause unwanted protocol deviations that may deteriorate quality of care. Several strategies have been suggested to improve guideline adherence. These strategies include common care pathways, ongoing education, and focus groups. 37 In terms of genetic testing, actively looking for familial manifestations of disease in patients and relative and acquiring a detailed family history are quintessential and may improve genetic yield dramatically.
Study limitations
In contrast to controlled clinical studies, our data are likely to be heterogeneous given the nature of the study design. As the structure of the registry requested to introduce patients from expert/tertiary centres, this may have caused a selection bias. Second limitation is that not all genes were tested in all patients, and centralized variant classification was not repeated in this study. Given that genetic testing was unknown in 110 patients, this might constitute another limitation. Even though myocarditis is formally an exclusion criterion, inflammation‐caused/mediated reasons for DCM are very difficult to diagnose. Therefore, we cannot exclude with certainty that a proportion of non‐familial DCM might be suspected for inflammatory diseases. As the EORP study was not designed for inter‐regional comparisons, and these analyses were not planned, such comparisons may be arbitrary and unrepresentative. Furthermore, sub‐analyses using ethnicity/race to provide insight into heterogeneity of populations are limited by poor accuracies of ethnicity/race in electronic health records. Importantly, even though we performed sensitivity analyses with and without patients with unknown classification, this still might have led to biased results. Lastly, the fact that no difference in outcome was detected in our DCM populations may be due to sample size and requires confirmation in larger studies.
Conclusions
Familial DCM and SDCM have significant differences at baseline. Patients with FDCM appear to present at earlier stage of disease. Patients with SDCM in this registry have less favourable clinical profile. Whether modification of associated cardiovascular risk factors provide opportunities for treatment remains to be investigated. Genetic testing confirms a prevalent role of genetics in FDCM but is largely neglected in SDCM. Although SDCM may probably be multifactorial, genetic influences need further understanding. Limited genetic testing provides a scaffold for improvement of guideline adherence.
Conflict of interest
F.W.A., A.S., A.L.P.C., C.L., J.R.G., P.E., P.P.R., R.J., L.C., and M.Z. have nothing to disclose. L.T. reports personal fees from Servier and CVIE Therapeutics outside the submitted work. M.T. reports personal fees from Bayer, Cadila Pharmaceuticals, Janssen‐Cilag, Kowa, OncoArendi, PERFUSE Group, Servier, and UCB Pharmaceuticals outside the submitted work. J.P.K. reports grants from British Heart Foundation and Medical Research Council Clinical Academic Research Partnership outside the submitted work. A.P.M. reports personal fees from Novartis, Bayer, and Fresenius outside the submitted work. H.T. reports other from cardiology consultant at Blueprint Genetics; personal fees from Sanofi‐Genzyme, Amgen, and Pfizer; and non‐financial support from Alnylam and MSD outside the submitted work. G.S. reports personal fees from Novartis, Vifor Pharma, Boston, Bayer, AstraZeneca, and Dompè outside the submitted work. I.O. reports grants and personal fees from Shire Takeda, Sanofi‐Genzyme, and Myokardia; grants from Amicus and Bayer; and grants Menarini International outside the submitted work. A.K. reports grants from Research Council of Lithuania; other from Novartis Int; and personal fees from Bayer, Berlin‐Chemie, and Menarini outside the submitted work. P.C. reports personal fees from Amicus, Pfizer, and Alnylam outside the submitted work.
Funding
Since the start of EORP, the following companies have supported the programme: Abbott Vascular Int. (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer AG (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific (2009–2012), The Bristol Myers Squibb and Pfizer Alliance (2011–2019), Daiichi Sankyo Europe GmbH (2011–2020), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Edwards (2016–2019), Gedeon Richter Plc. (2014–2016), Menarini Int. Op. (2009–2012), MSD‐Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed (2014–2016), Sanofi (2009–2011), SERVIER (2009–2021), and Vifor (2019–2022). Folkert Asselbergs is supported by UCL Hospitals NIHR Biomedical Research and CVON 2015‐12 eDETECT. Arjan Sammani is supported by the UMC Utrecht Alexandre Suerman Stipendium and CVON 2015‐12 eDETECT YTP. Juan Pablo Kaski is supported by the NIHR Great Ormond Street Hospital BRC and the British Heart Foundation and the Medical Research Council.
Supporting information
Data S1. Centre Inclusion Criteria.
Data S2. Supporting information.
Acknowledgements
We thank the EORP Oversight Committee, The Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection was conducted by the EORP Department from the ESC by Rachid Mir Hassaine as Clinical Project Manager; Emanuela Fiorucci, Myriam Glemot, and Patti‐Ann McNeill as Project Officers; and Marème Konté and Sebastien Authier as Data Managers. Statistical analyses were performed by Cécile Laroche. Overall activities were coordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator). All investigators are listed in Appendix A.
Appendix A.
EORP Oversight Committee
Christopher Peter Gale, Chair, GB, Branko Beleslin, RS, Andrzej Budaj, PL, Ovidiu Chioncel, RO, Nikolaos Dagres, DE, Nicolas Danchin, FR, Jonathan Emberson, GB, David Erlinge, SE, Michael Glikson, IL, Alastair Grey, GB, Meral Kayikcioglu, TR, Aldo Maggioni, IT, Klaudia Vivien Nagy, HU, Aleksandr Nedoshivin, RU, Anna‐Sonia Petronio, IT, Jolien Roos‐Hesselink, NL, Lars Wallentin, SE, Uwe Zeymer, DE.
Executive Committee
Alida Caforio (Chair), IT, Juan Ramon Gimeno Blanes, ES, Philippe Charron, FR, Perry Elliott, GB, Juan Pablo Kaski, GB, Aldo P. Maggioni, IT, Luigi Tavazzi, IT, Michal Tendera, PL.
Investigators
Belarus: Minsk: S. Komissarova, N. Chakova, S. Niyazova, Czech Republic: Prague: A. Linhart, P. Kuchynka, T. Palecek, J. Podzimkova, M. Fikrle, E. Nemecek, Denmark: Copenhagen: H. Bundgaard, J. Tfelt‐Hansen, J. Theilade, J. J. Thune, A. Axelsson, Odense: J. Mogensen, F. Henriksen, T. Hey, S. K. Nielsen, L. Videbaek, S. Andreasen, H. Arnsted, Egypt: Zagazig: A. Saad, M. Ali, Finland: Helsinki: J. Lommi, T. Helio, M. S. Nieminen, France: Boulogne‐Billancourt: O. Dubourg, N. Mansencal, M. Arslan, V. Siam Tsieu, Créteil: T. Damy, A. Guellich, S. Guendouz, C. M. Tissot, A. Lamine, S. Rappeneau, Paris: A. Hagege, M. Desnos, A. Bachet, M. Hamzaoui, Paris: P. Charron, R. Isnard, L. Legrand, C. Maupain, E. Gandjbakhch, M. Kerneis, J.‐F. Pruny, Germany: Crailsheim: A. Bauer, B. Pfeiffer, Greifswald: S. B. Felix, M. Dorr, S. Kaczmarek, K. Lehnert, A.‐L. Pedersen, D. Beug, M. Bruder, Homburg/Saar: M. Böhm, I. Kindermann, Y. Linicus, C. Werner, B. Neurath, M. Schild‐Ungerbuehler, Schweinfurt: H. Seggewiss, B. Pfeiffer, A. Neugebauer, Great Britain: Belfast: P. McKeown, A. Muir, J. McOsker, T. Jardine, G. Divine, London: P. Elliott, M. Lorenzini, O. Watkinson, E. Wicks, London: H. Iqbal, S. Mohiddin, C. O'Mahony, N. Sekri, London: G. Carr‐White, T. Bueser, R. Rajani, L. Clack, J. Damm, S. Jones, R. Sanchez‐Vidal, M. Smith, T. Walters, K. Wilson, London: S. Rosmini, Greece: Athens: A. Anastasakis, K. Ritsatos, V. Vlagkouli, Hungary: Szeged: T. Forster, R. Sepp, J. Borbas, V. Nagy, A. Tringer, K. Kakonyi, L. A. Szabo, Iran: Tehran: M. Maleki, F. Noohi Bezanjani, A. Amin, N. Naderi, M. Parsaee, S. Taghavi, B. Ghadrdoost, S. Jafari, M. Khoshavi, Italy: Bologna: C. Rapezzi, E. Biagini, A. Corsini, C. Gagliardi, M. Graziosi, S. Longhi, A. Milandri, L. Ragni, S. Palmieri, Florence: I. Olivotto, A. Arretini, G. Castelli, F. Cecchi, A. Fornaro, B. Tomberli, Genoa: P. Spirito, E. Devoto, Milan: P. Della Bella, G. Maccabelli, S. Sala, F. Guarracini, G. Peretto, Naples: M. G. Russo, R. Calabro, G. Pacileo, G. Limongelli, D. Masarone, V. Pazzanese, A. Rea, M. Rubino, S. Tramonte, F. Valente, M. Caiazza, A. Cirillo, G. Del Giorno, A. Esposito, R. Gravino, T. Marrazzo, Naples: B. Trimarco, M.‐A. Losi, C. Di Nardo, A. Giamundo, F. Musella, F. Pacelli, A. Scatteia, G. Canciello, Padua: A. Caforio, S. Iliceto, C. Calore, L. Leoni, M. Perazzolo Marra, I. Rigato, G. Tarantini, A. Schiavo, M. Testolina, Pavia: E. Arbustini, A. Di Toro, L. P. Giuliani, A. Serio, Rome: F. Fedele, A. Frustaci, M. Alfarano, C. Chimenti, Rome: F. Drago, A. Baban, Rome: L. Calò, C. Lanzillo, A. Martino, Rome: M. Uguccioni, E. Zachara, G. Halasz, F. Re, Trieste: G. Sinagra, C. Carriere, M. Merlo, F. Ramani, Lithuania: Kaunas: A. Kavoliuniene, A. Krivickiene, E. Tamuleviciute‐Prasciene, M. Viezelis, Vilnius: J. Celutkiene, L. Balkeviciene, M. Laukyte, E. Paleviciute, Netherlands: Amsterdam: Y. Pinto, A. Wilde, Utrecht: F. W. Asselbergs, A. Sammani, J. Van Der Heijden, L. Van Laake, N. De Jonge, R. Hassink, J. H. Kirkels, Nigeria: Lagos: J. Ajuluchukwu, A. Olusegun‐Joseph, E. Ekure, Poland: Katowice: K. Mizia‐Stec, M. Tendera, A. Czekaj, A. Sikora‐Puz, A. Skoczynska, M. Wybraniec, Krakow: P. Rubis, E. Dziewiecka, S. Wisniowska‐Smialek, Warsaw: Z. Bilinska, P. Chmielewski, B. Foss‐Nieradko, E. Michalak, M. Stepien‐Wojno, B. Mazek, Portugal: Almada: L. Rocha Lopes, A. R. Almeida, I. Cruz, A. C. Gomes, A. R. Pereira, Lisbon: D. Brito, H. Madeira, A. R. Francisco, M. Menezes, O. Moldovan, T. Oliveira Guimaraes, D. Silva, Romania: Bucharest: C. Ginghina, R. Jurcut, A. Mursa, B. A. Popescu, E. Apetrei, S. Militaru, I. Mircea Coman, Targu‐Mures: A. Frigy, Z. Fogarasi, I. Kocsis, I. A. Szabo, L. Fehervari, Russian Federation: Moscow: I. Nikitin, E. Resnik, M. Komissarova, V. Lazarev, M. Shebzukhova, D. Ustyuzhanin, Moscow: O. Blagova, I. Alieva, V. Kulikova, Y. Lutokhina, E. Pavlenko, N. Varionchik, Serbia: Belgrade: A. D. Ristic, P. M. Seferovic, I. Veljic, I. Zivkovic, I. Milinkovic, A. Pavlovic, G. Radovanovic, D. Simeunovic, Belgrade: M. Zdravkovic, M. Aleksic, J. Djokic, S. Hinic, S. Klasnja, K. Mircetic, Spain: A Coruna: L. Monserrat, X. Fernandez, D. Garcia‐Giustiniani, J. M. Larrañaga, M. Ortiz‐Genga, R. Barriales‐Villa, C. Martinez‐Veira, E. Veira, Barcelona: A. Cequier, J. Salazar‐Mendiguchia, N. Manito, J. Gonzalez, Madrid: F. Fernández‐Avilés, C. Medrano, R. Yotti, S. Cuenca, M. A. Espinosa, I. Mendez, E. Zatarain, R. Alvarez, Madrid: P. Garcia‐Pavia, A. Briceno, M. Cobo‐Marcos, F. Dominguez, Malaga: E. De Teresa Galvan, J. M. García Pinilla, N. Abdeselam‐Mohamed, M. A. Lopez‐Garrido, L. Morcillo Hidalgo, M. V. Ortega‐Jimenez, A. Robles Mezcua, A. Guijarro‐Contreras, D. Gomez‐Garcia, M. Robles‐Mezcua, Murcia: J. R. Gimeno Blanes, F. J. Castro, C. Munoz Esparza, M. Sabater Molina, M. Sorli García, D. Lopez Cuenca, Palma de Mallorca: T. Ripoll‐Vera, J. Alvarez, J. Nunez, Y. Gomez, Salamanca: P. L. Sanchez Fernandez, E. Villacorta, C. Avila, L. Bravo, E. Diaz‐Pelaez, M. Gallego‐Delgado, L. Garcia‐Cuenllas, B. Plata, Seville: J. E. Lopez‐Haldon, M. L. Pena Pena, E. M. Cantero Perez, Valencia: E. Zorio, M. A. Arnau, J. Sanz, E. Marques‐Sule.
Asselbergs, F. W. , Sammani, A. , Elliott, P. , Gimeno, J. R. , Tavazzi, L. , Tendera, M. , Kaski, J. P. , Maggioni, A. P. , Rubis, P. P. , Jurcut, R. , Heliö, T. , Calò, L. , Sinagra, G. , Zdravkovic, M. , Olivotto, I. , Kavoliūnienė, A. , Laroche, C. , Caforio, A. L. P. , Charron, P. , and Cardiomyopathy & Myocarditis Registry Investigators Group (2021) Differences between familial and sporadic dilated cardiomyopathy: ESC EORP Cardiomyopathy & Myocarditis registry. ESC Heart Failure, 8: 95–105. 10.1002/ehf2.13100.
References
- 1. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, Groote De P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio ALP, Charron P. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non‐dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016; 37: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 2. Grünig E, Tasman JA, Kücherer H, Franz W, Kübler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol 1998; 31: 186–194. [DOI] [PubMed] [Google Scholar]
- 3. Moretti M, Merlo M, Barbati G, di Lenarda A, Brun F, Pinamonti B, Gregori D, Mestroni L, Sinagra G. Prognostic impact of familial screening in dilated cardiomyopathy. Eur J Heart Fail 2010; 12: 922–927. [DOI] [PubMed] [Google Scholar]
- 4. Kushner JD, Nauman D, Burgess D, Ludwigsen S, Parks SB, Pantely G, Burkett E, Hershberger RE. Clinical characteristics of 304 kindreds evaluated for familial dilated cardiomyopathy. J Card Fail 2006; 12: 422–429. [DOI] [PubMed] [Google Scholar]
- 5. Mestroni L, Rocco C, Gregori D, Sinagra G, di Lenarda A, Miocic S, Vatta M, Pinamonti B, Muntoni F, Caforio ALP, McKenna WJ, Falaschi A, Giacca M. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. J Am Coll Cardiol 1999; 34: 181–190. [DOI] [PubMed] [Google Scholar]
- 6. Kayvanpour E, Sedaghat‐Hamedani F, Amr A, Lai A, Haas J, Holzer DB, Frese KS, Keller A, Jensen K, Katus HA, Meder B. Genotype‐phenotype associations in dilated cardiomyopathy: meta‐analysis on more than 8000 individuals. Clin Res Cardiol 2017; 106: 127–139. [DOI] [PubMed] [Google Scholar]
- 7. Harakalova M, Kummeling G, Sammani A, Linschoten M, Baas AF, van Der SJ, Doevendans PA, Van TJP, Dooijes D, Mokry M, Asselbergs FW. A systematic analysis of genetic dilated cardiomyopathy reveals numerous ubiquitously expressed and muscle‐specific genes. Eur J Heart Fail 2015; 17: 484–493. [DOI] [PubMed] [Google Scholar]
- 8. van Spaendonck‐Zwarts KY, van Rijsingen IAW, van den Berg MP, Lekanne Deprez RH, Post JG, van Mil AM, Asselbergs FW, Christiaans I, van Langen IM, Wilde AAM, de Boer RA, Jongbloed JDH, Pinto YM, van Tintelen JP. Genetic analysis in 418 index patients with idiopathic dilated cardiomyopathy: overview of 10 years' experience. Eur J Heart Fail 2013; 15: 628–636. [DOI] [PubMed] [Google Scholar]
- 9. Janin A, N'Guyen K, Habib G, Dauphin C, Chanavat V, Bouvagnet P, Eschalier R, Streichenberger N, Chevalier P, Millat G. Truncating mutations on myofibrillar myopathies causing genes as prevalent molecular explanations on patients with dilated cardiomyopathy. Clin Genet 2017; 92: 616–623. [DOI] [PubMed] [Google Scholar]
- 10. Begay RL, Graw S, Sinagra G, Merlo M, Slavov D, Gowan K, Jones KL, Barbati G, Spezzacatene A, Brun F, Di LA, Smith JE, Granzier HL, Mestroni L, Taylor M. Role of titin missense variants in dilated cardiomyopathy. J Am Heart Assoc 2015; 4: e002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubiś P. The diagnostic work‐up of genetic and inflammatory dilated cardiomyopathy. E‐J Cardiol Pract 2015: 19 https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/The-diagnostic‐work‐up‐of‐genetic‐and‐inflammatory‐dilated‐cardiomyopathy (15 December 2019). [Google Scholar]
- 12. Michels VV, Moll PP, Miller FA, Tajik AJ, Chu JS, Driscoll DJ, Burnett JC, Rodeheffer RJ, Chesebro JH, Tazelaar HD. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med 1992; 326: 77–82. [DOI] [PubMed] [Google Scholar]
- 13. Mahon NG, Murphy RT, MacRae CA, Caforio ALP, Elliott PM, McKenna WJ. Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease. Ann Intern Med 2005; 143: 108–115. [DOI] [PubMed] [Google Scholar]
- 14. Akinrinade O, Ollila L, Vattulainen S, Tallila J, Gentile M, Salmenperä P, Koillinen H, Kaartinen M, Nieminen MS, Myllykangas S, Alastalo TP, Koskenvuo JW, Heliö T. Genetics and genotype‐phenotype correlations in Finnish patients with dilated cardiomyopathy. Eur Heart J 2015; 36: 2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jansweijer JA, Hershberger R, van Spaendonck KY. Dilated cardiomyopathy. Clin Cardiogenetics Second Ed 2016; 390: 75–89. [Google Scholar]
- 16. Pugh TJ, Kelly MA, Gowrisankar S, Hynes E, Seidman MA, Baxter SM, Bowser M, Harrison B, Aaron D, Mahanta LM, Lakdawala NK, McDermott G, White ET, Rehm HL, Lebo M, Funke BH. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet Med 2014; 16: 601–608. [DOI] [PubMed] [Google Scholar]
- 17. Begay RL, Graw SL, Sinagra G, Asimaki A, Rowland TJ, Slavov DB, Gowan K, Jones KL, Brun F, Merlo M, Miani D, Sweet M, Devaraj K, Wartchow EP, Gigli M, Puggia I, Salcedo EE, Garrity DM, Ambardekar AV, Buttrick P, Reece TB, Bristow MR, Saffitz JE, Mestroni L, Taylor MRG, Filamin C. Truncation mutations are associated with arrhythmogenic dilated cardiomyopathy and changes in the cell–cell adhesion structures. JACC Clin Electrophysiol 2018; 4: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ware JS, Amor‐Salamanca A, Tayal U, Govind R, Serrano I, Salazar‐Mendiguchía J, García‐Pinilla JM, Pascual‐Figal DA, Nuñez J, Guzzo‐Merello G, Gonzalez‐Vioque E, Bardaji A, Manito N, López‐Garrido MA, Padron‐Barthe L, Edwards E, Whiffin N, Walsh R, Buchan RJ, Midwinter W, Wilk A, Prasad S, Pantazis A, Baski J, O'Regan DP, Alonso‐Pulpon L, Cook SA, Lara‐Pezzi E, Barton PJ, Garcia‐Pavia P. Genetic etiology for alcohol‐induced cardiac toxicity. J Am Coll Cardiol 2018; 71: 2293–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linschoten M, Teske AJ, Cramer MJ, van der Wall E, Asselbergs FW. Chemotherapy‐related cardiac dysfunction: a systematic review of genetic variants modulating individual risk. Circ Genomic Precis Med 2018; 11: e001753. [DOI] [PubMed] [Google Scholar]
- 20. Charron P, Elliott PM, Gimeno JR, Caforio ALP, Kaski JP, Tavazzi L, Tendera M, Maupain C, Laroche C, Rubis P, Jurcut R, Calò L, Heliö TM, Sinagra G, Zdravkovic M, Kavoliuniene A, Felix SB, Grzybowski J, Losi MA, Asselbergs FW, García‐Pinilla JM, Salazar‐Mendiguchia J, Mizia‐Stec K, Maggioni AP, Anastasakis A, Biagini E, Bilinska Z, Castro FJ, Celutkiene J, Chakova N. The cardiomyopathy registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J 2018; 39: 1784–1793. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der MP, Sisakian HS, Isayev E, Kurlianskaya A, Mullens W, Tokmakova M, Agathangelou P, Melenovsky V, Wiggers H, Hassanein M. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016 2016; 37: 2129–2200.27206819 [Google Scholar]
- 22. Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab 2019; 10 2042018819834869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keeling PJ, Goldman JH, Slade AKB, Elliott PM, Caforio ALP, Poloniecki J, McKenna WJ. Prognosis of idiopathic dilated cardiomyopathy. J Card Fail 1995; 1: 337–345. [DOI] [PubMed] [Google Scholar]
- 24. Merlo M, Cannatà A, Pio Loco C, Stolfo D, Barbati G, Artico J, Gentile P, de Paris V, Ramani F, Zecchin M, Gigli M, Pinamonti B, Korcova R, di Lenarda A, Giacca M, Mestroni L, Camici PG, Sinagra G. Contemporary survival trends and aetiological characterization in non‐ischaemic dilated cardiomyopathy. Eur J Heart Fail 2020; 22: 1111–1121. [DOI] [PubMed] [Google Scholar]
- 25. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham study. Am J Cardiol 1976; 38: 46–51. [DOI] [PubMed] [Google Scholar]
- 26. Gikas A, Lambadiari V, Sotiropoulos A, Panagiotakos D, Pappas S. Prevalence of major cardiovascular risk factors and coronary heart disease in a sample of Greek adults: the Saronikos study. Open Cardiovasc Med J 2016; 10: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park J, Lee HJ, Kim SK, Yi JE, Shin DG, Lee JM, Kim Y, Kim YJ, Joung B. Smoking aggravates ventricular arrhythmic events in non‐ischemic dilated cardiomyopathy associated with a late gadolinium enhancement in cardiac MRI. Sci Rep 2018; 8: 15609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michels VV, Driscoll DJ, Miller FA, Olson TM, Atkinson EJ, Olswold CL, Schaid DJ. Progression of familial and non‐familial dilated cardiomyopathy: long term follow up. Heart 2003; 89: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Csanady M, Hogye M, Kallai A, Forster T, Szarazajtai T. Familial dilated cardiomyopathy: a worse prognosis compared with sporadic forms. Br Heart J 1995; 74: 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sammani A, Kayvanpour E, Bosman LP, Sedaghat‐Hamedani F, Proctor T, Gi WT, Broezel A, Jensen K, Katus HA, Riele ASJM, Meder B, Asselbergs FW. Predicting sustained ventricular arrhythmias in dilated cardiomyopathy: a meta‐analysis and systematic review. ESC Hear Fail 2020; 7: 1430–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013; 10: 531–547. [DOI] [PubMed] [Google Scholar]
- 32. Tobita T, Nomura S, Fujita T, Morita H, Asano Y, Onoue K, Ito M, Imai Y, Suzuki A, Ko T, Satoh M, Fujita K, Naito AT, Furutani Y, Toko H, Harada M, Amiya E, Hatano M, Takimoto E, Shiga T, Nakanishi T, Sakata Y, Ono M, Saito Y, Takashima S, Hagiwara N, Aburatani H, Komuro I. Genetic basis of cardiomyopathy and the genotypes involved in prognosis and left ventricular reverse remodeling. Sci Rep 2018; 8: 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franaszczyk M, Chmielewski P, Truszkowska G, Stawinski P, Michalak E, Rydzanicz M, Sobieszczanska‐Malek M, Pollak A, Szczygieł J, Kosinska J, Parulski A, Stoklosa T, Tarnowska A, Machnicki MM, Foss‐Nieradko B, Szperl M, Sioma A, Kusmierczyk M, Grzybowski J, Zielinski T, Ploski R, Bilinska ZT. Titin truncating variants in dilated cardiomyopathy—prevalence and genotype‐phenotype correlations. PLoS One 2017; 12: e0169007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keren A, McKenna WJ. Successful launch of a comprehensive european registry for the cardiomyopathies. Eur Heart J 2016; 37: 174–176. [DOI] [PubMed] [Google Scholar]
- 35. Elliott P, Charron P, Blanes JRG, Tavazzi L, Tendera M, Konté M, Laroche C, Maggioni AP, Anastasakis A, Arbustini E, Asselbergs FW, Axelsson A, Brito D, Caforio ALP, Carr‐White G, Czekaj A, Damy T, Devoto E, Favalli V, Findlay I, Garcia‐Pavia P, Hagège A, Heliö T, Iliceto S, Isnard R, Jansweijer JA, Limongelli G, Linhart A, Cuenca DL, Mansencal N. European cardiomyopathy pilot registry: EURObservational research programme of the European society of cardiology. Eur Heart J 2016; 37: 164–173. [DOI] [PubMed] [Google Scholar]
- 36. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L, Alonso A, Ferrari R, Komajda M, Wood D, Manini M, Taylor C, Laroche C, Fiorucci E, Lucci D, Gonzini L. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 37. Hoorn CJGM, Crijns HJGM, Dierick‐Van Daele ATM, Dekker LRC. Review on factors influencing physician guideline adherence in cardiology. Cardiol Rev 2019; 27: 80–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Centre Inclusion Criteria.
Data S2. Supporting information.


