Abstract
Aims
Percutaneous veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) is utilized for patients with cardiogenic shock or cardiac arrest. However, the procedure protocol for weaning from VA‐ECMO has not been well established. The present study aimed to determine the usefulness of echocardiographic and pulmonary artery catheter parameters for predicting successful weaning from VA‐ECMO in patients with refractory cardiogenic shock.
Methods and results
We retrospectively studied 50 patients who were hospitalized and supported by VA‐ECMO for >48 h between January 2013 and March 2017. Patients successfully weaned from VA‐ECMO without reintroduction of VA‐ECMO or left ventricular assist device implantation were defined as 30 day survivors. Echocardiographic and pulmonary artery catheter parameters were evaluated when ECMO flow was limited to a maximum of 1.5–2.0 L/min. Twenty‐four patients were successfully weaned from VA‐ECMO, whereas 26 were not. Fractional shortening, corrected left ventricular ejection time (LVETc, defined as LVET divided by the square root of heart rate), left ventricular outflow tract velocity time integral, and LVETc divided by pulmonary artery wedge pressure (PAWP) were significantly larger in the 30 day survivor groups. Multivariable analysis revealed LVETc∕PAWP as a significant independent predictor of successful weaning (LVETc∕PAWP, odds ratio 0.82, 95% confidence interval 0.71–0.94, P = 0.005). Receiver operating characteristic curve analysis revealed 15.9 as the optimal LVETc∕PAWP for predicting successful weaning (area under the curve 0.82).
Conclusions
The present findings indicate that LVETc∕PAWP is a potential predictor of successful weaning from VA‐ECMO.
Keywords: Veno‐arterial extracorporeal membrane oxygenation weaning, Mechanical circulatory support, Refractory cardiogenic shock, Echocardiography, Pulmonary artery catheter
Introduction
The survival rate of patients with refractory cardiogenic shock (CS) remains low and is reported as 50–60% in 30 day CS survivors. 1 , 2 Recent studies have shown that percutaneous veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) is effective in selected patients for improving survival and can act as a bridge to recovery, heart transplantation, or left ventricular (LV) assist device (LVAD) implantation. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
Although assessment of cardiac function is necessary for the management of advanced heart failure, and haemodynamic assessment with echocardiography and pulmonary artery catheter (PAC) may be considered for patients with CS supported by mechanical circulatory support (MCS) devices, few studies have investigated which haemodynamic parameters can predict successful weaning from VA‐ECMO. 2 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24
Several studies have reported the following echocardiography and PAC parameters as tools available for the management of advanced heart failure with MCS devices: LV ejection time (LVET), LVET divided by left atrial pressure (LVET∕LAP) for patients with LVAD, and LV outflow tract velocity time integral (VTI) for patients with VA‐ECMO. 13 , 16 , 22 , 23
Therefore, the present study aimed to investigate whether the echocardiographic and PAC parameters of LVET, LVET divided by pulmonary artery wedge pressure (LVET∕PAWP), and VTI are useful for the assessment of cardiac function in patients with VA‐ECMO and whether they are predictors of successful weaning from VA‐ECMO.
Methods
Design
This retrospective cohort study was conducted at the National Cerebral and Cardiovascular Center, Osaka, Japan, and conformed to the principles of the Declaration of Helsinki. The study was approved by the National Cardiovascular Center Ethics Committee (No. M29‐156‐2).
Enrolled in the study were 84 consecutive patients with refractory CS or cardiac arrest who were supported by VA‐ECMO between January 2013 and March 2017. The exclusion criteria were cardiac arrest of non‐cardiac origin, an absence of echocardiographic data, an absence of PAC data including pulmonary artery wedge pressure (PAWP) values, severe aortic stenosis, severe aortic regurgitation, aortic valve replacement, and VA‐ECMO support within the past 48 h. A final total of 50 patients were selected (Figure 1 ).
Figure 1.
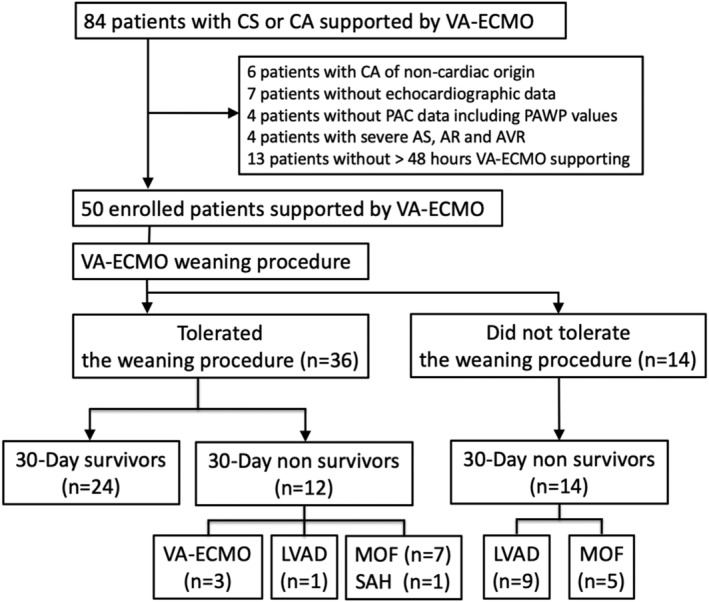
Flow chart showing patient enrolment and clinical outcomes following veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) support. AR, aortic regurgitation; AS, aortic stenosis; AVR, aortic valve replacement; CA, cardiac arrest; CS, cardiogenic shock; LVAD, left ventricular assist device; MOF, multiple organ failure; PAC, pulmonary artery catheter; PAWP, pulmonary artery wedge pressure; SAH, subarachnoid haemorrhage.
Echocardiographic parameters
All echocardiographic examinations were performed at least every 24 h using a Vivid Q cardiovascular ultrasound system (GE Healthcare Life Sciences, Piscataway, NJ). Standard two‐dimensional echocardiographic parameters were measured according to the recommendation of the American Society of Echocardiography. The duration of aortic valve opening, or LV ejection time (LVET), was measured by M‐mode in parasternal long‐axis view. LVET is related to heart rate, preload, LV systolic function, and afterload. Therefore, corrected LVET (LVETc) is defined as LVET divided by the square root of the RR interval, which is used to correct the QT interval of an electrocardiogram (a modification of Bazett's equation). VTI was measured by traced LVOT pulsed wave Doppler in standard apical three‐chamber or five‐chamber views of the left ventricle. All echocardiographic measurements were performed by experienced cardiologists or sonographers and confirmed by at least two experienced cardiologists and were taken as the average of three consecutive cycles for patients with sinus rhythm and as the average of seven consecutive cycles for patients with atrial fibrillation.
Pulmonary artery catheter parameters
A 7.5 Fr Swan‐Ganz thermodilution catheter (Model 744F75; Edwards Lifesciences, Irvine, CA) was inserted through the internal jugular vein or the femoral vein, and the following parameters were measured at least every 24 h: PAWP, pulmonary artery systolic pressure, pulmonary artery diastolic pressure, mean pulmonary artery pressure, and mean right atrial pressure (RAP). Mixed venous oxygen saturation (SVO2) via PAC was collected at least every 24 h. All PAC measurements were made by at least two experienced cardiologists.
End‐organ function parameters
The following blood samples were collected at least every 24 h; arterial blood gas analyses (pH, bicarbonate, and lactate levels), inflammation indexes (white blood cell count and C‐reactive protein levels), liver function (total bilirubin, aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels), renal function (blood urea nitrogen and serum creatinine levels), and sequential organ failure assessment score was calculated.
Veno‐arterial extracorporeal membrane oxygenation device and management
A percutaneous VA‐ECMO system is basically a femoro‐femoral bypass without a reservoir. This completely preconnected system is a compact integrated cardiopulmonary bypass unit comprising an artificial lung (Biocube TNC coating 6000, NIPRO, Osaka, Japan) and a centrifugal blood pump (Rotaflow, Maquet Getinge Group, Rastatt, Germany, or Gyropump, Medtronic, Inc., Minneapolis, MN).
Veno‐arterial extracorporeal membrane oxygenation weaning procedure
A VA‐ECMO weaning procedure was performed when the patient was judged to have recovered from myocardial damage and gained haemodynamic stability as reported previously. 25 Haemodynamic status was determined by at least two attending physicians using parameters of cardiac function and circulatory failure such as mean arterial blood pressure (MBP), heart rate, LV end‐diastolic diameter, LV end‐systolic diameter, fractional shortening (FS), LVETc, VTI, PAC parameters, SVO2, lactate level, and renal and liver function. After the patient was considered haemodynamically stable, the VA‐ECMO flow rate was decreased to 1.5–2.0 L/min and then to 0.5–1.0 L/min. If the haemodynamic status was unstable, that is, MBP < 60 mmHg, VA‐ECMO flow was returned to the initial rate and the procedure was stopped. VA‐ECMO removal was considered after the patient's cardiac function was partially or fully recovered and if the VA‐ECMO weaning procedure had been tolerated. The VA‐ECMO was removed surgically if the patient remained stable after complete‐circuit clamping. When VA‐ECMO weaning was deemed impossible, bridging to LVAD was considered.
We evaluated the echocardiographic and PAC parameters when VA‐ECMO flow was limited to a maximum of 1.5–2.0 L/min. If VA‐ECMO flow could not be reduced to this level, the most improved values were used for the analysis. All other parameters were measured at this time.
Outcome variables
Patients who underwent the VA‐ECMO weaning procedure were divided into two groups: 30 day survivor groups who were successfully weaned from VA‐ECMO and non‐survivor groups who were not weaned. We defined successful weaning as 30 day survival without reintroduction of VA‐ECMO or LVAD implantation due to recurring refractory CS or cardiac arrest. The outcome variables included 30 day survival, 3 day survival, duration of VA‐ECMO, and VA‐ECMO‐associated complications (e.g. major bleeding that required blood transfusions, limb ischaemia, stroke, and intracranial haemorrhage).
Statistical analysis
Statistical analyses were conducted using JMP 12.2 (SAS Institute, Cary, NC), Stata Version 15 (StataCorp LP, College Station, TX), and R Version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). All P values <0.05 were considered to indicate statistical significance. Data are expressed as mean ± standard deviation if normally distributed, or median (25th–75th percentiles) otherwise. For intergroup comparison of continuous variables, t‐test was used for normally distributed data, and Wilcoxon rank‐sum test was used otherwise. Nominal variables were compared using χ 2 test or Fisher's exact test. The association of the successful VA‐ECMO weaning with patients' baseline characteristics and haemodynamic and end‐organ function parameters obtained prior to VA‐ECMO weaning were evaluated. Using the variables showing P < 0.05 in the bivariable analysis including age and sex as pre‐specified covariate, multivariable logistic regression analysis by backward stepwise selection with a P value ≥0.05 for removal of variables was conducted to identify predictors of successful VA‐ECMO weaning. The ability of each variable to discriminate between the two groups was assessed using the area under the receiver operating characteristic curve (AUC) with their 95% confidence interval. AUC values of ≥0.8, 0.7–0.8, and <0.7 were considered good, moderate, and poor, respectively.
Results
Baseline characteristics
A total of 84 consecutive patients who were hospitalized and supported by VA‐ECMO between January 2013 and March 2017 were originally enrolled. After applying the exclusion criteria, 50 patients were admitted to the study. The following were present in these 50 patients: acute myocardial infarction (n = 22), prior myocardial infarction (n = 5), fulminant myocarditis (n = 12), end‐stage hypertrophic cardiomyopathy (n = 3), end‐stage dilated cardiomyopathy (n = 2), and other types of heart disease (n = 6). Table 1 lists the baseline characteristics of the 50 patients.
Table 1.
Comparisons of patients' baseline characteristics between the 30 day survivor and non‐survivor groups
| Overall (n = 50) | 30 day survivors (n = 24) | Non‐survivors (n = 26) | P value | |
|---|---|---|---|---|
| Age (years) | 67 (51–77) | 76 (54–82) | 64 (46–73) | 0.016 |
| Sex (male), n (%) | 37 (74) | 20 (83) | 17 (65) | 0.20 |
| Height (cm) | 165 (160–170) | 163 (160–171) | 165 (162–168) | 0.79 |
| Body weight (kg) | 61 (55–70) | 66 (56–72) | 58 (51–65) | 0.089 |
| BMI, n (%) | 22.9 (20.7–26.3) | 23.4 (21.0–26.8) | 22.9 (19.9–25.9) | 0.33 |
| Heart disease | 0.19 | |||
| Acute myocardial infarction, n (%) | 22 (44) | 12 (50) | 10 (38) | |
| Prior myocardial infarction, n (%) | 5 (10) | 3 (13) | 2 (8) | |
| Fulminant myocarditis, n (%) | 12 (24) | 5 (21) | 7 (27) | |
| Hypertrophic cardiomyopathy, n (%) | 3 (6) | 1 (4) | 2 (8) | |
| Dilated cardiomyopathy, n (%) | 2 (4) | 1 (4) | 1 (4) | |
| Other, n (%) | 6 (12) | 2 (8) | 4 (15) | |
| Indication for ECMO | ||||
| Cardiogenic shock, n (%) | 23 (46) | 14 (58) | 9 (35) | 0.16 |
| OHCA, n (%) | 6 (12) | 3 (13) | 3 (12) | 1.0 |
| IHCA, n (%) | 21 (42) | 7 (29) | 14 (54) | 0.093 |
| PCI, n (%) | 21 (42) | 11 (46) | 10 (38) | 0.77 |
| Catheter ablation, n (%) | 1 (2) | 0 (0) | 1 (4) | 1.0 |
| Other catheterization procedures, n (%) | 1 (2) | 1 (4) | 0 (0) | 0.48 |
| Cardiac surgery, n (%) | 2 (4) | 1 (4) | 1 (4) | 1.0 |
| Femoral ECMO, n (%) | 50 (100) | 24 (100) | 26 (100) | — |
| Bypass to peripheral FA, n (%) | 14 (28) | 6 (25) | 8 (31) | 0.76 |
| Mechanical ventilation, n (%) | 50 (100) | 24 (100) | 26 (100) | — |
| CRRT, n (%) | 20 (40) | 10 (42) | 10 (38) | 1.0 |
BMI, body mass index; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; FA, femoral artery; IHCA, in‐hospital cardiac arrest; OHCA, out‐of‐hospital cardiac arrest; PCI, percutaneous coronary intervention.
Patient outcomes
Figure 1 shows the patients' clinical outcomes. Among the 50 patients who received VA‐ECMO, 24 were successfully weaned from VA‐ECMO and 26 were not. Of the 26 patients who were not weaned, 14 were convincingly VA‐ECMO dependent and did not tolerate the VA‐ECMO weaning procedure (five patients died of multiple organ failure, and nine patients required implantation of LVAD). Of the other 12 patients who were not weaned and in whom the VA‐ECMO was removed, seven died of multiple organ failure, one died of subarachnoid haemorrhage, three required reintroduction of VA‐ECMO, and one required implantation of LVAD in the following 30 days. Thirty‐one patients survived more than 3 days after having the VA‐ECMO removed. There was no significant difference in terms of major complications associated with VA‐ECMO support. Median total VA‐ECMO duration was 4.5 days (inter‐quartile range 2.3–6.3).
Table 1 lists the comparison of patients' baseline characteristics between two groups. There was significant difference between the two groups in terms of age (P = 0.016) but not for the other baseline characteristics between the two groups.
Haemodynamic and end‐organ function parameters
Echocardiographic, PAC, other haemodynamic parameters, and end‐organ function parameters were evaluated when VA‐ECMO flow was limited to a maximum of 1.5–2.0 L/min during the 24 h preceding VA‐ECMO weaning.
Table 2 lists the haemodynamic parameters in the two groups. There was no significant difference between the groups in terms of mean blood pressure or VA‐ECMO flow. Under these conditions, FS (P = 0.003), VTI (P < 0.001), LVET (P = 0.004), LVETc (P < 0.001), and LVETc divided by PAWP (LVETc∕PAWP) (P < 0.001) were significantly larger and PAWP (P = 0.049) was significantly lower in the 30 day survivor groups than in the non‐survivor groups. There was no significant difference in terms of an intra‐aortic balloon pump use, vasoactive inotropic score, blood pressure, heart rate, pulse pressure, LV end‐diastolic diameter, LV end‐systolic diameter, mean pulmonary artery pressure, RAP, or SVO2. 26
Table 2.
Comparison of haemodynamic parameters obtained prior to VA‐ECMO weaning between the 30 day survivor and non‐survivor groups
| Overall (n = 50) | 30 day survivors (n = 24) | Non‐survivors (n = 26) | P value | |
|---|---|---|---|---|
| VA‐ECMO flow (L/min) | 1.9 (1.7–2.8) | 1.8 (1.7–2.0) | 2.0 (1.7–3.2) | 0.11 |
| VA‐ECMO duration (days) | 3.9 (2.2–5.8) | 3.9 (2.1–5.8) | 3.9 (2.2–6.0) | 0.95 |
| Total VA‐ECMO duration (days) | 4.5 (2.3–6.3) | 4.0 (2.2–5.9) | 4.8 (2.3–6.9) | 0.56 |
| IABP, n (%) | 48 (96) | 22 (92) | 26 (100) | 0.23 |
| Vasoactive inotropic score a | 7.1 (5.0–13.6) | 7.0 (5.0–13.7) | 8.1 (4.8–15.2) | 0.94 |
| Systolic BP (mmHg) | 104 (91–124) | 114 (93–127) | 100 (87–122) | 0.24 |
| Diastolic BP (mmHg) | 50 (47–65) | 54 (46–69) | 50 (47–56) | 0.47 |
| Mean BP (mmHg) | 70 (65–81) | 75 (65–85) | 69 (65–79) | 0.24 |
| Pulse pressure (mmHg) | 47 (39–66) | 50 (39–80) | 45 (38–58) | 0.44 |
| Heart rate (/min) | 82 (75–95) | 83 (80–94) | 82 (73–97) | 0.34 |
| Echocardiographic parameters | ||||
| LVDd (mm) | 49 (44–58) | 50 (44–55) | 48 (42–62) | 0.85 |
| LVDs (mm) | 43 (35–50) | 42 (36–49) | 44 (34–58) | 0.28 |
| FS (%) | 15 (7–19) | 18 (12–22) | 11 (6–16) | 0.003 |
| VTI (cm) | 9.0 (6.7–11.4) | 10.8 (9.0–12.1) | 7.3 (4.6–8.5) | <0.001 |
| LVET (ms) | 209 (170–250) | 237 (200–266) | 185 (140–222) | 0.004 |
| LVETc (ms) | 240 (198–282) | 265 (225–308) | 208 (159–251) | <0.001 |
| PA catheter parameters | ||||
| PAWP (mmHg) | 15 (10–17) | 14 (9–16) | 16 (13–18) | 0.049 |
| Mean PAP (mmHg) | 20 (16–23) | 20 (15–25) | 20 (16–23) | 0.94 |
| RAP (mmHg) | 9 (6–12) | 9 (6–11) | 10 (6–12) | 0.97 |
| Other haemodynamic parameters | ||||
| SVO2 (%) | 72 (63–78) | 76 (64–79) | 67 (61–74) | 0.072 |
| LVETc∕PAWP (ms/mmHg) | 16.8 (12.4–24.1) | 22.0 (16.5–28.7) | 12.9 (9.9–17.5) | <0.001 |
BP, blood pressure; FS, fractional shortening; IABP, intra‐aortic balloon pump; LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; LVET, left ventricular ejection time; LVETc, corrected left ventricular ejection time; PA, pulmonary artery; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; RAP, right atrial pressure; SVO2, mixed venous oxygen saturation; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation; VTI, left ventricular outflow tract velocity time integral.
Vasoactive inotropic score was calculated using the following formula: dopamine (μg/kg/min) + dobutamine (μg/kg/min) + 100 × norepinephrine (μg/kg/min) + 10 × phosphodiesterase inhibitor (μg/kg/min) + 10 000 × vasopressin (U/kg/min).
Table 3 shows the end‐organ function parameters in the two groups. Lactate dehydrogenase (P = 0.020) was significantly lower in the 30 day survivor groups than in the non‐survivor groups. There was no significant difference in terms of the other end‐organ function parameters.
Table 3.
Comparison of end‐organ function parameters obtained prior to VA‐ECMO weaning between the 30 day survivor and non‐survivor groups
| Overall (n = 50) | 30 day survivors (n = 24) | Non‐survivors (n = 26) | P value | |
|---|---|---|---|---|
| SOFA score | 11.0 (8.0–13.0) | 9.0 (7.0–12.0) | 11.0 (9.5–13.5) | 0.091 |
| ABG analysis | ||||
| pH | 7.47 (7.42–7.50) | 7.46 (7.43–7.50) | 7.47 (7.37–7.51) | 0.79 |
| Bicarbonate (mmol/L) | 23.9 (22.9–26.3) | 23.7 (22.0–25.9) | 24.6 (23.0–26.9) | 0.51 |
| Lactate (mg/dL) | 11.7 (9.6–17.6) | 11.2 (9.0–16.0) | 14.3 (10.1–21.7) | 0.073 |
| Laboratory data | ||||
| Total bilirubin (mg/dL) | 1.8 (1.0–4.4) | 1.3 (0.8–3.0) | 2.2 (1.2–5.1) | 0.13 |
| Aspartate aminotransferase (IU/L) | 107 (70–246) | 107 (59–192) | 106 (72–267) | 0.57 |
| Alanine aminotransferase (IU/L) | 50 (23–138) | 41 (21–250) | 56 (27–110) | 0.57 |
| Lactate dehydrogenase (IU/L) | 944 (576–1338) | 687 (464–1023) | 1047 (682–1562) | 0.020 |
| Creatine phosphokinase (IU/L) | 735 (398–2108) | 736 (362–2219) | 728 (359–2389) | 0.83 |
| Blood urine nitrogen (mg/dL) | 34 (21–46) | 31 (19–49) | 34 (25–45) | 0.70 |
| Serum creatinine (mg/dL) | 1.38 (0.82–2.28) | 0.90 (0.76–2.82) | 1.49 (0.88–2.19) | 0.78 |
| White blood cell count (/μL) | 9000 (6500–12 650) | 7000 (6075–11 950) | 11 000 (7125–13 700) | 0.079 |
| C‐reactive protein (mg/dL) | 11.2 (6.8–16.0) | 9.7 (6.3–14.4) | 12.0 (7.2–20.8) | 0.24 |
ABG, arterial blood gas; SOFA, sequential organ failure assessment; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Potential parameters for predicting 30 day survivors
Table 4 lists the potential parameters for predicting 30 day survival. Multivariable analysis revealed LVETc∕PAWP (P = 0.005) as a significant independent predictor of successful weaning.
Table 4.
Potential parameters for predicting 30 day survival: multivariable logistic regression analysis
| Multivariable analysis | |||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Age (years) | — | — | — |
| Sex (male) | — | — | — |
| FS (%) | — | — | — |
| VTI (cm) | — | — | — |
| LVETc (ms) | — | — | — |
| LVETc∕PAWP (ms/mmHg) | 0.82 | 0.71–0.94 | 0.005 |
| Lactate dehydrogenase (IU/L) | — | — | — |
CI, confidence interval; FS, fractional shortening; LVETc, corrected left ventricular ejection time; PAWP, pulmonary artery wedge pressure; VTI, left ventricular outflow tract velocity time integral.
Haemodynamic and end‐organ function variables that showed good or moderate discrimination for successful weaning were LVETc∕PAWP (AUC 0.82), VTI (AUC 0.80), LVETc (AUC 0.79), and FS (AUC 0.74). The highest AUC value was found for LVETc∕PAWP. The optimal cut‐off point for LVETc∕PAWP was identified; a threshold of 15.9 showed sensitivity of 88% and specificity of 69% for predicting successful weaning. Figure 2 shows the discriminate abilities of the variables, quantified as AUC values, and the receiver operating characteristic curves.
Figure 2.
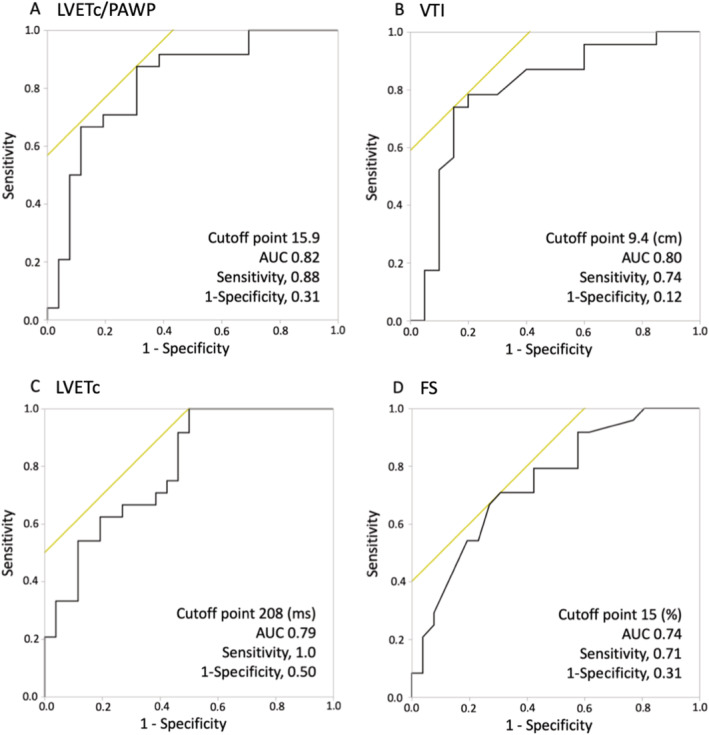
Comparison of the area under the receiver operating characteristic curve (AUC) values for the haemodynamic parameters and the receiver operating characteristic curves with (A) corrected left ventricular ejection time divided by pulmonary artery wedge pressure (LVETc∕PAWP), (B) left ventricular outflow tract velocity time integral (VTI), (C) corrected left ventricular ejection time (LVETc), and (D) fractional shortening (FS). The highest AUC value was found for (A) LVETc∕PAWP. The optimal cut‐off point for LVETc∕PAWP was identified; a threshold of 15.9 showed sensitivity of 88% and specificity of 69% for predicting successful weaning.
Figure 3 shows a scatter plot of PAWP vs. LVETc values that were measured when VA‐ECMO flow was limited to a maximum of 1.5–2.0 L/min prior to the weaning procedure. The patients were divided into four subsets based on thresholds of 208 ms for LVETc and 15 mmHg for PAWP, which were the optimal cut‐off points. These thresholds (LVETc ≥ 208 ms and PAWP ≤ 15 mmHg) showed sensitivity of 75% and specificity of 81% for predicting successful weaning.
Figure 3.
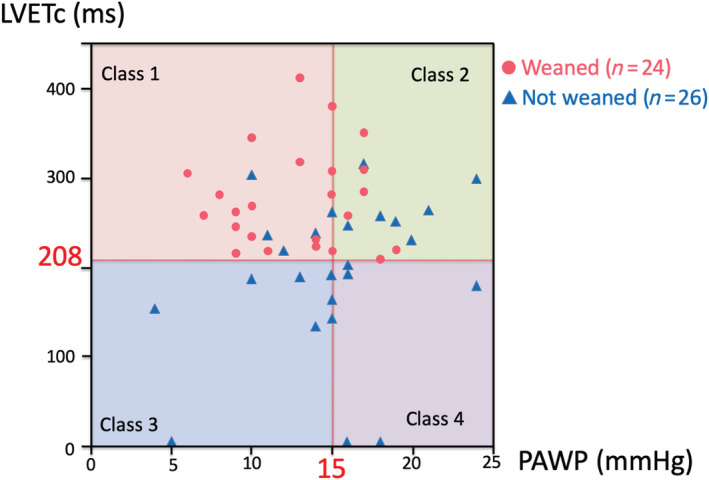
Four subsets of haemodynamic classification of patients supported by veno‐arterial extracorporeal membrane oxygenation. The patients were divided according to corrected left ventricular ejection time (LVETc) and pulmonary artery wedge pressure (PAWP) values measured prior to the weaning procedure, using thresholds of 208 ms for LVETc and 15.0 mmHg for PAWP, which had been determined as optimal cut‐off points.
Discussion
In this observational study, 48% of the present patients were successfully weaned from VA‐ECMO. Multivariable analysis revealed LVETc∕PAWP as a single significant independent predictor of successful weaning.
Comparison with previous studies
Few studies have investigated the optimal ways for VA‐ECMO weaning procedure or reported the haemodynamic parameters that predict successful weaning from VA‐ECMO.
In an observational study of 51 patients, Aissaoui et al. reported that echocardiographic parameters used for assessing systolic LV performance, such as VTI, LVEF, and lateral mitral annulus peak systolic velocity, were associated with successful weaning. 13 , 16 In a study of 10 patients, Aziz et al. recommended the following haemodynamic criteria for deciding the timing of weaning from a VA‐ECMO pump: cardiac index >2.4 L/min/m2, MBP > 60 mmHg, PAWP < 18 mmHg, and central venous pressure <18 mmHg. 24 In a study of 22 patients, Nakatani et al. and Aoyama et al. reported the utility of LVETc∕LAP for assessing cardiac function and predicting cardiac function recovery in patients with LVAD. 22 , 23 In addition, recent studies have supported the usefulness of PAC indexes as an indicator of improved survival in patients with CS and percutaneous LVAD. 24 , 25
These results suggest that in the management of patients with CS, it is of critical importance to perform haemodynamic evaluation of cardiac output and pulmonary congestion using the parameters of PAC as well as those of echocardiography. However, there remain unanswered questions regarding the clinical utility of these parameters of haemodynamic evaluation for predicting successful weaning from VA‐ECMO. Therefore, the present study investigated and showed that usefulness.
Possible explanations and implications of the present study
The present findings revealed that the parameter LVETc∕PAWP could potentially predict the success of weaning from VA‐ECMO in patients with refractory CS. Possible explanations for its suitability are as follows.
First, LVETc∕PAWP is a haemodynamic parameter that is based on both cardiac output and pulmonary congestion. Several studies have reported that LVETc correlated with stroke volume and cardiac output. 27 , 28 , 29 , 30 , 31 , 32 Because high PAWP values generally correlate with severe pulmonary congestion, PAWP is considered as an objective and reliable method for its assessment. As haemodynamic evaluation based on cardiac output and pulmonary congestion is important for the management of advanced heart failure, LVETc∕PAWP might be a useful parameter for predicting successful weaning from VA‐ECMO in these patients.
Second, we considered that LVETc should be corrected for LV preload, that is, PAWP due to the following reasons. As is the case for cardiac output, LVETc depends on both preload and afterload besides systolic LV performance. As preload and afterload may be strongly influenced by the VA‐ECMO flow rate and the native cardiovascular status, we compared preload and afterload between two groups. In the present study, PAWP showed significant difference, but MBP (indicator of afterload) did not differ significantly between the groups. For these reasons, we corrected LVETc by PAWP for an index to predict successful weaning.
Third, LVETc∕PAWP is a simple haemodynamic parameter with high reproducibility (Supporting Information, Table S1 ). It is easier to obtain adequate and complete visualization of LVET than other haemodynamic parameters with transthoracic echocardiography because it can be measured despite the restrictions on the position of the transducer experienced in patients with MCS devices.
Clinical relevance of haemodynamic classification by corrected left ventricular ejection time and pulmonary artery wedge pressure
To further investigate the relationship between LVETc and PAWP measured prior to the weaning procedure, we divided the patients into four subsets according to the thresholds of 208 ms for LVETc and 15 mmHg for PAWP, which were determined as the optimal cut‐off points. In the scatter plot in Figure 3 , the patients in Class 1 (LVETc ≥ 208 ms and PAWP ≤ 15 mmHg) had no pulmonary congestion or peripheral hypoperfusion and a low in‐hospital mortality following VA‐ECMO weaning. The successful weaning rate in this class was ~78% (18/23 patients). The patients in Class 2 (LVETc ≥ 208 ms and PAWP > 15 mmHg) had pulmonary congestion but no peripheral hypoperfusion. The successful weaning rate in this class was ~43% (6/14 patients). Among the eight patients who did not survive more than 30 days, five survived more than 3 days after withdrawal of VA‐ECMO. This finding indicates that in some patients of this class, more aggressive approaches for myocardial recovery other than medications, catheter intervention, and cardiac surgery could be considered; these could include LVAD implantation and heart transplantation. The patients in Class 3 (LVETc < 208 ms and PAWP ≤ 15 mmHg) had peripheral hypoperfusion but no pulmonary congestion, and none of these patients were weaned. Among the eight patients in this class, three tended to have relatively low PAWP along with high RAP, which suggests that some patients in this class might be complicated by severe right heart failure and PAC may be useful for evaluating right heart failure and the right ventricular–LV relationship. In some patients in this class, right ventricular assist device implantation may be considered as well as LVAD implantation. The patients in Class 4 (LVETc < 208 ms and PAWP > 15 mmHg) had peripheral hypoperfusion and pulmonary congestion, and none of these patients were weaned. Accordingly, LVAD implantation and heart transplantation should be considered in these patients.
Furthermore, the management of ventricular loading and pulmonary congestion during the VA‐ECMO support is essentially important. The four class subsets with PAC values may potentially extract which subsets successfully wean and require additional LV and/or right ventricular unloading strategies for the recovery of myocardial function in patients with VA‐ECMO support.
Study limitations
This study had the following potential limitations. First, it was a retrospective observational study with a relatively small population conducted in a single centre. Second, in each case, the attending physicians made the final decision to remove the patient's VA‐ECMO based on their haemodynamic status. Third, the evaluation for weaning from VA‐ECMO was performed with measurements obtained when ECMO flow was limited to 1.5–2.0 L/min. It may be difficult to evaluate the parameters promptly and accurately under the condition of flow reduced to <1.5 L/min after partial circuit clamping. Fourth, the PAC providing simultaneous monitoring may be beneficial to detect relative haemodynamic changes in the VA‐ECMO setting. However, further studies are needed to determine whether the PAC measurements are reliable in central VA‐ECMO support. Fifth, although the tissue Doppler imaging and the echocardiographic assessment of right ventricular function and the right ventricular–LV relationship are reported to be useful for the successful weaning, those data are not available in the present study. 13 , 16 , 17 , 18 , 19 Lastly, it should be mentioned that VA‐ECMO support is characterized by a rise in afterload of the left ventricle, which either further impair or delay cardiac contractility improvement. Percutaneous approaches utilizing unloading devices are becoming an option and require accumulating evidence. 33
Conclusions
In conclusion, the present findings indicate that LVETc∕PAWP is a potential predictor of successful weaning from VA‐ECMO. Further prospective studies are required for validation of the present findings.
Conflict of interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Supporting information
Table S1. Reproducibility of LVETc/PAWP
Sawada, K. , Kawakami, S. , Murata, S. , Nishimura, K. , Tahara, Y. , Hosoda, H. , Nakashima, T. , Kataoka, Y. , Asaumi, Y. , Noguchi, T. , Sugimachi, M. , Fujita, T. , Kobayashi, J. , and Yasuda, S. (2021) Predicting Parameters for Successful Weaning from Veno‐Arterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. ESC Heart Failure, 8: 471–480. 10.1002/ehf2.13097.
References
- 1. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K, IABP‐SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 2. Thiele H, Ohman EM, de Waha S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019; 40: 2671–2683. [DOI] [PubMed] [Google Scholar]
- 3. Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014; 63: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 4. Combes A, Brodie D, Chen YS, Fan E, Henriques JPS, Hodgson C, Lepper PM, Leprince P, Maekawa K, Muller T, Nuding S, Ouweneel DM, Roch A, Schmidt M, Takayama H, Vuylsteke A, Werdan K, Papazian L. The ICM research agenda on extracorporeal life support. Intensive Care Med 2017; 43: 1306–1318. [DOI] [PubMed] [Google Scholar]
- 5. Abrams D, Garan AR, Abdelbary A, Bacchetta M, Bartlett RH, Beck J, Belohlavek J, Chen YS, Fan E, Ferguson ND, Fowles JA, Fraser J, Gong M, Hassan IF, Hodgson C, Hou X, Hryniewicz K, Ichiba S, Jakobleff WA, Lorusso R, MacLaren G, McGuinness S, Mueller T, Park PK, Peek G, Pellegrino V, Price S, Rosenzweig EB, Sakamoto T, Salazar L, Schmidt M, Slutsky AS, Spaulding C, Takayama H, Takeda K, Vuylsteke A, Combes A, Brodie D, International ECMO Network (ECMONet) and The Extracorporeal Life Support Organization (ELSO) . Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med 2018; 44: 717–729. [DOI] [PubMed] [Google Scholar]
- 6. Le Gall A, Follin A, Cholley B, Mantz J, Aissaoui N, Pirracchio R. Veno‐arterial‐ECMO in the intensive care unit: from technical aspects to clinical practice. Anaesth Crit Care Pain Med 2018; 37: 259–268. [DOI] [PubMed] [Google Scholar]
- 7. Mebazaa A, Combes A, van Diepen S, Hollinger A, Katz JN, Landoni G, Hajjar LA, Lassus J, Lebreton G, Montalescot G, Park JJ, Price S, Sionis A, Yannopolos D, Harjola VP, Levy B, Thiele H. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med 2018; 44: 760–773. [DOI] [PubMed] [Google Scholar]
- 8. Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, Mohr FW. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010; 139: 302–311. [DOI] [PubMed] [Google Scholar]
- 9. Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Léger P, Pavie A, Chastre J. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008; 36: 1404–1411. [DOI] [PubMed] [Google Scholar]
- 10. Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, Huang SC, Lin FY, Wang SS. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol 2003; 41: 197–203. [DOI] [PubMed] [Google Scholar]
- 11. Chang WW, Tsai FC, Tsai TY, Chang CH, Jenq CC, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. PLoS One 2012; 7: e42687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aso S, Matsui H, Fushimi K, Yasunaga H. In‐hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care 2016; 20: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aissaoui N, Luyt CE, Leprince P, Trouillet JL, Léger P, Pavie A, Diebold B, Chastre J, Combes A. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 2011; 37: 1738–1745. [DOI] [PubMed] [Google Scholar]
- 14. Cavarocchi NC, Pitcher HT, Yang Q, Karbowski P, Miessau J, Hastings HM, Hirose H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg 2013; 146: 1474–1479. [DOI] [PubMed] [Google Scholar]
- 15. Pappalardo F, Pieri M, Arnaez Corada B, Ajello S, Melisurgo G, De Bonis M, Zangrillo A. Timing and strategy for weaning from venoarterial ECMO are complex issues. J Cardiothorac Vasc Anesth 2015; 29: 906–911. [DOI] [PubMed] [Google Scholar]
- 16. Aissaoui N, El‐Banayosy A, Combes A. How to wean a patient from veno‐arterial extracorporeal membrane oxygenation. Intensive Care Med 2015; 41: 902–905. [DOI] [PubMed] [Google Scholar]
- 17. Huang KC, Lin LY, Chen YS, Lai CH, Hwang JJ, Lin LC. Three‐dimensional echocardiography‐derived right ventricular ejection fraction correlates with success of decannulation and prognosis in patients stabilized by venoarterial extracorporeal life support. J Am Soc Echocardiogr 2018; 31: 169–179. [DOI] [PubMed] [Google Scholar]
- 18. Aissaoui N, Caudron J, Leprince P, Fagon JY, Lebreton G, Combes A, Diebold B. Right–left ventricular interdependence: a promising predictor of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 2017; 43: 592–524. [DOI] [PubMed] [Google Scholar]
- 19. Ortuno S, Delmas C, Diehl JL, Bailleul C, Lancelot A, Naili M, Cholley B, Pirracchio R, Aissaoui N. Weaning from veno‐arterial extra‐corporeal membrane oxygenation: which strategy to use? Ann Cardiothorac Surg 2019; 8: E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Cardiac Fail 2019; 25: 364–371. [DOI] [PubMed] [Google Scholar]
- 21. Nalluri N, Patel NJ, Atti V, Kumar V, Basir MB, O'Neill WW. Temporal trends in utilization of right‐sided heart catheterization among percutaneous ventricular assist device recipients in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol 2018; 122: 2014–2017. [DOI] [PubMed] [Google Scholar]
- 22. Nakatani T, Takano H, Beppu S, Noda H, Taenaka Y, Kumon K, Kito Y, Fujita T, Kawashima Y. Practical assessment of natural heart function using echocardiography in mechanically assisted patients. ASAIO Trans 1991; 37: M420–M421. [PubMed] [Google Scholar]
- 23. Aoyama N, Izumi T, Hiramori K, Isobe M, Kawana M, Hiroe M, Hishida H, Kitaura Y, Imaizumi T, Japanese Investigators of Fulminant Myocarditis . National survey of fulminant myocarditis in Japan: therapeutic guidelines and long‐term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee). Circ J 2002; 66: 133–144. [DOI] [PubMed] [Google Scholar]
- 24. Aziz TA, Singh G, Popjes E, Stephenson E, Mulvey S, Pae W, El‐Banayosy A. Initial experience with CentriMag extracorporal membrane oxygenation for support of critically ill patients with refractory cardiogenic shock. J Heart Lung Transplant 2010; 29: 66–71. [DOI] [PubMed] [Google Scholar]
- 25. Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, Sasako Y, Nakatani T, Nonogi H, Miyazaki S. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J 2005; 26: 2185–2192. [DOI] [PubMed] [Google Scholar]
- 26. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11: 234–238. [DOI] [PubMed] [Google Scholar]
- 27. Braunwald E, Sarnoff SJ, Stainsby WN. Determinants of duration and mean rate of ventricular ejection. Circ Res 1958; 6: 319–325. [DOI] [PubMed] [Google Scholar]
- 28. Weissler AM, Harris LC, White GD. Left ventricular ejection time index in man. J Appl Physiol 1963; 18: 919–923. [DOI] [PubMed] [Google Scholar]
- 29. Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation 1968; 37: 149–159. [DOI] [PubMed] [Google Scholar]
- 30. Lewis RP, Rittogers SE, Froester WF. A critical review of the systolic time intervals. Circulation 1977; 56: 146–158. [DOI] [PubMed] [Google Scholar]
- 31. Denardo SJ, Nandyala R, Freeman GL, Pierce GL, Nichols WW. Pulse wave analysis of the aortic pressure waveform in severe left ventricular systolic dysfunction. Circ Heart Fail 2010; 3: 149–156. [DOI] [PubMed] [Google Scholar]
- 32. Haiden A, Eber B, Weber T. U‐shaped relationship of left ventricular ejection time index and all‐cause mortality. Am J Hypertens 2014; 27: 702–709. [DOI] [PubMed] [Google Scholar]
- 33. Donker DW, Brodie D, Henriques JPS, Broome M. Left ventricular unloading during veno‐arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion 2019; 34: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reproducibility of LVETc/PAWP


