Abstract
Aims
Exercise intolerance is the leading manifestation of heart failure with preserved or mid‐range ejection fraction (HFpEF or HFmrEF), and left atrial (LA) function might contribute to modulating left ventricular filling and pulmonary venous pressures. We aim to assess the association between LA function and maximal exercise capacity in patients with HFpEF or HFmrEF.
Methods and results
Sixty‐five patients, prospectively enrolled in the German HFpEF Registry, were analysed. Inclusion criteria were New York Heart Association functional class ≥ II, left ventricular ejection fraction > 40%, structural heart disease or diastolic dysfunction, and elevated levels of N terminal pro brain natriuretic peptide (NT‐proBNP). LA function was evaluated through speckle‐tracking echocardiography by central reading in the Charité Academic Echocardiography core lab. All patients underwent maximal cardiopulmonary exercise test and were classified according to a peak VO2 cut‐off of prognostic value (14 mL/kg/min). NT‐pro‐BNP was measured. Twenty‐nine patients (45%) reached a peak VO2 < 14 mL/kg/min (mean value 12.4 ± 1.5) and 36 patients (55%) peak VO2 ≥ 14 mL/kg/min (mean value 19.4 ± 3.9). There was no significant difference in left ventricular ejection fraction (60 ± 9 vs. 59 ± 8%), left ventricular mass (109 ± 23 vs. 112 ± 32 g/m2), LA volume index (45 ± 17 vs. 47 ± 22 mL/m2), or E/e´ (13.1 ± 4.7 vs. 13.0 ± 6.0) between these groups. In contrast, all LA strain measures were impaired in patients with lower peak VO2 (reservoir strain 14 ± 5 vs. 21 ± 9%, P = 0.002; conduit strain 9 ± 2 vs. 13 ± 4%, P = 0.001; contractile strain 7 ± 4 vs. 11 ± 6%, P = 0.02; reported lower limits of normality for LA reservoir, conduit and contractile strains: 26.1%, 12.0%, and 7.7%). In linear regression analysis, lower values of LA reservoir strain were associated with impaired peak VO2 after adjustment for age, sex, body mass index, heart rhythm (sinus/AFib), and log‐NTproBNP [β 0.29, 95% confidence interval (CI) 0.02–0.30, P = 0.02], with an odds ratio 1.22 (95% CI 1.05–1.42, P = 0.01) for peak VO2 < 14 mL/kg/min for LA reservoir strain decrease after adjustment for these five covariates. Adding left ventricular ejection fraction, it did not influence the results. On the other hand, the addition of LA strain to the adjustment parameters alone described above provided a significant increase of the predictive value for lower peak VO2 values (R 2 0.50 vs. 0.45, P = 0.02). With receiver operating characteristic curve analysis, we identified LA reservoir strain < 22% to have 93% sensitivity and 49% specificity in predicting peak VO2 < 14 mL/kg/min. Using this cut‐off, LA reservoir strain < 22% was associated with peak VO2 < 14 mL/kg/min in logistic regression analysis after comprehensive adjustment for age, sex, body mass index, heart rhythm, and log‐NTproBNP [odds ratio 95% CI 10.4 (1.4–74), P = 0.02].
Conclusions
In this HFpEF and HFmrEF cohort, a reduction in LA reservoir strain was a sensible marker of decreased peak exercise capacity. Therefore, LA reservoir strain might be of clinical value in predicting exercise capacity in patients with HFpEF or HFmrEF.
Keywords: Left atrial strain, Cardiopulmonary exercise test, Heart failure, Preserved ejection fraction, Mid‐range ejection fraction
Introduction
Heart failure (HF) with preserved and mid‐range ejection fraction (HFpEF, HFmrEF) accounted for more than half of patients with HF syndrome and are associated with high morbidity and mortality. 1 , 2 Exercise intolerance, objectivized by means of peak oxygen consumption, is the leading manifestation, but the underlying pathophysiology is still uncertain.
Several cardiac and peripheral mechanisms are involved in determining a reduced exercise capacity, and an exaggerated increase in pulmonary capillary wedge pressure (PCWP) with exercise is one of the most consistent. 3 However, neither echocardiographic parameters of diastolic function nor neurohumoral activation were independently associated with exercise capacity in clinically stable HFpEF patients. 4 Also, single echocardiographic variables at rest cannot reliably estimate cardiac pressure when left ventricular ejection fraction (LVEF) is >40%, so an integrated approach evidencing structural or functional alterations is suggested in the effort to diagnose HFpEF and HFmrEF. 5 Interestingly, the left atrium (LA) contributes to modulate left ventricular (LV) filling pressure through its reservoir, conduit, and booster functions both at rest and with exercise. In particular, LA compliance allows LA volume to increase during the reservoir phase without an increase in filling pressure and at the same time providing an adequate LV filling during diastole through a preload mechanism.
The ideal method to evaluate LA function is by invasively determined LA pressure–volume curves. Speckle‐tracking echocardiography (STE) is a promising non‐invasive semi‐automated less load‐dependent method to measure LA function and indirectly estimate LV filling pressure. In fact, a negative correlation was found between impaired LA strain and invasively measured LV end‐diastolic pressure and PCWP. 6 , 7 , 8
In patients with preserved LVEF, first evidences showed that LA strain was associated with exercise capacity expressed as estimated metabolic equivalent among patients with clinical indication to perform an exercise test 9 and haemodynamic and cardiopulmonary exercise test (CPET) variables, especially in younger patients with less comorbidities, in an HFpEF population. 10 In line with these findings, we aimed to assess the value of LA functional remodelling in predicting exercise intolerance in a cohort of HFpEF or HFmrEF patients above traditional echocardiographic parameters.
Methods
Study population
Between August 2016 and December 2019, consecutive patients with a diagnosis of HFpEF or HFmrEF evaluated in an outpatients setting or during hospitalization for HF at the Charité University Hospital were prospectively enrolled in the German HFpEF Registry. Inclusion criteria of the German HFpEF Registry were (1) LVEF > 40% (for patients with LVEF 40–50%, irrespective of a previous history of HFpEF with subsequent LVEF impairment < 50%); (2) age ≥ 18 years; (3) New York Heart Association (NYHA) functional class ≥ II; (4) elevated levels of natriuretic peptides [N terminal pro brain natriuretic peptide (NT‐proBNP) > 125 pg/mL]; and (5) at least one additional criterion for structural heart disease or diastolic dysfunction (LV mass index ≥ 115 g/m2 for men and ≥95 g/m2 for women; LA volume index > 34 mL/m2; mean E/e´ ≥ 13 and mean E´ < 9 cm/s). 11 Registry exclusion criteria were acute coronary syndrome or cardiac surgery/percutaneous intervention during the past 3 months, haemodynamic relevant pericardial disease, and severe kidney disease. For the present study, we applied more restrictive exclusion criteria. Patients enrolled in the registry were excluded from the final study population in presence of further conditions, which could affect the cardiopulmonary haemodynamic or bias the evaluation of atrial and/or ventricular function, that is if they had more than moderate valve disease, significant mitral annular calcification, congenital heart disease, previous cardiac transplantation, restrictive cardiomyopathy, severe chronic obstructive pulmonary disease, severe kidney disease (estimated GFRMDRD ≤ 30 mL/min/1.73 m2 or requiring dialysis), or severe liver disease (Child–Pugh class B and C or with indication for liver transplantation). Moreover, patients were excluded if they could not perform echocardiography in stable condition or could not perform a maximal exercise testing. The study complies with the Declaration of Helsinki. The Ethics Committee of Charité University Hospital approved the research project and written informed consent was obtained from all subjects.
Clinical characteristics
The following data were collected in all study participants: demographics, body mass index (BMI), cardiovascular risk factors, comorbidities, clinical history, NYHA functional class, and medications. An electrocardiogram was performed at the time of the first clinical evaluation after enrolment. Blood samples were collected for laboratory testing, including haemoglobin, creatinine, HbA1c, hs‐RP, and NT‐proBNP.
Echocardiography
All study participants underwent comprehensive 2D echocardiography at rest using commercially available ultrasound systems (Philips EPIQ 7, Philips Medical Systems, Andover, MA). Echocardiography was performed in conditions of respiratory (<20 breaths/min), haemodynamic (systolic blood pressure 90–140 mmHg), and electrical (51–99 beats/min) stability. All images acquired for STE analysis were obtained at a frame rate of 50 to 80 frame/s, and a minimum of three cardiac cycles (for patients in sinus rhythm) or five cardiac cycles (for patients in atrial fibrillation) were acquired. All sonographers were trained in accordance with a pre‐specified standard operation procedure. All 2D, Doppler, and strain measurements were performed offline, at the echocardiographic core laboratory, using a customized software package (TomTec Image Arena, Unterschleissheim, Germany). All analyses were performed according to ASE/EACVI recommendations 12 , 13 , 14 by a single investigator, with over‐reading by a second investigator. Both researchers were blinded to the clinical characteristics of the patients. The presence of sinus rhythm or atrial fibrillation during echocardiography was recorded.
Left ventricular endocardial longitudinal strain was measured with an algorithm designed for the LV in apical four‐chamber and two‐chamber view and averaged; the biplane longitudinal strain was considered for the analyses as global longitudinal strain. LV endocardial border was contoured at LV end‐diastole and end‐systole and manually adjusted when required. When in two or more segments out of six there were dropout or poor tracking, LV strain was not measured.
LA maximal and minimal volumes were measured in apical four‐chamber and two‐chamber view and LA ejection fraction (EF) was calculated. LA strain was measured in an LA‐focused apical four‐chamber and two‐chamber views, with an algorithm designed for the LA (Figure 1 ). The onset of QRS was used as the referent point, and the average of three consecutive measurements was made. LA endocardial border was manually contoured at LV end‐diastole and end‐systole, with visual tracking quality and manual adjustment when required. When in one out of three segments there were dropout or poor tracking due to inadequate image quality, LA strain and strain rate were not measured and patients without measurable LA strain in four‐chamber view were excluded. Overall, a total of 122/131 (93%) LA apical four‐chamber and 90/131 (69%) LA apical two‐chamber tracings were suitable for analysis. In 41 patients (31%), the image quality in apical two‐chamber view was inadequate for the analysis due to poor tracking, especially in the anterior LA segment. Therefore, only four‐chamber volumes and strain parameters were used for further LA analyses. Three components of LA function were evaluated: reservoir (the LA filling phase, corresponding to LV systole), conduit (the passive LA empting phase, from mitral valve opening to P‐wave), and contractile (the active LA empting phase, from the onset of P‐wave to mitral valve closure). For each phase, strain and strain rate were measured.
Figure 1.
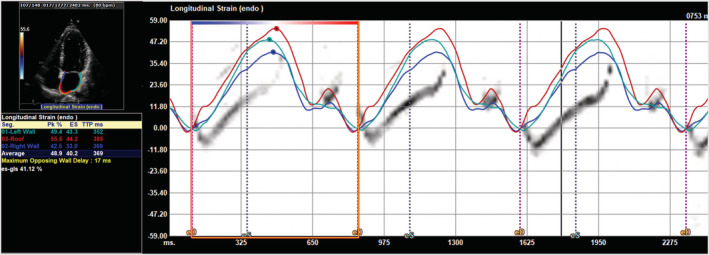
Left atrial function assessed by the speckle tracking echocardiography. Example of three‐beat strain curves in the three atrial segments in apical four‐chamber view. The mean value of the first positive peak of the three curves represent the reservoir strain, and that of the second lower positive peak represents the contractile strain.
The intraobserver variability for key measures was estimated by means of intraclass correlation coefficients (ICCs). Reproducibility was excellent for strain parameters (ICC 91–92) and good for strain rate (ICC 78–85).
Cardiopulmonary exercise test
All patients performed a symptom‐limited CPET using a cycle ergometer protocol, cycling with a pedal speed of 60 rpm, starting at a workload of 20 W, followed by a stepwise 20 W increment every 2 min until exhaustion. Heart rate was continuously monitored by electrocardiography at rest and during exercise; blood pressure was measured at rest and every 2 min. Breath‐by‐breath oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE) were acquired and averaged over 30 s intervals using a ventilator expired gas analysis system. Test termination criteria considered were patients' request due to symptoms, ventricular arrhythmia, ST segment depression ≥ 2.0 mm, and drop in systolic blood pressure ≥ 20 mmHg. All oral medications were continued beforev and through CPET.
Peak VO2 (pVO2) was defined as the highest averaged VO2 during the last stage of exercise. Percentage values of predicted pVO2 were calculated using the Wasserman formula. 15 VE/VCO2 slope was calculated to estimate the ventilator response to exercise. The maximal respiratory exchange ratio (RER) was calculated as the VCO2/VO2 ratio during the last stage of exercise before recovery and was considered as index of maximal exercise. The ability to perform maximal exercise testing was considered a mandatory inclusion criterion. Therefore, patients with maximal RER < 1.0 were excluded from the study analyses. 16
Statistical analysis
Data are presented as mean ± standard deviation or absolute values and percentages, as appropriate. Patients were categorized in two groups based on a validated pVO2 cut‐off considered of prognostic value in HF patients' populations (pVO2 14 mL/kg/min). 17 , 18 , 19 , 20 Student's t‐test and χ2 test were used to compare continuous and categorical variables between groups, respectively. Pearson's correlation coefficient was used to evaluate the relationship between echocardiographic parameters as continuous variables. The independent association of LA strain with pVO2 was studied with regression analysis. As independent variables, we considered age, gender, and BMI, which are non‐cardiac factors known to have an influence on pVO2 values 20 ; heart rhythm [sinus or atrial fibrillation (AFib)], because lower values of LA strain are expected in patients with AFib 21 ; and NT‐proBNP as validated marker associated with HF symptoms. Because NT‐proBNP distribution was skewed, it was log‐transformed for analysis. Logistic regression analysis was performed to assess the predictability of pVO2 < 14 mL/kg/min for LA strain and key echocardiographic variables [LVEF, LV mass, left atrial volume (LAV) index, and E/e´]. Continuous values of the described covariates were used. Moreover, receiver operatic characteristic curve and Youden test were performed to identify LA strain cut‐off value to be used in subsequent logistic regression analysis to assess the predictability of pVO2 < 14 mL/kg/min. All tests were two‐tailed. A P value < 0.05 was considered statistically significant. Analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL).
Results
Population characteristics
Of the 131 patients enrolled in the German HFpEF Registry, 44 patients were excluded due to submaximal exercise testing (premature stop of testing with peak RER < 1.0), 10 due to not performing CPET, 3 due to resting heart rate < 45 bpm or >100 bpm, 1 due to severe valve disease, and 8 due to unsuitable LA strain analysis. Sixty‐five patients formed the final study population. Average age was 72 ± 8 years; 37 (57%) were men, and 17 (26%) were obese (BMI ≥ 30 kg/m2). Median NT‐proBNP was 510 pg/mL (interquartile range 308–906). Comorbidities were common, especially hypertension, dyslipidaemia, diabetes, ischaemic heart disease, and chronic kidney injury. Fifteen patients (23%) presented AFib during echocardiography, and among these, pacemaker rhythm was present in five patients.
Overall, average pVO2 was 16.3 ± 4.7 mL/kg/min, with median (interquartile range) 15.3 (13.0–19.1) mL/kg/min. Twenty‐nine patients (45%) had a pVO2 < 14 mL/kg/min (mean value 12.4 ± 1.5), and 36 patients (55%) reached a pVO2 ≥ 14 mL/kg/min (mean value 19.4 ± 4.0). Echocardiography and cardiopulmonary exercise test were performed in the same day or with 1 day time interval in all patients. Fifty‐two patients assumed beta‐blockers regularly (81%), but any effect of the medication was noted on exercise tolerance. Compared with patients who didn't assume beta‐blockers, there was no significant difference in terms of maximal workload, rest heart rate, maximal exercise heart rate, differential heart rate, Borg score, RER, pVO2, and VE/VCO2 (P > 0.05 for all). Clinical and echocardiographic characteristics of patients divided according to pVO2 are shown in Tables 1 and 2 . Patients with severely reduced functional capacity were older, with greater BMI, more symptomatic (NYHA class III vs. II; no one was classified as NYHA IV in either groups), and with higher values of NT‐proBNP but did not present significant differences in terms of comorbidities, medical therapy, and other laboratory tests. Overall, patients had LV of normal dimensions with concentric remodelling in 20 (29%), concentric hypertrophy in 21 (32%), eccentric hypertrophy in 6 (9%), preserved or mildly reduced EF (55 and 10 patients with EF > 50% and 40–50%, respectively), decreased mean tissue Doppler‐derived S′ (5.9 ± 0.9 and 6.6 ± 1.6 cm/s in patients with EF > 50% and ≤50%, respectively, P = 0.1). Among patients with LVEF > 50%, 24 (44%) reached a pVO2 < 14 and 31 (56%) a pVO2 ≥ 14 mL/kg/min; among patients with LVEF 40–50%, five (50%) reached a pVO2 < 14 and five (50%) a pVO2 ≥ 14 mL/kg/min. Patients with pVO2 < 14 mL/kg/min had lower stroke volume and LV S′ and slightly higher peak tricuspid regurgitation velocity, but there was no difference in terms of LVEF, LV remodelling pattern, mitral inflow parameters, or E/e´. Mild mitral regurgitation was slightly more prevalent in patients with severely reduced pVO2 and only one patient presented moderate mitral regurgitation (Table 2 ).
Table 1.
Demographic and clinical characteristics and cardiopulmonary exercise test variables
| Peak VO2 < 14 mL/kg/min (n = 29) | Peak VO2 ≥ 14 mL/kg/min (n = 36) | P value | |
|---|---|---|---|
| Age, years | 75 ± 6 | 70 ± 9 | 0.03 |
| Male sex, n (%) | 14 (48) | 23 (64) | 0.2 |
| Body mass index, kg/m2 | 30 ± 5 | 26 ± 4 | 0.002 |
| Systolic blood pressure, mmHg | 132 ± 18 | 141 ± 22 | 0.09 |
| Heart rate, bpm | 68 ± 9 | 65 ± 10 | 0.3 |
| Atrial fibrillation, n (%) | 8 (28) | 7 (19) | 0.4 |
| NYHA class III, n (%) | 10 (34) | 2 (5) | 0.003 |
| Medical history | |||
| Hypertension, n (%) | 27 (93) | 31 (86) | 0.3 |
| Dyslipidaemia, n (%) | 22 (76) | 18 (50) | 0.04 |
| Diabetes mellitus, n (%) | 10 (34) | 12 (33) | 0.9 |
| Sleep apnoea syndrome, n (%) | 1 (3) | 9 (25) | 0.02 |
| Smoke, n (%) | 13 (45) | 20 (56) | 0.5 |
| Ischaemic heart disease, n (%) | 15 (52) | 18 (50) | 0.9 |
| Valve percutaneous intervention, n (%) | 7 (24) | 4 (11) | 0.1 |
| Peripheral artery disease, n (%) | 3 (10) | 3 (8) | 0.8 |
| Stroke/TIA, n (%) | 4 (14) | 6 (16) | 0.7 |
| Chronic obstructive pulmonary disease, n (%) | 4 (14) | 2 (5) | 0.3 |
| Chronic liver disease, n (%) | 1 (3) | 0 (0) | 0.3 |
| Chronic kidney injury, n (%) | 9 (31) | 7 (19) | 0.3 |
| Medications | |||
| ACE inhibitors or ARBs, n (%) | 22 (76) | 31 (86) | 0.3 |
| Beta‐blockers, n (%) | 25 (86) | 27 (75) | 0.3 |
| Anti‐aldosterone, n (%) | 5 (17) | 9 (25) | 0.4 |
| Diuretics, n (%) | 22 (76) | 19 (52) | 0.06 |
| Laboratory | |||
| Haemoglobin, g/dL | 13.4 ± 1.2 | 13.5 ± 1.4 | 0.7 |
| Total cholesterol, mg/dL | 159 ± 41 | 180 ± 51 | 0.08 |
| LDL cholesterol, mg/dL | 95 ± 32 | 114 ± 49 | 0.08 |
| HbA1c, % | 5.5 ± 0.9 | 5.5 ± 0.9 | 0.9 |
| Creatinine, mg/dL | 1.1 ± 0.4 | 1.0 ± 0.4 | 0.4 |
| Hs‐C‐reactive protein, mg/L | 2.6 ± 3.0 | 4.2 ± 6.8 | 0.3 |
| Ferritin, μg/L | 113 ± 82 | 115 ± 100 | 0.9 |
| NT‐proBNP, pg/mL | 640 (436–1022) | 413 (249–786) | 0.01 |
| Cardiopulmonary exercise test | |||
| Maximal work, Watt | 75 ± 16 | 111 ± 27 | <0.0001 |
| Time of exercise, min | 7 ± 1 | 11 ± 3 | <0.0001 |
| Peak VO2, mL/kg/min | 12.4 ± 1.5 | 19.4 ± 4.0 | <0.0001 |
| Percent predicted peak VO2, % | 74 ± 16 | 94 ± 18 | <0.0001 |
| Peak RER | 1.05 ± 0.03 | 1.05 ± 0.05 | 0.5 |
| VE/VCO2 slope | 39 ± 7 | 34 ± 6 | 0.001 |
| Rest heart rate, bpm | 73 ± 13 | 69 ± 10 | 0.1 |
| Exercise maximal heart rate, bpm | 102 ± 21 | 121 ± 25 | 0.002 |
| Δheart rate, bpm | 30 ± 18 | 52 ± 21 | <0.0001 |
| Borge score (6–20) | 15.6 ± 2.2 | 15.9 ± 1.7 | 0.6 |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; NT‐proBNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; RER, respiratory exchange ratio; TIA, transient ischaemic attack.
Table 2.
Echocardiographic parameters
| Peak VO2 < 14 mL/kg/min (n = 29) | Peak VO2 ≥ 14 mL/kg/min (n = 36) | P value | Coefficient for correlation with peak VO2, mL/kg/min | P value for correlation | |
|---|---|---|---|---|---|
| LV structure and function | |||||
| LV EDV‐i, mL/m2 | 51 ± 16 | 57 ± 14 | 0.2 | 0.10 | 0.5 |
| LV‐mass‐i, g/m2 | 109 ± 23 | 112 ± 32 | 0.7 | −0.07 | 0.6 |
| RWT | 0.45 ± 0.07 | 0.45 ± 0.09 | 0.8 | −0.05 | 0.7 |
| LVEF, % | 60 ± 9 | 59 ± 8 | 0.9 | 0.13 | 0.3 |
| LV GLS, % | −17.9 ± 3.6 | −18.3 ± 2.9 | 0.7 | −0.12 | 0.4 |
| S′, cm/s | 6.1 ± 1.3 | 6.8 ± 1.6 | 0.09 | 0.27 | 0.03 |
| SV‐LVOT‐i, mL/m2 | 27 ± 5 | 32 ± 7 | 0.008 | 0.21 | 0.1 |
| LA structure and function | |||||
| LAV‐max‐i, mL/m2 | 45 ± 17 | 47 ± 22 | 0.6 | −0.07 | 0.6 |
| LAV‐min‐i, mL/m2 | 29 ± 14 | 30 ± 19 | 0.8 | −0.08 | 0.5 |
| LA EF, % | 35 ± 10 | 39 ± 13 | 0.1 | 0.19 | 0.1 |
| LA reservoir strain, % | 14 ± 5 | 21 ± 9 | 0.002 | 0.36 | 0.003 |
| LA conduit strain, % | 9 ± 2 | 13 ± 4 | 0.001 | 0.29 | 0.04 |
| LA contractile strain, % | 7 ± 4 | 11 ± 6 | 0.02 | 0.33 | 0.02 |
| LA systolic SR, %/s | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.01 | 0.32 | 0.01 |
| LA early diastolic SR, %/s | −0.4 ± 0.1 | −0.5 ± 0.2 | 0.03 | −0.16 | 0.2 |
| LA late diastolic SR, %/S | −0.5 ± 0.2 | −0.6 ± 0.3 | 0.1 | −0.29 | 0.04 |
| Doppler | |||||
| E, cm/s | 83 ± 32 | 76 ± 23 | 0.3 | −0.21 | 0.1 |
| A, cm/s | 76 ± 26 | 70 ± 24 | 0.4 | −0.09 | 0.5 |
| DTE, ms | 213 ± 73 | 213 ± 51 | 0.9 | 0.08 | 0.5 |
| E/A | 1.1 ± 0.6 | 1.1 ± 0.7 | 0.9 | −0.13 | 0.4 |
| E´, cm/s | 6.5 ± 1.8 | 6.3 ± 1.8 | 0.7 | 0.01 | 0.9 |
| E/e´ | 13.1 ± 4.7 | 13.0 ± 6.0 | 0.9 | −0.16 | 0.2 |
| MR, n (%) | 25 (86) | 23 (64) | 0.05 | −0.21 | 0.04 |
| TR, n (%) | 23 (79) | 23 (64) | 0.1 | −0.09 | 0.1 |
| Peak TR velocity, m/s | 2.7 ± 0.6 | 2.4 ± 0.4 | 0.08 | −0.31 | 0.03 |
| SPAP, mmHg | 37 ± 19 | 29 ± 10 | 0.1 | −0.30 | 0.06 |
DTE, E wave deceleration time; EDV‐i, Simpson‐biplane end‐diastolic volume index; EF, Simpson‐biplane ejection fraction; GLS, global endocardial longitudinal strain [GLS was measured in apical four‐chamber and two‐chamber view and averaged; biplane GLS was available in 54 (79%) patients]; LAV, left atrial volume; LV, left ventricular; RWT, relative wall thickness; SPAP, systolic pulmonary artery pressure [TR velocity and SPAP were measurable in 46 (68%) of total patients, 92% of patients with TR]; SR, strain rate; SV‐LVOT, left ventricular outflow tract stroke volume; TR, tricuspid regurgitation [it was present in 50 (77%) patients].
Relationship between left atrium and exercise capacity
In the study cohort, LA enlargement was present in 49 patients (75%), with mean LA maximal volume 46 ± 19 mL/m2. All LA strain parameters (reservoir strain 18 ± 8%, conduit strain 11 ± 4%, and contractile strain 9 ± 5%) were impaired in comparison with described values in healthy populations. 22 , 23 Volume‐derived morphological (maximal and minimal volumes) and functional (LA EF) LA indices were not significantly different according to pVO2, whereas a correlation was found between LA STE‐derived functional parameters and pVO2 in terms of reservoir, conduit, and contractile functions, with the stronger correlation between pVO2 and LA reservoir function (Table 2 and Figure 2 ). Moreover, among all volume‐derived and STE‐derived LA parameters, only LA reservoir strain and LA systolic strain rate correlated with VE/VCO2 slope (P = 0.02 and P = 0.004, respectively).
Figure 2.
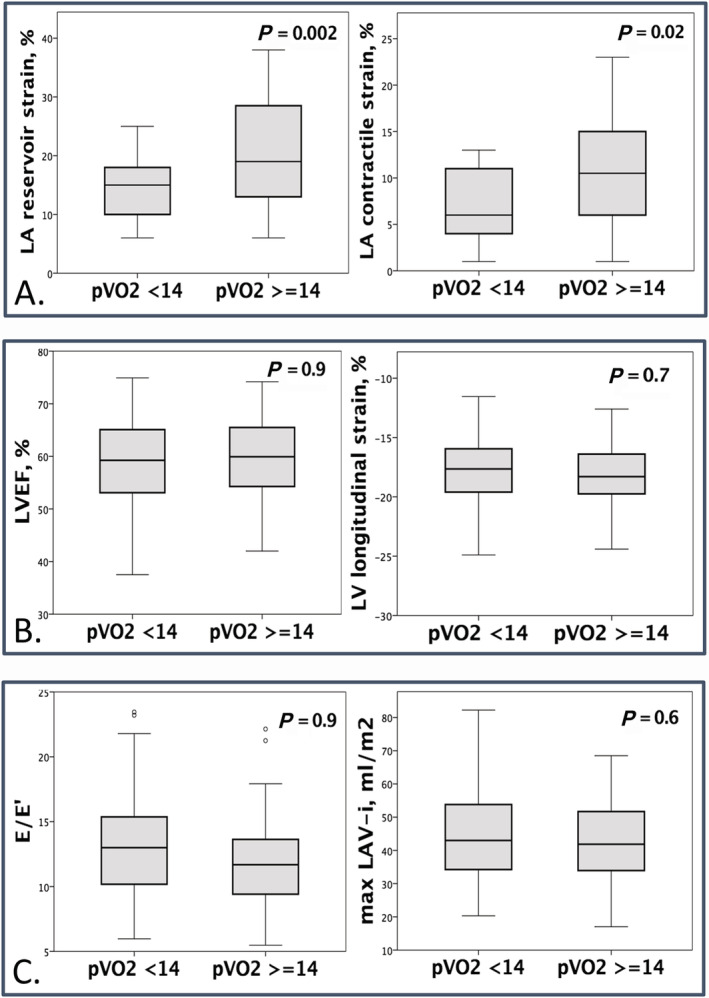
Association of left atrial reservoir strain and contractile strain (A), left ventricular ejection fraction and left ventricular global longitudinal strain (B), and E/e´ and left atrial volume index (C) with peak VO2 (mL/kg/min).
We further tested the correlation between LA strain and pVO2 by means of linear regression analysis. Both LA reservoir strain and contractile strain were univariate associated with pVO2 [β 0.36, P = 0.003, 95% confidence interval (CI) 0.07–0.34 and β 0.33, P = 0.02, 95% CI 0.05–0.53, respectively]. The association with LA reservoir strain persisted after a comprehensive adjustment for highly regarded determinants of exercise capacity (i.e. age, sex, and BMI), heart rhythm (sinus/AFib), and log‐NT‐proBNP (β 0.29, P = 0.02, 95% CI 0.02–0.30). The further addiction of NYHA class as covariate didn't change the result (β 0.29, P = 0.02, 95% CI 0.03–0.31). On the other hand, of note, the addition of LA reservoir strain to the adjustment parameters alone described above provided a significant increase of the predictive value for lower pVO2 values (R 2 0.50 vs. 0.45, P = 0.02).
At logistic regression analysis, lower LA reservoir strain values were associated with higher probability to reach pVO2 < 14 mL/kg/min after adjustment for age, sex, BMI, heart rhythm (sinus/AFib), and log‐NT‐proBNP [for each 1 unit LA reservoir strain decrease odds ratio (OR) 95% CI 1.22 (1.05–1.42), P = 0.01].
Adding LVEF to the covariates described above, the association between LA reservoir strain and pVO2 was not affected (β 0.27, P = 0.02, 95% CI 0.01–0.29), with an OR 95% CI 1.22 (1.04–1.44), P = 0.01, for each 1 unit LA reservoir strain decrease.
Left atrial strain vs. traditional echocardiographic parameters and association with exercise capacity
Left atrium reservoir strain was correlated to all other LA morphological and functional parameters, mitral inflow variables (E wave, A wave, E/A), LV tissue Doppler S′, and SV‐i (Table 3 ) but not to peak tricuspidal regurgitation (TR) velocity. However, among all echocardiographic parameters, only S′, LA reservoir strain, and peak TR velocity were associated with pVO2 (Table 2 and Figure 3 ). Moreover, LA reservoir strain was associated with pVO2 independently from S′ and peak TR velocity (Supporting Information, Table S1 ), and of note, the addition of LA reservoir strain to peak TR velocity alone provided a significant increase of the predictive value for lower pVO2 values (R 2 = 0.27 vs. R 2 = 0.1; P = 0.002).
Table 3.
Pearson correlation of left atrial reservoir strain with echocardiographic variables
| Correlation coefficient | P value | |
|---|---|---|
| LA structure and function | ||
| LAV‐max‐i, mL/m2 | −0.49 | <0.0001 |
| LAV‐min‐i, mL/m2 | −0.59 | <0.0001 |
| LA EF, % | 0.77 | <0.0001 |
| LA conduit strain, % | 0.78 | <0.0001 |
| LA contractile strain, % | 0.88 | <0.0001 |
| LA systolic SR, %/s | 0.89 | <0.0001 |
| LA early diastolic SR, %/s | −0.46 | <0.0001 |
| LA late diastolic SR, %/s | −0.75 | <0.0001 |
| LV structure and function | ||
| LV EDV‐i, mL/m2 | 0.21 | 0.08 |
| LV‐mass‐i, g/m2 | −0.15 | 0.2 |
| LVEF, % | −0.01 | 0.9 |
| LV GLS, % | −0.13 | 0.3 |
| LV S′, cm/s | 0.39 | 0.001 |
| SV‐LVOT‐i, mL/m2 | 0.30 | 0.03 |
| E, cm/s | −0.30 | 0.02 |
| A, cm/s | 0.32 | 0.02 |
| DTE, ms | 0.09 | 0.5 |
| E/A | −0.39 | 0.005 |
| E´, cm/s | −0.25 | 0.05 |
| E/e´ | −0.03 | 0.8 |
DTE, E wave deceleration time; EDV‐i, Simpson‐biplane end‐diastolic volume index; EF, ejection fraction; GLS, global endocardial longitudinal strain; LAV, left atrial volume; LV, left ventricular; SR, strain rate; SV‐LVOT, left ventricular outflow tract stroke volume; TR, tricuspid regurgitation.
Figure 3.
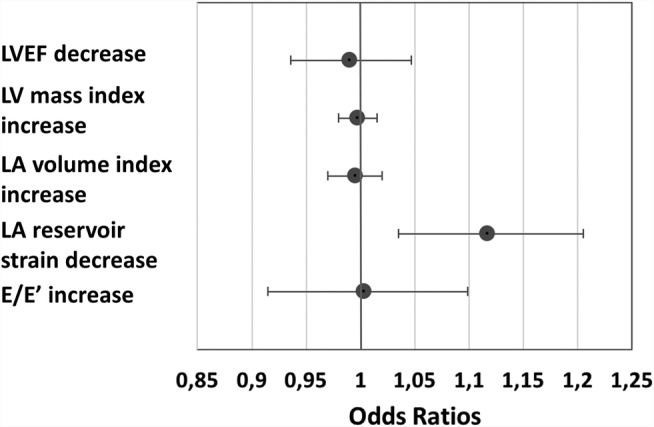
Association of LV and LA parameters with severely reduced exercise capacity (peak VO2 < 14 mL/kg/min). Logistic regression analysis for predictability of peak VO2 < 14 mL/kg/min: LVEF 1 unit (%) decrease odds ratio (OR) 95% confidence interval (CI) 0.99 (0.93–1.05), LV mass index 1 unit (g/m2) increase OR 95% CI 0.99 (0.98–1.01), LA volume index 1 unit (mL/m2) increase OR 95% CI 0.99 (0.97–1.02), LA reservoir strain 1 unit (%) decrease OR 95% CI 1.12 (1.03–1.20), E/e´ 1 unit increase OR 95% CI 1.01 (0.91–1.10). LA, left atrium; LVEF, left ventricular ejection fraction.
A receiver operating characteristic curve analysis was performed to identify the cut‐off of LA reservoir strain that could predict pVO2 < 14 mL/kg/min. LA reservoir strain < 22% showed 93% sensitivity and 49% specificity to predict pVO2 (area under the receiver operating characteristic curve 0.69, P = 0.008, 95% CI 0.56–0.82). On the contrary, any predictive capacity was not found by receiver operating characteristic curve analyses for LAV index and E/e´ regarding pVO2 < 14 mL/kg/min (area under the receiver operating characteristic curve 0.49 and 0.54, respectively). A logistic regression analysis was performed to ascertain the additive effect of LA reservoir strain < 22% on pVO2 < 14 mL/kg/min prediction on top of age, sex, BMI, heart rhythm (sinus/AFib), and log‐NT‐proBNP. LA reservoir strain added a significant contribution to the predictability of the model (χ2 30.704 vs. 23.831, P = 0.009), which correctly classified 75% of cases. In particular, patients showing LA reservoir strain < 22% and higher BMI were more likely to exhibit pVO2 < 14 mL/kg/min [OR 95% CI 10.4 (1.4–74), P = 0.02, OR 95% CI 1.4 (1.1–1.7), P = 0.004, respectively].
Left atrial strain and exercise capacity in heart failure with preserved ejection fraction
With the intention to validate the study results in the HFpEF population, we tested the association between LA reservoir strain and pVO2 in patients who met the most recent HFpEF diagnostic criteria. Within the entire cohort, 55 (85%) and 47 (72%) patients had 2016 European Society of Cardiology (ESC) HF guideline 11 and 2020 Heart Failure Association of the ESC consensus recommendation 24 criteria for HFpEF, respectively. Moreover, as further confirmation, among the cohort of 55 patients with HFpEF (according to 2016 ESC criteria), we decided to exclude from the analysis patients presenting characteristics that could interfere in the evaluation of LA function, that is, atrial fibrillation, previous pacemaker implantation, and previous severe valve disease requiring interventional procedure (e.g. transcatheter aortic valve implantation). With such approach, 40 patients were identified. In these three patient subgroups, LA reservoir, conduit, and contractile strains were impaired in patients with pVO2 < 14 mL/kg/min. On the contrary, LAV index and E/e´ did not differentiate between patients with lower and higher functional capacity (Supporting Information, Table S2). In linear regression analysis, lower LA reservoir strain and lower pVO2 values were positively associated at univariate analysis and after adjustment for age, sex, and BMI (Table 4 ).
Table 4.
Linear regression analysis for association between left atrial reservoir strain and peak VO2 according to different patient selection criteria
| LA reservoir strain, % | 2016 ESC HF guideline (n = 55) | 2020 HFA of the ESC consensus recommendation (n = 47) | LVEF ≥ 50%, sinus rhythm, without valve procedure (n = 40) |
|---|---|---|---|
| Unadjusted | β 0.36, P = 0.006, 95% CI 0.06–0.33 | β 0.32, P = 0.03, 95% CI 0.01–0.29 | β 0.37, P = 0.01, 95% CI 0.04–0.38 |
| Adjusted for age, sex, BMI | β 0.36, P = 0.005, 95% CI 0.06–0.32 | β 0.31, P = 0.03, 95% CI 0.01–0.29 | β 0.36, P = 0.009, 95% CI 0.05–0.35 |
BMI, body mass index; CI, confidence interval; ESC, European Society of Cardiology; HFA, Heart Failure Association; LA, left atrium; LVEF, left ventricular ejection fraction.
Discussion
In a cohort of patients enrolled in the German HFpEF Registry, we found that (1) all STE‐derived measures of LA function were associated with exercise intolerance in terms of pVO2, whereas traditional indices of LV diastolic dysfunction and filling pressure (LAV, mitral inflow Doppler parameters, E/e´, and LV structure and function) didn't discriminate patients according to pVO2 and (2) the assessment of LA reservoir strain added value to predict lower pVO2 independently from age, sex, BMI, rhythm of presentation, and NT‐proBNP levels. Moreover, LA reservoir strain < 22% predict pVO2 < 14 mL/kg/min with high sensitivity. Results suggest that STE‐derived LA function parameters portend clinical utility in clinical judgement as well as risk stratification and prognostic assessment of patients with clinically diagnosed HFpEF and HFmrEF.
Left atrial function in heart failure with preserved and mid‐range ejection fraction haemodynamic
Several mechanisms are implicated in exercise intolerance and reduced oxygen consumption. In a pooled analysis of 910 HFpEF patients from 17 cohorts, the most consistent haemodynamic reserve alteration was an exaggerated increase in filling pressure, together with reduced chronotropic reserve. 3 Patients with HFmrEF seem to exhibit a haemodynamic performance more similar to HFpEF than HFrEF. 25 In HFmrEF, chronotropic incompetence, peripheral factors, limited stroke volume reserve, right ventricle dysfunction, and mitral regurgitation have been called into question, 26 but there is a paucity of studies addressing mechanism of exercise intolerance in HFmrEF.
Left ventricular diastolic dysfunction has long been considered mainly responsible for the development of overt HFpEF, with elevation in LV, LA, and pulmonary pressure. Diastolic dysfunction could be of relevance also in haemodynamic of HFmrEF, as far as the current HFmrEF diagnostic criteria are parallel to those for HFpEF, requiring elevated natriuretic peptides and structural heart disease or diastolic dysfunction. Also, LV systolic functional impairment characterize both HFpEF (in terms of longitudinal systolic function) 27 and HFmrEF. However, the relative contribution of systolic and diastolic impairment is not completely understood. LV and LA function are linked to each other and a relevant role in modulating the relationship between LV and pulmonary circle haemodynamic has been increasingly recognized to the LA. 28 Under physiological conditions, a preserved LA reservoir and contractile function allow an adequate LV filling during exercise, so this impairment due to chronic pressure overload and progressive reduced LA compliance in HFpEF patients contributes to poor augmentation in stroke volume and increase in filling pressure during exercise. 29
The impairment in LA function reflects the close interplay between LA and LV. In fact, LA reservoir function is affected by LA relaxation and compliance and modulated by LV longitudinal systolic contraction through the downward motion of the mitral annular plane in systole; LA conduit function is influenced by LV stiffness and early diastolic relaxation; and LA booster function reflects intrinsic LA contractility and LV end‐diastolic compliance and pressure.
Therefore, as LV systolic and diastolic function worsens, the increase in pressure overload leads the LA to remodel and two phases have been described: in the early stages, LA reservoir and contractile function slightly increase to augment LV filling and preserve cardiac output; chronically elevated LV afterload determines progressive LA compliance impairment with decrease in LA reservoir and contractile function due to work mismatch. Such failing LA leads to high LA pressure and pulmonary congestion. 30 At this stage, LA dilation occurs primarily as consequence of long‐standing elevated LA pressure, at rest or at least during exercise.
Consistent with previous HFpEF and HFmrEF studies, 21 , 31 we found an impaired LA haemodynamic with decline in all phases of LA function. Interestingly, LA strain was associated with S′, indices of basal LV systolic function, but not with LVEF or global longitudinal strain. Moreover, LA strain didn't correlate with E/e´ or peak TR velocity, two major components for LA pressure estimation. 12 However, a relevant proportion of HFpEF patients is known to develop high filling pressure only during exercise 32 and E/e´ and peak TR velocity could both significantly rise during exercise induced loading conditions.
Left atrium and prognosis in heart failure with preserved and mid‐range ejection fraction
In the spectrum of HF, HFpEF pathophysiology is characterized by great heterogeneity in cardiac and extra‐cardiac underlying mechanisms, which hamper diagnosis, risk stratification, and treating options development. In fact, hospitalization and mortality rates of HFpEF patients are similar to HFrEF, but evidences of disease‐modifying therapies in HFpEF are lacking. 33 , 34 , 35 Pathophysiology of HFmrEF is further unclear, and it has been hypothesized that it represents a transition category from or to lower LVEF and not a distinct pathophysiological entity, with long‐term prognosis similar to that of HFpEF. 36 The identification of key parameters that help in risk and prognosis assessment is of paramount importance, because patients' stratification represents a first step for orientation in the conundrum of HFpEF syndrome and definition of therapeutic targets.
In the present study, we indirectly investigated the prognostic role of LA function through its association with CPET variables, which demonstrated high prognostic value in HFpEF, even if with contrasting results regarding the best parameter to predict hospitalization for HF and death (pVO2, VE/VCO2 slope). 37 , 38 , 39 Interestingly, Nadruz et al. observed a relevant association between pVO2 and VE/VCO2 slope and adverse outcome in HFpEF, even stronger compared with HFrEF, demonstrating a great ability of CPET in discriminating HFpEF patients' risk profile. 18 Evidence from CPET studies showed pVO2 predictive ability also in HFmrEF, with a rate of adverse events similar between HFmrEF and HFpEF. 39
The association between LA mechanics and prognosis finds its rationale in the development of elevated LA pressure and PCWP, especially during exercise, which are mainly responsible for HF symptoms. The prognostic role of LA in HFpEF has been extensively demonstrated for LA size, 40 , 41 , 42 being LA dilation expression of chronically elevated LV filling pressure and disease progression. However, LA size was not prognostic in 1.097 patients enrolled in the PARAGON‐HF, probably due to the high prevalence of LA structural remodelling in the substudy population. 43 Similarly, in our study, the majority of patients had a moderately dilated LA, suggesting a more advanced syndrome stage, in which LA size capability in patients' stratification could be reduced.
It has been extensively demonstrated that all LA strain parameters are significantly reduced in HFpEF patients, 21 , 44 so that LA function evaluation has been proposed to discriminate asymptomatic diastolic dysfunction from HFpEF. 45 An association exists between LA functional and structural remodelling, 21 but recent evidences demonstrated the strong independent prognostic value of LA dysfunction in HFpEF, more powerful than LV longitudinal function, whereas LAV was actually not associated with adverse outcomes in some studies. 10 Also, in patients enrolled in the TOPCAT trial, a worse LA function was associated with HF hospitalization; however, these are not independent from LV systolic deformation and filling pressure. 46
In our study, LA reservoir strain was a powerful predictor of CPET pVO2, whereas no association was found for LAV and E/e´. In the study cohort, all LA strain parameters were well below the reported reference ranges and were comparable with the lower LA strain value range in previous HFpEF study populations, 21 , 46 highlighting the relevant LA functional impairment in our population. Comparable enlarged LAV and impaired LA strain values were present in patients described by Hummel et al. Interestingly, they found that LA reservoir strain was a better predictor of invasively measured PCWP than LAV, irrespective of the underlying rhythm, whereas the correlation between E/e´ and PWCP was only poor. 47
E/e´ ratio has long been used as single parameter to estimate elevated LV filling pressure, because it was well validated against invasive measurements. 48 , 49 Moreover, it was the strongest haemodynamic predictor of outcome in an HFpEF study 50 and the most robust index of elevated filling pressure among parameters included in the 2016 ESC HF guidelines 11 and in the 2016 ASE/EACVI recommendations. 12 However, its value has been recently questioned, because its correlation with high LV filling pressure and sensitivity are actually both very poor. Also, the multiparametric approaches 12 , 45 were shown to be highly insufficient to reliably estimate PCWP. 32
However, resting assessment of LV filling pressure in HFpEF is probably very insensitive per se, because a great proportion of patients develop elevated filling pressure only during exercise. Recently, Telles et al. demonstrated that LA reservoir and contractile strains were independently associated with exercise PCWP even after adjustment for diastolic dysfunction variables. 8 Moreover, they used an LA reservoir strain value ≤ 33% as cut‐off to demonstrate a greater diagnostic sensitivity and specificity in comparison with the 2016 ESC criteria. 11 In our study, we identified LA reservoir strain value < 22% to predict pVO2 < 14 mL/kg/min with high sensitivity.
All together, these evidences suggest LA function as reliable marker of impaired haemodynamic in HFpEF and HFmrEF, particularly useful in evaluation at rest when other well‐established echocardiographic parameters could be non‐discriminative. Of note, the value of LA function showed to overcome that of LAV in advanced stages of disease, supporting its relevant role in prognostic stratification of patients with clinical HFpEF and HFmrEF diagnosis.
Limitations
The major limitation of the study is the, to date, limited cohort size and single‐centre setting. Therefore, results should be considered with caution. However, these preliminary results from the German HFpEF Registry are highly encouraging and highlight the powerful association between LA function and consolidated prognostic marker in HFpEF and HFmrEF patients. We used pVO2 14 mL/kg/min value as cut‐off to divide our cohort, because it is of prognostic value in HF patients. However, as confirmatory analysis, we divided the study population according to pVO2 median value with comparable results.
Second, patients enrolled comprehend two types of HF (HFpEF and HFmrEF) according to the most recent guidelines. 11 In our cohort, the small proportion of patients with HFmrEF prevented us from analysing HFmrEF patients separately. However, LVEF > 40% or ≥45% were the cut‐offs used as inclusion criteria in most HFpEF clinical trials. 43 We performed the analyses also in cohort subgroups with preserved LVEF according to recently recommended inclusion criteria 11 , 24 and the association between LA functional parameters and pVO2 did not change.
Even though there is a plausible relationship between atrial dysfunction, AFib, and exercise capacity, we did not analyse separately patients with AFib due to the small sample size. However, we noted the aligned linear association between LA strain and pVO2 also after exclusion of patients with atrial fibrillation and/or pacemaker implantation, which are potential confounding factors.
Conclusions
In a cohort of stable HFpEF and HFmrEF patients, the assessment of LA function in addition to LA structural remodelling and E/e´ could help in further characterization of patients' haemodynamic. An impaired LA function, in particular LA reservoir strain, emerged as sensitive and powerful marker of exercise intolerance, above echocardiographic parameters routinely used to estimate LV filling pressure and independently from known determinants of exercise capacity (age, sex, and BMI), heart rhythm, and NT‐proBNP. Therefore, the evidence and degree of LA functional impairment could portend clinical utility in evaluation of HFpEF and HFmrEF patients.
Conflict of interest
None declared.
Funding
This work was supported by a research grant from Servier.
Supporting information
Table S1. Association between echocardiographic variables and peakVO2 (ml/kg/min).
Table S2. Volumetric and speckle‐tracking‐echocardiography derived left atrial parameters and E/e' in patients with peakVO2 < or≥14 mL/kg/min according to different patients' selection criteria.
Acknowledgement
Open access funding enabled and organized by Projekt DEAL.
Maffeis, C. , Morris, D. A. , Belyavskiy, E. , Kropf, M. , Radhakrishnan, A. K. , Zach, V. , Rozados da Conceicao, C. , Trippel, T. D. , Pieske‐Kraigher, E. , Rossi, A. , Pieske, B. , and Edelmann, F. (2021) Left atrial function and maximal exercise capacity in heart failure with preserved and mid‐range ejection fraction. ESC Heart Failure, 8: 116–128. 10.1002/ehf2.13143.
References
- 1. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2017; 376: 897. [DOI] [PubMed] [Google Scholar]
- 2. Nauta JF, Hummel YM, van Melle JP, van der Meer P, Lam CSP, Ponikowski P, Voors AA. What have we learned about heart failure with mid‐range ejection fraction one year after its introduction? Eur J Heart Fail 2017; 19: 1569–1573. [DOI] [PubMed] [Google Scholar]
- 3. Pandley A, Khera R, Park B, Haykowsky M, Borlaug BA, Lewis GD, Kitzman DW, Butler J, Berry JD. Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction: a study‐level pooled analysis. JACC Heart Fail 2018; 6: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edelmann F, Gelbrichb G, Duvinage A, Stahrenberg R, Behrens A, Prettin C. Differential interaction of clinical characteristics with key functional parameters in heart failure with preserved ejection fraction—results of the Aldo‐DHF trial. Int J Cardiol 2013; 169: 408–417. [DOI] [PubMed] [Google Scholar]
- 5. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2018; 20: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 6. Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, Narita H, Kimura G. Correlation between left ventricular end‐diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr 2009; 22: 847–851. [DOI] [PubMed] [Google Scholar]
- 7. Hewing B, Theres L, Spethmann S, Stangl K, Dreger H, Knebel F. Left atrial strain predicts hemodynamic parameters in cardiovascular patients. Echocardiography 2017; 34: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 8. Telles F, Nanayakkara S, Evans S, Patel HC, Mariani JA, Vizi D, William J, Marwick TH, Kaye DM. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 495–505. [DOI] [PubMed] [Google Scholar]
- 9. Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 2012; 98: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 10. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen‐Torvik LJ, Maganti K, Shah SJ. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 2016; 9: e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) Developed With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 14. Badano L, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry TaskForce to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018; 19: 591–600. [DOI] [PubMed] [Google Scholar]
- 15. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J, European Association for Cardiovascular Prevention & Rehabilitation; American Heart Association . EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012; 126: 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Roeder M, Rommel CP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuß G, Lücke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017; 10: e005467. [DOI] [PubMed] [Google Scholar]
- 17. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research . Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010; 122: 191–225. [DOI] [PubMed] [Google Scholar]
- 18. Nadruz W, West E, Sengelov M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H, Shah AM. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc 2017; 6: e006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991; 83: 778–786. [DOI] [PubMed] [Google Scholar]
- 20. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 2016; 4: 607–616. [DOI] [PubMed] [Google Scholar]
- 21. Santos AB, Kraigher‐Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD, Investigators PARAMOUNT. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle‐tracking echocardiography: a systematic review and meta‐analysis. J Am Soc Echocardiogr 2017; 30: 59–70.e8. [DOI] [PubMed] [Google Scholar]
- 23. Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Lopez T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, Von Bardeleben RS, Vinereanu D, Zamorano JL, Go YY, Marchetta S, Nchimi A, Rosca M, Calin A, Moonen M, Cimino S, Magne J, Cosyns B, Galli E, Donal E, Habib G, Esposito R, Galderisi M, Badano LP, Lang RM, Lancellotti P. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2018; 19: 630–638. [DOI] [PubMed] [Google Scholar]
- 24. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020; 22: 391–412. [DOI] [PubMed] [Google Scholar]
- 25. Pugliese NR, Fabiani I, Santini C, Rovai I, Pedrinelli R, Natali A, Dini FL. Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid‐range ejection fraction. Eur Heart J Cardiovasc Imaging 2019; 20: 828–836. [DOI] [PubMed] [Google Scholar]
- 26. Topilsky Y, Rozenbaum Z, Khoury S, Pressman GS, Gura Y, Sherez J, Man A, Shimiaie J, Edwards S, Berookhim J, Le Tourneau T, Halkin A, Biner S, Keren G, Aviram G. Mechanisms of effort intolerance in patients with heart failure and borderline ejection fraction. Am J Cardiol 2017; 119: 416–422. [DOI] [PubMed] [Google Scholar]
- 27. Morris DA, Ma X‐X, Belyavskiy E, Aravind Kumar R, Kropf M, Kraft R, Frydas A, Osmanoglou E, Marquez E, Donal E, Edelmann F, Tschöpe C, Pieske B, Pieske‐Kraigher E. Left ventricular longitudinal systolic function analysed by 2D speckle‐tracking echocardiography in heart failure with preserved ejection fraction: a meta analysis. Open Heart 2017; 4: e000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, Drago A, Guazzi M, Ribichini FL, Cicoira M. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail 2020; 22: 499–506. [DOI] [PubMed] [Google Scholar]
- 29. Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure. Pathophysiological implications on the right heart and exercise ventilation inefficiency. J Am Coll Cardiol Img 2017; 10: 1253–1264. [DOI] [PubMed] [Google Scholar]
- 30. Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction structure, function, and significance. Circ Heart Fail 2014; 7: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 31. Al Saikhan L, Hughes AD, Chung WS, Alsharqi M, Nihoyannopoulos P. Left atrial function in heart failure with mid‐range ejection fraction differs from that of heart failure with preserved ejection fraction: a 2D speckle‐tracking echocardiographic study. Eur Heart J Cardiovasc Imaging 2019; 20: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation 2017; 135: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; 20: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 34. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 36. Martone R, Marchionni N, Cappelli F. Heart failure with mid‐range ejection fraction: current evidence and uncertainties. Monaldi Arch Chest Dis 2019; 89: 63–66. [DOI] [PubMed] [Google Scholar]
- 37. Guazzi M, Labate V, Cahalin LP, Arena R. Cardiopulmonary exercise testing reflects similar pathophysiology and disease severity in heart failure patients with reduced and preserved ejection fraction. Eur J Prev Cardiol 2014; 21: 847–854. [DOI] [PubMed] [Google Scholar]
- 38. Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, Schairer JR, Ehrman JK, Velez M, Selektor Y, Lanfear DE, Keteyian SJ. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT‐CPX) project. Am Heart J 2016; 174: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sato T, Yoshihisa A, Kanno Y, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Ishida T, Takeishi Y. Cardiopulmonary exercise testing as prognostic indicators: comparisons among heart failure patients with reduced, mid‐range and preserved ejection fraction. Eur J Prev Cardiol 2017; 24: 1979–1987. [DOI] [PubMed] [Google Scholar]
- 40. Rossi A, Cicoira M, Florea VG, Golia G, Florea ND, Khan AA, Murray ST, Nguyen JT, O'Callaghan P, Anand IS, Coats A, Zardini P, Vassanelli C, Henein M. Chronic heart failure with preserved left ventricular ejection fraction: diagnostic and prognostic value of left atrial size. Int J Cardiol 2006; 110: 386–392. [DOI] [PubMed] [Google Scholar]
- 41. Kaneko H, Koike A, Senoo K, Tanaka S, Suzuki S, Nagayama O, Sagara K, Otsuka T, Matsuno S, Funada R, Uejima T, Oikawa Y, Yajima J, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yamashita T. Role of cardiopulmonary dysfunction and left atrial remodeling in development of acute decompensated heart failure in chronic heart failure with preserved left ventricular ejection fraction. J Cardiol 2012; 59: 359–365. [DOI] [PubMed] [Google Scholar]
- 42. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE, I‐PRESERVE Investigators . Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011; 124: 2491–2501. [DOI] [PubMed] [Google Scholar]
- 43. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O'Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD, PARAGON‐HF Investigators . Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2019; 74: 2858–2873. [DOI] [PubMed] [Google Scholar]
- 44. Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, Triposkiadis F, Lam CSP, Shah AM, Butler J, Shah SJ. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail 2020; 22: 472–485. [DOI] [PubMed] [Google Scholar]
- 45. Kurt M, Wang J, Torre‐Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2009; 2: 10–15. [DOI] [PubMed] [Google Scholar]
- 46. Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 2016; 9: e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hummel YM, Liu LCY, Lam CSP, Fonseca‐Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, Hoendermis ES. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail 2017; 19: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 48. Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, Zuckerman J, Thomas JD. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol 2000; 36: 1664–1669. [DOI] [PubMed] [Google Scholar]
- 49. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997; 30: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 50. Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long‐term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3103–3112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between echocardiographic variables and peakVO2 (ml/kg/min).
Table S2. Volumetric and speckle‐tracking‐echocardiography derived left atrial parameters and E/e' in patients with peakVO2 < or≥14 mL/kg/min according to different patients' selection criteria.


