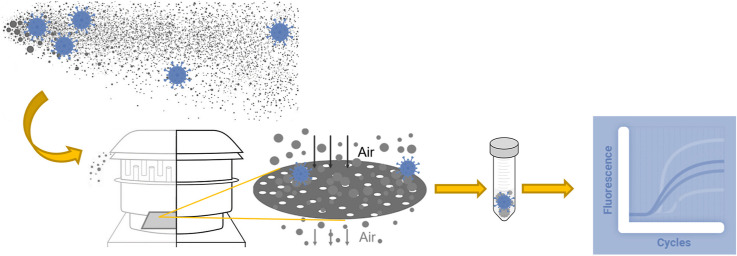
Abstract
Although much has been discovered regarding the characteristics of SARS-CoV-2, its presence in aerosols and their implications in the context of the pandemic is still controversial. More research on this topic is needed to contribute to these discussions. Presented herein are the results of ongoing research to detect SARS-CoV-2 RNA in aerosol in different hospital facilities (indoor environments) and public spaces (outdoor environments) of a metropolitan center in Brazil. From May to August 2020, 62 samples were collected using active sampling method (air samplers with filters) and passive method (petri dishes) in two hospitals, with different occupancies and infrastructure for contamination control. Outdoor public spaces such as sidewalks and a bus station were also investigated. Five air samples from four facilities in a hospital tested positive for SARS-CoV-2 in suspended and sedimentable particles. SARS-CoV-2 was found in aerosols inside the Intensive Care Unit (ICU), in the protective apparel removal room, in the room containing patient mobile toilets and used clothes (room with natural ventilation) and in an external corridor adjacent to the ICU, probably coming from infected patients and/or from aerosolization of virus-laden particles on material/equipment. Our findings reinforce the hypothesis of airborne transmission of the new coronavirus, contributing to the planning of effective practices for pandemic control.
Keywords: Aerosol, Hospital facilities, Indoor air, Outdoor air, SARS-CoV-2
Graphical abstract
1. Introduction
In December 2019, a new infectious disease capable of being transmitted between humans was identified in Wuhan, China. Named coronavirus disease 2019 (COVID-19), this severe acute respiratory syndrome caused by a new coronavirus, identified as SARS-CoV-2 (Chen et al., 2020; Zheng, 2020; Zhou et al., 2020), later turned out to be a rapidly spreading global pandemic. As of October 2020, the world has already registered more than 36 million cases of the disease and more than 1 million deaths (WHO, 2020). In Brazil, the first official case was registered on February 26th of 2020, and more than 5 million confirmed cases and almost 150,000 deaths had been registered across the country as of October 8th (Dong et al., 2020). As in some other countries, contamination cases in Brazil show that the pandemic is not yet under control. Although much progress has been made in curbing the disease with the establishment of quarantines and lockdowns around the world, the characteristics of SARS-CoV-2 are not yet fully understood and the exposure pathways are still being explored. More (and new) control measures are needed to contain the spread of the pandemic.
Primary concern about COVID-19 transmission laid emphasis on saliva droplets from an infected person to another in a close contact, direct contact (person-to-person), and indirect contact (by fomite and contaminated surfaces). Public health guidelines issued since the beginning of the pandemic were based on these potential routes of contamination and resulted in recommendation for social distance, lockdown, hand washing, decontamination of surfaces and some guidelines to encourage respiratory protection. Other potential pathways, such as inhalation of contaminated aerosol particles, have not received as much attention, nor have generated specific public health guidelines to contain or prevent the continued spread of the virus (Anderson et al., 2020). Although airborne transmission was determined to play an important role during the SARS outbreak in 2003 (Anderson et al., 2020; Asadi et al., 2020; Morawska and Cao, 2020; Tellier et al., 2019), many countries have not yet acknowledged airborne transmission by contaminated aerosol particles as a possible pathway for the SARS-CoV-2 transmission (Morawska and Cao, 2020; Tang et al., 2020). However, more recently aerosol transmission is being increasingly indicated as a relevant route to be considered for SARS-CoV-2 too. Scientists around the world have reported the presence of the new coronavirus in aerosols and/or warning about this real possibility. Pioneer publications in this effort (Anderson et al., 2020; Asadi et al., 2020; Buonanno et al., 2020b; Gorbunov, 2020; Kohanski et al., 2020; Kumar and Morawska, 2019; Liu et al., 2020; Morawska and Cao, 2020; Ong et al., 2020; Tang et al., 2020; Wang and Du, 2020) had suggested that aerosols produced by asymptomatic or pre-symptomatic individuals during coughs, sneezes, speaking and breathing can cause airborne transmission of the virus, which appears to explain a large proportion of the spread of COVID-19. Researchers have elucidated transmission pathways of COVID-19 by retrospective assessments of documented outbreaks, suggesting that the airborne transmission route is highly virulent and dominant for the spread of the SARS-Cov-2 indoors, under certain conditions (Buonanno et al., 2020a; Miller et al., 2020). In these cases, most likely the airborne transmission of the virus is prevalent in the so-called superspreading events, in which scenarios such as indoor events, with little ventilation, long duration, with people speaking aloud, singing or engaged in heavy exercising activity are considered the worst-case for the transmission of the COVID-19, not necessarily depending on the unlikely presence of a superspreader (Buonanno et al., 2020a; Miller et al., 2020). Airborne transmission could also partially account for the high secondary transmission rates to medical staff, as well as outbreaks in nursing facilities (Prather et al., 2020; Tellier et al., 2019).
Droplets are classically described in the literature as larger particles that rapidly drop to the ground by force of gravity, and aerosols as smaller particles that are small enough to remain suspended in the air, although there is no consensus regarding the diameter that limits the two entities. When it comes to contaminated aerosols, the health implications can be very worrying. Due to the small aerodynamic diameter of the inhalable aerosol particles, the virus can remain suspended in air for hours and accumulates over time. Smaller particles are more impactful when taking into account the health risk, as they can stay longer suspended in the air and also penetrate more easily the respiratory tract. Aerosols with aerodynamic diameter < 5 μm, that researches have denominate “droplet nuclei” (Tang et al., 2020), can easily stay floating in air for many minutes (Netz, 2020) and be inhaled deep into the lungs (Prather et al., 2020). Particulate matter smaller than 2.5 μm (PM2.5) can penetrate deep into the respiratory tract and even reach other vital organs (Rychlik et al., 2019; Wu et al., 2019). It is possible that virus-containing aerosols in the submicron size are transported deep into the alveolar region of the lungs, where immune responses seem to be temporarily bypassed (Prather et al., 2020), leading to more severe infection. Evaporation has a significant role in the evaluation of the route of transmission of the virus by aerosols since it acts reducing the size and weight of the droplets initially emitted, causing them to stay suspended longer, thus significantly increasing the viral load in the air (Netz, 2020). Wells (1934) suggested that droplets with a diameter < 100 μm completely evaporate before reaching the ground and stay sedimenting as “droplet nuclei” for a long time. Regarding the distances that contaminated aerosols can reach, discussions and modelling studies suggest that emission events (e.g. talking, coughs, sneezes) can create thousands of aerosols and propel them meters or tens of meters in indoor air (Gorbunov, 2020; Morawska and Cao, 2020).
Despite the great probability of aerosol transmission of SARS-CoV-2, one of the three pillars for this definitive conclusion or strengthening of this hypothesis is the amount of evidence of virus-containing aerosols (Tang et al., 2020), based on the of Jones and Brosseau criteria (Jones and Brosseau, 2015). Therefore, more experimental confirmation of positive SARS-CoV-2 samples in air particles collected in the field are needed. The small number of studies on this topic is partially because of the difficulties in sampling virus-containing aerosols in real-world settings and the challenges with their quantification in low concentrations (Liu et al., 2020). In addition, full monitoring process is generally very time-consuming and there is often a fear about the negative disclosure of monitoring results in some locations, which can lead to access difficulties for researchers, restrictions on the installation of equipment, etc. In a recent mini review about sampling and detection of corona viruses in air samples, researches highlight that in the most of the studies reviewed, sampling was performed in the patient's room, sometimes very close to (or even in front of) patients not wearing a mask, which makes it difficult to discriminate whether the virus is airborne or transmitted via larger droplets (Rahmani et al., 2020). Despite the obstacles, research continues to emerge. The more studies that are carried out, the sooner it will be possible to better understand the route of transmission through the air, its particularities and its relative relevance among the known modes of transmission. Reports from different locations, with different characteristics (such as climate, pandemic evolution, control policies), can contribute to this effort. Studies on these topics are crucial to the planning of effective practices and methods for pandemic control.
The preset study analyzed the occurrence of SARS-CoV-2 in suspended and sedimentable aerosol particles in 52 monitoring events in hospitals and 10 monitoring events in outdoor public areas in Belo Horizonte, metropolitan center in Brazil.
2. Material and methods
2.1. Study location
The study was carried out in Belo Horizonte, the capital of Minas Gerais, which is one of the most populous metropolitan regions in Brazil (about 6 million people). On the first day of the monitoring (May 25th), 1444 confirmed cases and 42 deaths due to COVID-19 were officially registered in the city (SMS/PBH, 2020). On October 8th the numbers increased to 44,127 confirmed cases and 1331 deaths (SMS/PBH, 2020). The areas selected for monitoring were those recognized as the most critical in terms of infection rates, where most of the deaths and confirmed cases occurred. These correspond to areas with public squares, bus stations/terminals, and hospital areas, i.e., where a large circulation and concentration of people is commonly observed.
2.2. Hospital facilities
Aerosol samples were collected in two hospitals (with different levels of bed occupancy by infected patients and different available infrastructure for contamination control), in both COVID-19 dedicated facilities and non-COVID-19 areas. The first hospital (Hospital 1) was monitored with 33% of ICU occupancy at the beginning of the study (3 patients, being 2 with mild symptoms and 1 intubated, with severe symptoms) and 67% at the end (6 patients, being 5 with mild symptoms and 1 intubated, with severe symptoms), in a better situation regarding the COVID-19 outbreak in the city (1444 confirmed cases of contamination and 42 deaths at the beginning of the monitoring campaign, and 2144 confirmed cases of contamination and 55 deaths at the end (SMS/PBH, 2020)). Hospital 1 had an ICU dedicated to COVID-19 with negative pressure in the entire facility and individual rooms for patients.
The second hospital (Hospital 2) was monitored during a period when 100% of intensive care beds were occupied all the time (10 patients in the ICU, being 9 with mild/moderate symptoms and 1 with severe symptoms, at the beginning). The epidemiological situation during this monitoring period suggested an accelerated spread of the virus in the city, with 2549 confirmed cases of contamination and 62 deaths at the beginning of the monitoring campaign and 13,700 confirmed cases of contamination and 329 deaths at the end (SMS/PBH, 2020). Hospital 2 also had a COVID-19 dedicated ICU, but there was no negative pressure in the room and there was no physical division between patients’ beds. In both hospitals, there was a strict control policy regarding the use of personal protective equipment (PPE), which was strictly followed by medical staff, and surface cleaning and disinfection were performed at least three times a day by a trained cleaning staff.
Non-COVID-19 areas dedicated to patients and medical staff were monitored, including different zones of the restaurant, passageway, medical staff break room, sitting rooms (were all kind of patients, including possible infected patients, were initially received and examined), as well as in more peripheral areas of the hospital, such as a stairway for employee access and ventilation equipment room. Exhaustive sampling was performed inside the ICUs exclusively dedicated to COVID-19 patients or those suspected of infection, and in external areas near ICUs: patient boxes, patient and staff restrooms, corridors, ward units, protective apparel removal rooms (PARR), patient mobile toilet room, room containing patient mobile toilets and used clothes, passageways, staff change rooms, workstation, elevator.
2.3. Outdoor public areas
Open public places were also monitored. Aerosol samples were collected on sidewalks near the hospitals, outdoor outpatient hall, open car parking near hospitals and at a bus station with intense movement of people.
Approximately 3000 passengers circulated daily at the monitored bus station. Although the place was always crowded, security guards were intensively checking the place for the use of masks by passengers and there was a routine of spraying the surfaces with chlorinated disinfectant at least three times a day.
2.4. Sample collection
During the planning of this work (in March 2020), there were no published papers on aerosol sampling for the identification of SARS-CoV-2. To date, there is no guidance on the most effective methods and adequate equipment to monitor the new coronavirus in the air, due to insufficient experimental evidence. Therefore, we decided to carry out an exploratory evaluation using different materials based on experiments with other viruses. When possible, some comparisons about the possible aerodynamic characteristics of the particles were made, taking into account the type of material used and the pore size of the filter medium (a rough estimate).
In general, active sampling methods to suspended aerosol particles (air samplers with filters) and passive methods for sedimentable particles were used (petri dishes). In indoor areas there were severe limitations due to the rules and access restrictions to hospitals facilities. Therefore, the selection of equipment to be used in each monitored facility was a function of imposed limitations and other issues such as noise generated, available space, availability of energy sources and time allowed for monitoring. The equipment, samplers, accessories and common objects were routinely cleaned after each sampling campaign. Before each new sampling, the experimental setups were disinfected using 70% ethanol solutions and prepared as described on item 2.5.
The researchers used appropriate dischargeable PPE (masks, protective eyewear, face shield, gloves and surgical gown) during all the procedures. Additional PPE such as special ICU clothes were worn when necessary, following the protocols already established in the hospitals. COVID-19 protocols such as social distancing and proper prevention hygiene procedures before and after each monitoring were strictly followed by the researchers and hospital staff involved in this work. Cleaning, disinfection, protective and social distance procedures were based on guidelines from Centers for Disease Control and Prevention (CDC, 2020). All monitoring points were signposted and each hospital defined a representative responsible for ensuring the safety and protection of the equipment in the absence of the researchers (security guards in outdoor public areas). Only the researchers handled the equipment and performed the setup and collection procedures.
2.5. Laboratory preparation
Before each monitoring campaign, all materials used in the field (e.g., tweezers, spatula, tongs, scissors, styrene filter cassettes, acrylic apparatus, petri dishes and hoses of the samplers) were sanitized with 70% isopropyl alcohol and placed in sealed pre-sterilized plastic pouches (VEDAMAX®), to be opened at the monitoring site only. Filters were preloaded inside the cassettes and samplers in a laminar flow hood of a controlled area and placed inside of sealed pre-sterilized plastic pouches (VEDAMAX®) for opening at the monitoring site only. Cassettes with filters were also sealed with plastic paraffin tape (PARAFILM®) before packing. Only new filters and materials were used, still with the factory packaging. Air samplers were calibrated according to the manufacturer's manual.
2.6. Indoor environment monitoring
Two types of aerosol samples in indoor environments were collected: (1) aerosol samples of suspended particles using air samplers with filters, in order to quantify the concentrations of SARS-CoV-2 in aerosols and to estimate the size of airborne particulates. In this case, the lower limit was estimated by the filter porosity and the upper limit defined by a cyclone separator (<4 μm at a flowrate of 2.5 L min−1; or with no cyclone, no upper size limit), and/or by approximate comparison between results of sampling with different filters (pore sizes), at the same location; and (2) aerosol deposition samples, in order to determine the deposition rate of airborne SARS-CoV-2.
Cellulose nitrate membrane filters (Sartorius®) with pore size of 0.2, 1.2, 3.0 and 5.0 μm, PTFE (polytetrafluorethylene) membrane filters (Whatman®) with pore size of 2.0 μm, and high-purity quartz microfiber filters (Whatman®) with pore size of 0.3 μm, all measuring 37 mm dimeter, were previously tested in laboratories for their ability to retain low viral loads, by using viral particles dissolved in 5 ml of a solution containing isothiocyanate guanidine (4 M) (Promega®), at cycle threshold values (Ct) of 35/36. With the exception of the membrane filter with a pore size of 5.0 μm, in which the virus passed through and then was identified on the support below the filter, all other filters were able to retain the applied viral load on the surface. The viral load retained on these filters was able to be almost fully recovered (the same Ct was observed before and after the retention test and no virus RNA was identified on the support below the filter). Regarding the 5.0 μm membrane filter, although other retention mechanisms are expected to occur in the sampling of air particles in the field - that can contribute to higher collection efficiency, in addition to what occurred in this test performed with liquid in the laboratory (Verreault et al., 2008) - it was not used for monitoring. Pre-sterilized gelatin filters measuring 80 mm in dimeter (Sartorius®) were also utilized in two sampling events. It is important to note that all of these filter materials have already been used successfully in aerosol virus sampling (Meiklejohn et al., 1961; Pan et al., 2019; Verreault et al., 2008).
Indoor samples of suspended aerosol particles were obtained using portable/personal low flow air samplers and hand-held samplers at higher flows: (a) Portable low flow samplers (CRIFFER®), calibrated to 2.5 L min−1, with 37 mm diameter filters loaded into styrene filter cassettes (SKN Inc.); (b) a hand-held programmable impactor air sampler (AIRIDEAL 3P, Biomérieux®), calibrated to sample a 2000 L volume per run, with a 65 mm diameter filter on a adapted perforated plate inside; (c) a hand-held programmable air sampler (MD-8 AirPort, Sartorius®), calibrated to sample a 2000 L volume per run, with a 80 mm diameter gelatin filter; (d) hand-held vacuum pumps (821T, Fisatom®), 18 L min−1, connected to an adapted acrylic collector containing 37 mm diameter filters; (e) a hand-held high-volume pump (HANDI-VOL, Energética®), calibrated to 150 L min−1, with 100 mm diameter filter inside. The total volume collected in each monitoring event was recorded by the equipment itself or by manual recording. Passive sampling of aerosol deposition was performed using 90 mm diameter quartz microfiber filters (Whatman®) packed into a petri dish, with an effective deposition area of 63.6 cm2.
Because of the need to use pumps, the duration of sampling suspended aerosol particles depended on the conditions and restrictions in each monitored location. The duration of passive sampling for aerosol deposition was defined as approximately one week, according to a study conducted by researchers from Wuhan, China, a pioneering study on the subject, which reports the results of deposition samples for 7 monitoring days (Liu et al., 2020).
To aid in the analysis, some surface samples were taken with a swab, to assess the possibility of surface contamination by hand touch or to evaluate aerosol deposition in places where the petri dish could not be placed. In this case, swabs with sterile phosphate-buffered saline were rubbed on surfaces, in a 10 cm2 area, and then transferred to tubes containing 1 mL of guanidine isothiocyanate buffer, 4M (Promega®), which was used as the transport solution.
At the end of the sampling, filters were collected on-site with laboratory tweezers, folded with the collection surface facing inwards, packaged in pre-sterilized sealed plastic bags and sent immediately to the laboratory (when not possible, on a few occasions, the filters were refrigerated at 4 °C until receipt at the laboratory the next day). Having determined the viral content in the particulate samples on the filters, in terms of the number of genomic units, it was possible to estimate the viral concentrations in the air (genomic units m−3) or the virus aerosol deposition rate (genomic units m−2 h−1).
Relative humidity and temperature inside the units were measured by a conventional digital thermo hygrometer (AKSO AK-28® similar, ±1 °C, ± 5%RH).
2.7. Outdoor environment monitoring
Aerosol samples in external environments were collected using high-volume air samplers, HVS (AGV, Energética®), designed to collect ambient particulate matter with an aerodynamic diameter of 10 μm (PM10) or 2.5 μm (PM2.5) (±5%), operating with a mean flow rate of 1130 L min−1.
Before each sampling procedure, the equipment was calibrated according to the manufacturer's guidelines to obtain the actual operating flow rate, which may vary with the weather and equipment conditions. The total duration of each sampling event was checked with an analog hour meter at the time of collection. Quartz microfiber filters (Whatman®), in a 203 × 254 mm rectangular sheet, were used to retain the particles in the HVS. The sampled air volume, corrected for standard conditions (298.15 K, 760 mmHg), was determined from the measured flow and real sampling time. At the end of the sampling, the filters were collected on site with laboratory tweezers, folded with the collection surface facing inwards, packaged in presterilized sealed plastic bags, weighted on a precision balance and sent immediately to the laboratory (when it was not possible, on a few occasions, the filters were refrigerated at 4 °C prior to receipt at the laboratory the next day). The particulate concentrations in the air, expressed in micrograms per cubic meter (μg m−3), were obtained by dividing the mass of the particles collected in each sample by the filtered air volume.
The ambient relative humidity and temperature were also measured by a digital thermo hygrometer (AKSO AK-28® similar, ±1 °C, ± 5% RH). Absolute atmospheric pressure, necessary for equipment calibration and data correction, was measured with a digital barometer (TESTO 511®, ±3 hPa).
2.8. Analytical method and data analysis
Air particles retained on the cellulose, PTFE and quartz microfiber filters were removed by swabbing the entire surface of each filter (swab with 1 mL of guanidine isothiocyanate buffer, 4 M) and triturating the remaining filter. Then, both (swab tip + filter) were mixing with 4 mL of sterile deionized water for liquid extraction in a mixer. In the larger rectangular quartz microfiber filters (used in the HVS), the same procedure was performed on a 100 × 100 mm piece removed from the center of the filter. Regarding the samples with gelatin filters, each was transferred to a clean 15 mL conical tube right after the sampling campaign and 10 mL of sterile deionized water per sample was added immediately to each tube. The tubes were then centrifuged and incubated at 37 °C for 10 min in a block heater to dissolve the gelatin.
Nucleic acid extraction and the RT-qPCR analysis were performed at the Virus Lab, similarly to recent work for detection of SARS-CoV-2 RNA on surfaces carried out by this same group (Abrahão et al., 2020). Briefly, for each sample after particle extraction, 70 μL of transport solution was submitted to nucleic acid extraction using the QIAmp Viral RNA Mini Kit (QIAGEN®). Total RNA (5 μL) was used as a template for one-step qPCR (Promega®) (in a final volume of 20 μL per reaction, GoTaq1-sept qPCR system, Promega®), using primers and probes specific for the N1 and N2 regions of the SARS-CoV-2 genome (CDC, USA, 2020). Samples were considered positive if amplification of the N1 and N2 target regions occurred and had a cycle threshold value (Ct) for quantification less than 40 (CDC, USA, 2020), since the range of 37–40 indicate minimal quantities of DNA. RNA extraction was performed in batches of 13 samples plus one negative control. The negative controls consisted of the extraction control and a non-template control. Positive controls consisted of RNA extracted from inactivated SARS-CoV-2, kindly provided by Dr. E. Durigon and Dr. D. Durigon of USP, Brazil. Following the MIQE Guidelines (Bustin et al., 2009), results of the RT-qPCR runs were manually inspected for the correction of baseline and threshold parameters whenever necessary due to heterogeneity in the amount of input RNA among different samples. To confirm the results, all positive samples were submitted to a second round of RNA extraction and RT-qPCR. Quantification of viral RNA in the aerosol samples was based on a standard curve generated from serial dilutions (1:10) of SARS-CoV-2 RNA and converted to genomic units per volume (N1 gene). The SARS-CoV-2 RNA control was previously quantified as described in a previous study (Almeida et al., 2020). To date, for PCR quantification (based on standard curve) we considered 1 SARS-CoV-2 plaque forming unit as 1 SARS-CoV-2 genomic unit. Quantification was based only on the N1 target gene given the high efficiency of our standard curve (Abrahao et al., 2020). N2 target gene quantification was not performed due to a low efficiency achieved for the standard curve for this target, as described elsewhere (Abrahao et al., 2020).
3. Results and discussion
A summary of the monitoring can be seen in Table S1, Supplementary Material. The table is divided into “Hospital 1” facilities, “Hospital 2” facilities (indoor environments), and “Outdoor areas/public spaces”. The points monitored in hospitals are classified as “COVID-19 dedicated facilities” and “Non-COVID-19 facilities”. Sampling events are numbered. There is also a description of the location, equipment used, type of filter, start and end time of monitoring, total sampled volume, relevant observations and result of the concentration of genomic units in the aerosol particles (for suspended aerosol samples) or deposition rate (for sedimentable particle samples). Five aerosols samples tested positive for SARS-CoV-2 RNA in four indoor hospital facilities. The main results are then presented and discussed, including discussion on possible explanations and suggestions for control methods.
3.1. Positive samples to SARS-CoV-2 RNA in aerosols
Air samples from four facilities in Hospital 2 tested positive for SARS-CoV-2 in suspended particles retained in filters of pore size >0.2 μm and >0.3 μm, and in sedimentables particles. The relative humidity and temperature inside the units varied from 46% to 15.6 °C (minimum) to 62% and 24.3 °C (maximum), respectively. SARS-CoV-2 RNA was found in suspended air particles inside the COVID-19 ICU, in the PARR, and in the room for patient mobile toilets and used clothes (at the ward). SARS-CoV-2 RNA was found in sedimentable particles in the external corridor adjacent to the ICU. Results of surfaces samples (swabs) collected in the same places (inside the ICU and PARR) were all negative.
3.1.1. Intensive Care Unit (ICU)
SARS-CoV-2 was detected in an aerosol sample inside the ICU dedicated to 12 symptomatic patients known to be, or suspected to be, infected with COVID-19. A concentration of 0.33 genomic units m−3 was obtained from the genomic material found in a 0.3 μm pore size quartz microfiber filter adapted to a hand-held air sampler (sampling event no. 19 in Table S1, Supplementary Material). There was no negative pressure in the ICU and no physical division between patients. In this case, the sampling device was placed on a table near the wall, 1.2 m high and at least 2 m from the nearest patient (but not directly in front of). Patients remained in their bed during the whole stay in the ICU. No aerosol-generating medical procedures were performed during the monitoring period, strengthening the hypothesis of SARS-CoV-2 in small aerosol particles released in the air by contaminated patients. Monitoring performed in the ICU during the same period with a low-flow pump and a 0.2 μm pore cellulose membrane showed a negative result (sampling event no. 20 in Table S1, Supplementary Material), however, in this event the volume of air collected was 8 times smaller. Swab sampling on high-touch surfaces in the same ICU environment, carried out at the end of the air monitoring period, were negative (sampling events no. 24, 25 in Table S1, Supplementary Material). In Nebraska, USA, air samples from hospital and residential isolation rooms housing individuals testing positive for SARS-CoV-2 were 63.2% positive, with mean concentration of 2.9 copies/L of air (Santarpia et al., 2020). In Singapore, air samples from two of three airborne infection isolation rooms tested positive for SARS-CoV-2, in particle sizes >4 μm and 1–4 μm in diameter. Samples from the fractionated size <1 μm were all negative in that study, as were all non-size-fractionated PTFE filter cassette samples (3 μm pores). SARS-CoV-2 concentrations in the air ranged from about 916 to 2000 RNA copies per m3 of air sampled. In the rooms where positive samples were found, patients remained in bed within 1 m from all samplers and one of them was also talking on the phone for a significant proportion of time during sampling (Chia et al., 2020). Another study, in Wuhan, China, found contamination in air samples for 35% (14 samples positive/40 samples tested) of ICU samples; at two points <1 m from patients (average virus concentration of 3.8 and 1.4 copies L−1) and at one point ≈4 m from patients (3.8 and 0.52 copies L−1) (Guo et al., 2020).
To minimize the effect of this contamination source, an airborne infection isolation room, a specific place where patients infected with highly airborne infectious diseases are treated, can be adopted wherever possible. For example, negative pressure isolation rooms can be implemented in high-risk settings, although these measures are not completely sufficient to prevent the transmission of COVID-19. Researchers described the factors involved in aerosol transmission in hospitals and presented how the dispersion of the droplet nuclei is also affected by airflows from open windows, ventilation systems and door opening (Tang et al., 2006). When airborne infection isolation rooms are not available, the importance of monitoring airflow, air exchange, and ventilation and filtration aspects in closed environments is paramount. Regarding filtration, centers treating infected SARS-CoV-2 patients should assure that the air delivered to external areas passes through a high-efficient filtration system. The hospital 2 exhaustion system was evaluated in this work using a hand-held vacuum pump with quartz microfiber filters, placed on a floor stand, 0.5 m high, near the air outlet coming from the ICUs (after passing through filtration systems using High Efficiency Particulate Air, HEPA, filters), and by a swab test on the wall in the same place. PCR indicated no detectable virus RNA in the samples (sampling events no. 50, 51 and 52 in Table S1, Supplementary Material).
3.1.2. Protective apparel removal room (PARR)
In the PARR, a positive sample was observed by monitoring using a 0.2 μm pore size cellulose membrane filter coupled to an acrylic collector adapted to a vacuum pump (since higher noise levels were allowed at certain times), placed in an isolated and protected spot in the corner, 0.5 m above the floor (sampling event no. 18 in Table S1, Supplementary Material). The room was approximately 4 m × 2 m (3 m height). A concentration of 0.14 genomic units m−3 of filtered air was found. Results of parallel and later monitoring at the same place, using 1.2 μm and 2 μm pore size membranes with the same type of equipment and same flowrate were negative, suggesting possible submicron contaminated particles. In this room, the medical staff removes gowns, surgical caps, gloves and masks used in the ICU and arranged them in containers. The room does not have air disinfection system (e.g. UV irradiation), negative pressure or exhaustion. The doors are constantly opened and closed by the staff, causing air movement. During this procedure, small contaminated particles from the PPE can become aerosols by resuspension and carry the SARS-CoV-2 into the air. Greater mobility of submicron particles facilitates resuspension from PPE surface after gaining the initial velocity while being removed (Liu et al., 2020). In one of the first reports of the SARS-CoV-2 presence in aerosols, these authors reported positive results in three PARRs in a hospital in Wuhan, China, epicentre of the pandemic, with concentrations ranging from 16 to 42 copies m−3 and most copies in the diameter size range of 0.25–1.0 μm.
3.1.3. Room for patient mobile toilet and used clothes disposal
A positive sample was also observed in a place with natural ventilation, in the room for disposal patient mobile toilet and used clothes before washing and disinfecting, in a COVID-19 ward. The room was approximately 5 m × 2 m (3 m height), with a single window and no air disinfection system. Because higher noise levels were allowed at certain times, monitoring was performed at higher flow rates, with a hand-held vacuum pump coupled to an acrylic collector (sampling event no. 42 in Table S1, Supplementary Material, using 0.3 μm pore size quartz microfiber filter). The equipment was placed in an isolated spot near wall, 5 m in front of the window (in the opposite wall), 1.0 m high. Due to the configuration of the room, the wind entered the window and blew through the materials used by contaminated patients before reaching the monitoring point. There was no cross ventilation or other air circulation strategy, which can result in low rates of air change in the room. Access to the site was restricted to a small number of professionals responsible for placing/collecting the materials. A concentration of 0.19 genomic units m−3 was encountered, probably resulting from the aerosolization of virus-laden particles from the materials temporarily stored in the room. This result may be considered a warning regarding the importance of studies on air circulation in closed environments with low rates of dilution ventilation and the importance of using masks in the presence of a potentially contaminated person, even though this environment has natural ventilation. A positive result with 19 copies m−3 in a patient mobile toilet closed room (a similar place) was reported in a hospital in Wuhan (Liu et al., 2020).
3.1.4. External corridor
Two aerosol deposition samples tested positive in the external corridor near the ICU. In the first monitoring event (no. 22 in Table S1, Supplementary Material), a petri dish with a quartz microfiber filter was placed on the floor, in an isolated, signposted and protected spot near the entrance door to the ICU, for seven days. In the other (sampling event no. 36 in Table S1, Supplementary Material), the same apparatus was placed in an isolated, signposted and protected spot on a windowsill situated almost in front of the same door, 1.5 m high, for 7 day as well. Estimated deposition rates were 66.4 and 7.1 genomic units m−2 h−1, respectively. Although the two monitoring events were carried out in different weeks, the fact that the petri dish is on the floor and closer to the ICU entrance door may explain the higher value of the aerosol deposition rate in the first. Two positive results of aerosol deposition in a COVID-19 ICU in Wuhan were reported (Liu et al., 2020), at deposition rates of 31 and 113 copies m−2 h−1, which were also monitored for seven days. Due to the long monitoring time, it is unlikely that any virus deposited in the first days will survive. Therefore, it is expected that the positive results obtained through this method are related to aerosol generated and deposited in the last days of the monitoring period (a limitation for the calculation of deposition rates). On the other hand, longer times can facilitate the deposition of smaller aerosol particles. In this work, because it is a passive method, it was possible to maintain the monitoring period reported by these authors, allowing for a more direct comparison between the data. Samples of suspended aerosol particles in the same corridor were negative (sampling events no. 44 and 45 in Table S1, Supplementary Material). In a research preprint, authors reported one air sample from a corridor that was weakly positive for SARS-CoV-2, in a hospital to COVID-19 patients in Nanjing, China. It was the only positive among 46 air samples in the hospital (Ding et al., 2020).
Due to the strict control in this area, it is likely that airborne virus came from the ICU, probably due to the exchange of air between the two environments. According to literature, it is known that human movement and air circulation can affect the dispersion of aerosols. Depending on aerodynamic size and initial exhaled velocity, particles can behave differently from the gaseous portion of the air under the influence of human walking (Wang and Chow, 2011). At Hospital 2, elevated healthcare staff movement between the two areas was observed close to the ICU door (a hinged door), to receive medical supplies, equipment and medicines. This may have had significant effects on the dispersion and deposition of aerosols on the monitoring petri dishes outside the ICU. People moving and walking, equipment transfer and the simple opening movement of the hinged door can generate local pressure drop and/or recirculation zones, leading to a large exchange of air across the doorway and contributing to the escape of contaminated aerosols from the ICU to the corridor. Although the flow of staff was well defined in this ICU, the entry of healthcare material should be reformulated. Pass-through systems or appropriate doors may be good solutions. The problem noted with hinged doors may also be reduced by the use of sliding doors (Wang and Chow, 2011).
3.2. Applications
All positive results in the hospital facilities were discussed with the hospital management team and contributed to changes in the layout of adjacent ICU areas, adjustment of the staff flow between areas and improvement in protection measures. Although the results were observed in hospital facilities, the confirmation of the presence of the virus in the air reinforces the concern with the most critical scenarios for the COVID-19 transmission through this route, which are poorly ventilated environments in the presence of individuals coughing, sneezing, speaking loudly, shouting, singing and/or with accelerated breathing (during intense physical exercises, for example). These factors, associated with long exposure times, increase the likelihood of airborne transmission. In this sense, greater attention should be paid to environments such as gyms, offices, stores, churches, choirs, pubs, nightclubs, among others.
It is important to emphasize that all factors must be evaluated together for a more complete assessment of risk and assistance in planning restrictive or control measures. An example of a prospective approach to quantitative risk assessment in closed spaces can be seen in (Buonanno et al., (2020a)). More complex fluid dynamics studies, such as using the CFD (computational fluid dynamics) tool, can also provide insights into the particular conditions of an environment and contribute to the improvement of the risk assessment, including in the analysis relevant factors such as geometry and boundary conditions related to the environment, positioning of ventilation devices, air flows, aerosols characteristics and many others.
3.3. Negative results and limitations
Air samples from Hospital 1 tested negative for SARS-CoV-2 RNA in suspended and deposition particles (sampling events no. 1 to 16 in Table S1, Supplementary Material). Although it is not possible to determine the exact reason, it is important to remember that Hospital 1 was in a better situation with regards to advance of the COVID-19 pandemic in the city and bed occupancy. Moreover, the entire ICU dedicated to COVID-19 was under negative pressure, including in the individual patient rooms, in which no equipment was allowed.
When monitoring open public spaces, air sample were all negatives for SARS-CoV-2 RNA (one sample in a car parking lot near the hospital, four samples on sidewalks near the hospitals and five samples at a bus station). The mean daily relative humidity and temperature varied from 51% to 9.6 °C (minimum) to 62% and 26.1 °C (maximum), respectively. Negative results were expected in these areas, where excellent air circulation was observed (wind perception, suggesting high air renovation), as well as the correct use of protective masks by the people who were circulating close to the monitoring sites. It is possible that the frequent use of the protective masks was a function of the presence of the equipment, researchers and/or security guards on the site (since in the city, mask use was not completely followed by the population). In fact, in entirely open environments, risks are expected to be reduced due to the large dilution and dispersion that may occur with aerosols due to the action of winds and other environmental factors. On the other hand, the high operating flow of the HVSs, desiccation effects, strong impaction and the high concentration of contaminants in the air (particulate matter) may have contributed to complicate extraction of the genetic material of the virus under viable conditions for detection. Information on the concentration of particulate matter in the air (PM10 or PM2.5) is also shown in Table S1, Supplementary Material. Upon determination of the viral content in the particulate samples on the filters, besides the viral concentrations in the air (genomic units m−3), it is possible to obtain the concentration in terms of mass of the total suspended particles (genomic units g−1), which is an information not yet obtained and reported in literature.
Many negative results were obtained in hospital indoor places where the opposite was expected. In addition to the possible interferences mentioned above, some more general interferences can also contribute to the explain the results. In the laboratory, losses are expected in the extraction of the viral genetic content from the filters, caused by injury in post-sampling processes (Pan et al., 2019). There are also intrinsic uncertainties and variations regarding the monitored conditions in the field (e.g., bed occupancy rate, severity patient symptoms, viral load and distance to the possible sources). Temperature, radiation, oxygen, ozone and other exposures can also damage viral proteins, lipids and nucleic acids (Cox, 1989). Short-time or low-volume samplings (due to restrictions on presence of equipment or noise) or even sampling with very long monitoring time and/or high air flow through the filters can affect the results. Short-time or low-volume samplings can decrease the chances of trapping the virus when indoors. On the other hand, very long monitoring times and/or high air flow can cause physical damage by desiccation effects, strong impaction and viruses being trapped by the inlet or the samplers’ wall (Pan et al., 2019; Rahmani et al., 2020), resulting in the losses of some or all the collected viruses components. All these factors contribute to limit our understanding of the negative results. These factors can also explain the low RNA concentration values in the air found in the present work, when compared to similar studies. According (Fennelly, 2020), these multiple factors, as well as inherent physical inefficiencies of air samplers, also suggest that most infectious aerosol data are probably underestimates of the actual exposure to the virus. The results presented in this work may be underestimated in terms quantity of positive samples and concentrations of virus particles due to many factors responsible for losses and degradation of the virus RNA during field sampling and analytical procedures in the laboratory, which further reinforces the importance of the positive results.
4. Practical aspects of the monitoring
Although the use of different techniques can generate uncertainty when comparing results, the main objective was to assess the presence of SARS-CoV-2 RNA in aerosol particles. At the same time, it was considered that preliminary results by using different equipment and filter media could serve as an additional reference for the next steps of this study and for other researchers. Some studies have been recently published on the monitoring of SARS-CoV-2 in aerosol, however the lack of sampling standardization of airborne viruses, and the impact on development of general recommendations should be addressed. Until now, no single device has been demonstrated capable of serving as the gold standard sampler to SARS-Cov-2, as well as in the detection of another airborne viruses, as noted by (Pan et al., (2019)). Parameters such as monitoring time, air volume and extraction methods are not clearly established for indoor or outdoor monitoring. The results of this work may contribute to monitoring practices and equipment selection, however, considering a future possibility that aerosol monitoring for SARS-CoV-2 be adopted as a routine control measure in some environments, it is important that more specific studies emerge which focus on the most appropriate techniques for both indoor and outdoor monitoring. Different air samplers are commercially available and innovative systems are being disclosed. Membrane filters are more efficient for determining viral loads in aerosols, but can damage viruses (Verreault et al., 2008). An adequate sampling device and material for airborne viruses must be proposed so the potential risk of infection could be predicted more accurately. “The fact that there are no simple methods for detecting the virus in the air does not mean that the viruses do not travel in the air” (Morawska and Cao, 2020).
From the exploratory evaluation and the use of various sampling techniques and methods, it was possible to make some practical considerations based on the results of this work. (a) Cellulose membrane filters are fragile and can break during long-term monitoring and when using higher flow rates. We had many missing samples related to these cases, in which surface cracking was observed and may have occurred due to dryness in conjunction with clogging of the filter medium. PTFE and quartz microfiber filters were resistant to longer monitoring periods and higher flow rates. (b) Despite being more costly, gelatin filters were more practical to use because they did not demand a stage of physical extraction of particulates, which can prevent damage to genetic material and increase virus recovery efficiency. Although only a few of these units were used in this work, these filters are highly recommended for microorganism sampling (Verreault et al., 2008) and most studies with positive test results with corona viruses have used gelatin filters and PTFE filters (Rahmani et al., 2020). Their use can be limited by environmental conditions, since low humidity can cause them to dry out and break, while high humidity or water droplets can cause them to dissolve before the end of the monitoring event (Pan et al., 2019; Verreault et al., 2008). (c) Portable low flow samplers are practical and more versatile to use in different environments, because they are easily moved and set up for experiment. However, they may have a short battery life (usually less than 8 h), which is further reduced by the use of low porosity filters, essential for studies with aerosols. The pressure loss caused by the filters means that the power of the sampler has to be increased to maintain the flow rate, which considerably reduces battery life and monitoring time. On the other hand, vacuum pumps (connected to a collector) had the advantage of allowing longer monitoring time and higher flow rates, but the noise produced is not compatible with environments such as hospitals and workstations.
5. Conclusions
In general, our findings contribute to reinforce the hypothesis of airborne transmission of the new coronavirus. To the best of our knowledge, this work is the first to assess SARS-CoV-2 in the air of indoor and outdoor locations in Brazil, and it is one of few published papers that present scientific evidence of the occurrence of the new coronavirus in aerosols. The results provide insights to a better understanding the dynamics of viral dissemination within critical COVID-19 areas inside a hospital and insights regarding possible control measures.
SARS-CoV-2 RNA was found in both suspended and sedimentable particles in indoor environments. Positive results of SARS-CoV-2 RNA in aerosols were restricted to Hospital 2, in which all of the intensive care beds were occupied, there was no negative pressure in the ICU and no physical division between patients. Critical points in the hospital facilities and possible virus circulation through the air was identified, as well as approximate particle diameters, suggesting possible submicron contaminated particles (a rough estimative based on the filter pore size), and aerosol deposition rates. Positive results for SARS-CoV-2 RNA were not restricted to inside the ICU, and even in the ICU sample, were not reported for closed patient rooms or monitoring events performed very close to patients. The virus was also found in aerosol particles collected in a room with natural ventilation, but with no cross ventilation or other circulation strategy, suggesting low rates of air change. Because of strict control policies regarding the use of personal protective equipment in these places, which were appropriately strictly followed by the staff, positive results to SARS-CoV-2 RNA in the particles were considered to be airborne, probably dispersing from contaminated patients in controlled environments to external areas or by the aerosolization and dispersion of virus-laden particles from materials, apparels and equipment.
These findings represent an alert about the transmission of the virus through the air. Although observed in hospital facilities (controlled environments), the presence of SARS-CoV-2 in aerosols and the possibility of dispersion from contaminated persons or material (aerosolization) over longer distances also draws attention to other environments; these are mainly closed spaces with insufficient air circulation, in which the air-exchange rate is usually much lower than necessary, in the presence of individuals coughing, sneezing, speaking loudly, shouting, singing or in intense expiratory activities (during intense physical exercises, for example), especially when policies on masks wearing and concentration of people may not be strictly followed. These findings may also reinforce the importance of controlling the occupancy of spaces (number of people in certain environments) and the need for specific studies to define air circulation strategies in closed environments (e.g., local fluid dynamics studies and models) or even air disinfection (e.g., ultraviolet irradiation), which may be especially necessary in more vulnerable places, such as hospitals and nursing homes.
In outdoors environments, air samples were all negatives for SARS-CoV-2 RNA. It is expected that the risks are well reduced in open spaces, due to the high dilution/dispersion that can occur with aerosols due to winds and other environmental factors. However, special attention should be given to crowded sites with insufficient air circulation, which are factors that can eventually override the “intrinsic security” of open places.
In order to consolidate the understanding of the aerosol transmission route and pandemic control policies to be adopted around the world, more studies on the airborne transmission potential of SARS-CoV-2 are urgent and necessary, including air monitoring in the field, further studies on the aerodynamic characteristics, infectious load, main places of occurrence and transmission pathways of SARS-CoV-2 related to aerosols, to contribute to the planning of effective practices and methods for pandemic control.
Authorship contribution statement
R. Passos, Conceptualization, Methodology, Validation, Writing. M. Silveira, Conceptualization, Methodology, Validation, Writing. J. Abrahão: Methodology, Validation, Writing. The manuscript was written through contributions of all authors. All authors contributed equally.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank the staff in the hospitals for their support with field operations, especially Washington, Ângela, Max and Helvécio, and the BHTRANS/PBH for the support with the fieldwork at the bus stations and public spaces. We thank the CDTN for the continued support to this research, especially Dr. Luiz. C. D. Ladeira (CDTN/Director), Dra. Amenônia M. F. Pinto (CDTN/SEAMA) and Dr. Márcio T. Pereira (CDTN/SERFI). We also thank our colleagues from Laboratório de Vírus da UFMG (Betania Drumond, Giliane Trindade, Erna Kroon and the whole team), Gabinete da Reitora da UFMG, the Pró-Reitoria de Pesquisa da UFMG/Secretaria de Educação Superior/ Ministério da Educação (number 23072.211119/2020-10), Finep/RTR/PRPq/Rede COVID-19 (number 0494/20-0120002600). J.S.A. is a CNPq researcher and member of the Rede Vírus MCTI.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.110808.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abrahão J.S., Sacchetto L., Rezende I.M., Rodrigues R.A.L., Crispim A.P.C., Moura C., Mendonça D.C., Reis E., Souza F., Oliveira G.F.G., Domingos I., Boratto P.V. de M., Silva P.H.B., Queiroz V.F., Machado T.B., Andrade L.A.F., Lourenço K.L., Silva T., Oliveira G.P., Alves V. de S., Alves P.A., Kroon E.G., Trindade G. de S., Drumond B.P. Detection of SARS-CoV-2 RNA on public surfaces in a densely populated urban area of Brazil: a potential tool for monitoring the circulation of infected patients. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.142645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P.R., Demoliner M., Antunes Eisen A.K., Heldt F.H., Hansen A.W., Schallenberger K., Fleck J.D., Spilki F.R. SARS-CoV2 quantification using RT-dPCR: a faster and safer alternative to assist viral genomic copies assessment using RT-qPCR. bioRxiv. 2020 doi: 10.1101/2020.05.01.072728. 2020.05.01. [DOI] [Google Scholar]
- Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40:902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno G., Morawska L., Stabile L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: prospective and retrospective applications. Environ. Int. 2020;145:106112. doi: 10.1016/j.envint.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- CDC Prevent getting sick. National center for immunization and respiratory diseases (NCIRD), division of viral diseases. 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/index.html
- Chen L., Liu W., Zhang Q., Xu K., Ye G., Wu W., Sun Z., Liu F., Wu K., Zhong B., Mei Y., Zhang W., Chen Y., Li Y., Shi M., Lan K., Liu Y. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microb. Infect. 2020;9:313–319. doi: 10.1080/22221751.2020.1725399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y.S., Ng O.T., Wong M.S.Y., Marimuthu K., Lye D.C., Lim P.L., Lee C.C., Ling L.M., Lee L., Lee T.H., Wong C.S., Sadarangani S., Lin R.J., Ng D.H.L., Sadasiv M., Yeo T.W., Choy C.Y., Tan G.S.E., Dimatatac F., Santos I.F., Go C.J., Chan Y.K., Tay J.Y., Tan J.Y.L., Pandit N., Ho B.C.H., Mendis S., Chen Y.Y.C., Abdad M.Y., Moses D. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.S. Airborne bacteria and viruses. Sci. Prog. 1989;73:469–499. [PubMed] [Google Scholar]
- Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.-L., Xiao S., Cui L., Wu X., Shao W., Song Y., Sha L., Zhou L., Xu Y., Zhu B., Li Y. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. 2020. [DOI] [PMC free article] [PubMed]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunov B. Aerosol particles laden with COVID-19 travel over 30m distance 1–18. 2020. [DOI]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y., Zhang M.-Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.-W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, wuhan, China, 2020. Emerg. Infect. Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Brosseau L.M. Aerosol transmission of infectious disease. J. Occup. Environ. Med. 2015;57:501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- Kohanski M.A., Palmer J.N., Cohen N.A. Aerosol or droplet: critical definitions in the COVID-19 era. Int. Forum Allergy Rhinol. 2020;10:968–969. doi: 10.1002/alr.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Morawska L. Could fighting airborne transmission be the next line of defence against COVID-19 spread? City Environ. Interact. 2019;4:100033. doi: 10.1016/j.cacint.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yuan, Ning Z., Chen Y., Guo M., Liu Yingle, Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K., fai Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Meiklejohn G., Kempe C.H., Downie A.W., Berge T.O., St Vincent L., Rao A.R. Air sampling to recover variola virus in the environment of a smallpox hospital. Bull. World Health Organ. 1961;25:63–67. [PMC free article] [PubMed] [Google Scholar]
- Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., Kurnitski J., Marr L.C., Morawska L., Noakes C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020:1–10. doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz R.R. Mechanisms of airborne infection via evaporating and sedimenting droplets produced by speaking. J. Phys. Chem. B. 2020;124(33):7093–7710. doi: 10.1021/acs.jpcb.0c05229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA, J. Am. Med. Assoc. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Lednicky J.A., Wu C.Y. Collection, particle sizing and detection of airborne viruses. J. Appl. Microbiol. 2019;127:1596–1611. doi: 10.1111/jam.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science 84. 2020;368:1422. doi: 10.1126/science.abc6197. LP – 1424. [DOI] [PubMed] [Google Scholar]
- Rahmani A.R., Leili M., Azarian G., Poormohammadi A. Sampling and detection of corona viruses in air: a mini review. Sci. Total Environ. 2020;740:140207. doi: 10.1016/j.scitotenv.2020.140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik K.A., Secrest J.R., Lau C., Pulczinski J., Zamora M.L., Leal J., Langley R., Myatt L.G., Raju M., Chang R.C.A., Li Y., Golding M.C., Rodrigues-Hoffmann A., Molina M.J., Zhang R., Johnson N.M. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc. Natl. Acad. Sci. U.S.A. 2019;116:3443–3448. doi: 10.1073/pnas.1816103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., Brett-Major D.M., Schnaubelt E.R., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMS/PBH . Belo Horizonte; MG: 2020. Boletins Epidemiológicos COVID-19. [Google Scholar]
- Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., Shen J., Lv Y., Pan L., Ding P., Wang X., Wang Y., Macintyre C.R., Shi X. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. J. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:1–9. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008;72:413–444. doi: 10.1128/mmbr.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chow T.T. Numerical investigation of influence of human walking on dispersion and deposition of expiratory droplets in airborne infection isolation room. Build. Environ. 2011;46:1993–2002. doi: 10.1016/j.buildenv.2011.04.008. [DOI] [Google Scholar]
- Wang J., Du G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. 2020:5–6. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells W.F. On air-borne infection. Study II: droplets and droplet nuclei. Am. J. Epidemiol. 1934;20:611. doi: 10.1093/oxfordjournals.aje.a118097. [DOI] [Google Scholar]
- WHO Coronavirus disease 2019, coronavirus disease (COVID-19) situation report – 182. 2020. [DOI]
- Wu G., Brown J., Zamora M.L., Miller A., Satterfield M.C., Meininger C.J., Steinhauser C.B., Johnson G.A., Burghardt R.C., Bazer F.W., Li Y., Johnson N.M., Molina M.J., Zhang R. Adverse organogenesis and predisposed long-term metabolic syndrome from prenatal exposure to fine particulate matter. Proc. Natl. Acad. Sci. U.S.A. 2019;116:11590–11595. doi: 10.1073/pnas.1902925116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. SARS-coV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X., Lou Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R. Di, Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.