Abstract
Aims
Heart failure (HF) confers potentially negative effects on the brain and autonomic nervous system. The measurement cerebral tissue oxygen saturation (SctO2) may aid in understanding such effects. We aimed to investigate if compensated HF affects SctO2 at rest and during orthostatic challenge.
Methods and results
Non‐invasive haemodynamic monitoring and near‐infrared spectroscopy were applied during head‐up tilt (HUT) in 61 HF patients [mean (SD) 71 (11) years, 82% male, New York Heart Association (NYHA) class I–III] and 60 controls [60 (12) years, 42% male). Group differences in continuous variables were compared using Student's t‐test. Associations between HF and SctO2 were studied using multivariable linear regression models adjusted for age, sex, diabetes, smoking, systolic blood pressure (SBP), and heart rate in supine position and after 10 min of HUT.
Mean SctO2 was lower in HF patients compared with controls both in the supine position (67 vs. 71%; P < 0.001) and after 10 min of HUT (64 vs. 69%; P < 0.001). The HUT‐induced SctO2 decrease was greater in HF patients compared with controls (P = 0.026). SBP did not change in neither HF patients nor controls during HUT, whereas diastolic blood pressure and heart rate increased in both groups. HF was associated with lower SctO2 in supine (B = −2.5%, P = 0.023) and after 10 min of HUT (B = −2.6%, P = 0.007) after multivariable adjustments.
Conclusions
Cerebral tissue oxygenation is lower in HF patients both at rest and during orthostasis compared with subjects without HF. Future studies should test if the lower cerebral oxygenation associates with negative prognosis and with impaired cognitive function.
Keywords: Heart failure, Cerebral oximetry, Head‐up tilt test, Orthostatic provocation
Introduction
Heart failure (HF) is a clinical syndrome characterized by impaired cardiac output (CO) and/or elevated intracardiac pressures, resulting in symptoms of fatigue, dyspnoea, and peripheral and/or pulmonary congestion. 1 , 2 In response to decreased CO with subsequent tissue hypoperfusion, HF patients often have chronically elevated sympathetic and neurohormonal activation. 3 An under‐investigated complication of HF is the potentially negative effects on the brain 4 and the autonomic nervous system, 5 possibly secondary to cerebral hypoperfusion due to systemic hypotension. This could partially explain the well‐known overrepresentation of cognitive dysfunction in HF patients, as well as the demonstrated faster decline in global cognitive ability in HF patients compared with controls. 6
In healthy individuals, standing causes pooling of venous blood in the lower extremities and splanchnic area, leading to decreased venous return and reduced CO. In turn, decreased CO initiates various autonomic responses that if insufficient or impaired, ultimately may result in decreased cerebral blood flow (CBF). 7 In HF, where CO already is limited, upright posture may increase the vulnerability to cerebral hypoperfusion, further increasing the risk of chronic cerebral ischemia and cognitive impairment. 8
Previous research indicates that HF patients show an abnormal haemodynamic response to upright posture 8 , 9 , 10 and a greater decrease in CBF 9 , 11 during orthostatic stress compared with healthy individuals. However, changes in cerebral tissue oxygenation (SctO2) during orthostatic stress have not been systematically investigated in patients with HF. Cerebral oximetry assessed by near‐infrared spectroscopy can non‐invasively provide absolute values of SctO2 in real‐time and synchronized with the measurement of haemodynamic parameters during orthostatic provocation. 12
Here, we investigated if HF affects SctO2 relative to changes in haemodynamic parameters during controlled orthostatic provocation.
Methods
Study population
Patients
Sixty‐one patients with compensated HF [New York Heart Association (NYHA) class I–III] were included from the HeArt and bRain failure inVESTigation project (HARVEST‐Malmö). 13 HARVEST‐Malmö is a prospective study at Skåne University Hospital, Malmö, Sweden, including all patients, with HF diagnosis admitted to the cardiology and internal medicine wards, regardless of aetiology, duration, or HF severity. However, the current study excluded patients from HARVEST‐Malmö that were in NYHA class IV at the time of enrolment.
Controls
Sixty control subjects (age > 40 years) were included from the Syncope Study of Unselected Population in Malmö (SYSTEMA). The SYSTEMA cohort consists of patients with syncope and orthostatic intolerance evaluated at Skåne University Hospital, Malmö, Sweden. Control participants with a normal response to passive head‐up tilt test (HUT; described below) and with no signs of heart disease were included in the present study. The SYSTEMA cohort has been described in detail elsewhere. 14 All patients gave written informed consent. The study was approved by the regional ethical review board in Lund, and all procedures were performed in accordance with the Helsinki Declaration.
Head‐up tilt test
The HUT is an established method for the evaluation of unexplained syncope and orthostatic intolerance, 15 and the method used in our study has been previously described in detail. 16 Following supine rest of 15 min, HF patients and controls were tilted head‐up to an angle of 70° for 20 min. Both HF patients and controls that were included in the study completed the HUT protocol; however, HF patients were tilted back immediately if there were any symptoms indicating presyncope or clinical instability. For the current study, nitroglycerine was not administered as part of the protocol. Arterial blood pressure was continuously recorded using a photoplethysmographic device (Nexfin, BMEYE, Amsterdam, The Netherlands or Finapres Nova, Finapres Medical Systems, PH Enschede, The Netherlands) and electrocardiogram.
Cerebral oximetry
Cerebral tissue oxygenation was measured by cerebral oximetry using near‐infrared spectroscopy (NIRS) at rest and during HUT. NIRS is a non‐invasive method yielding mixed blood oxygen saturation levels in cerebral tissue by determining the ratio of oxygenated haemoglobin to total haemoglobin. It reflects a proportional mix of arterial and venous blood in the outer regions of the frontal hemispheres. 17 Normal range of SctO2 is 60–80%. 18 SctO2 was assessed using the Fore‐Sight absolute cerebral oximeter (CAS Medical Systems Inc., Branford, CT, USA), which was utilized in a previous study in the SYSTEMA cohort. 16 Absolute cerebral oximetry and haemodynamic parameters were measured simultaneously and synchronized.
Echocardiography
Transthoracic echocardiograms in HF patients were obtained by experienced sonographers using a Philips IE33 (Philips, Andover, MA, USA) with a 1–5 MHz transducer (S5–1) or with a GE Vingmed Vivid 7 Ultrasound (GE, Vingmed Ultrasound, Horten, Norway) with a 1–4 MHz transducer (M3S). Measurements were done offline using Xcelera 4.1.1 (Philips Medical Systems, The Netherlands) according to the recommendations of the American Society of Echocardiography. 19 Left ventricular volumes were calculated using the biplane Simpson method of discs, by manual tracing (papillary muscles included in the cavity) in two‐dimensional end‐diastolic and end‐systolic frames defined as the largest and smallest left ventricular cavities, respectively, in apical four‐chamber and two‐chamber projections. Ejection fraction (EF) was calculated automatically from end‐diastolic (EDV) and end‐systolic volume (ESV) using the following formula: EF = (EDV − ESV) ∕ EDV.
Statistical analysis and included variables
Cerebral tissue oxygen saturation and haemodynamic parameters were recorded in supine position at 1, 3, and 10 min of HUT, respectively. Delta SBP, HR, and SctO2 were defined as the difference between the values of supine position and after 10 min of HUT. Group differences in continuous variables between HF patients and controls were compared using independent‐samples t‐test. Changes within the same group during HUT were compared using paired‐samples t‐test. Apart from the continuous variables, the proportion of patients with SctO2 < 65% (indicating the lower limit of normal in subjects with normal HUT 16 ) and SctO2 < 60% (indicating the lower limit for when patients experience syncope 16 ) after 10 min of HUT was compared between HF patients and controls, using Pearson's χ 2 test, as for other dichotomous variables. The associations between HF and SctO2 levels in supine position and after 10 min of HUT were studied using multivariable‐adjusted linear regression models, including age, sex, smoking, diabetes, SBP in supine position and after 10 min of HUT, and heart rate (HR) in supine position and after 10 min of HUT. In addition, univariable linear regression was performed separately in HF patients and controls in order to analyse the association between SctO2 and age, sex, smoking, diabetes, SBP, and HR in each group, respectively. Diabetes was defined as either a self‐reported diagnosis of diabetes or use of antidiabetic medication. Data were analysed using SPSS software Version 25 (SPSS, Chicago, IL, USA). A P value of <0.05 was considered significant for all tests.
Results
Study population characteristics
Heart failure patients were older and more often male than controls. Moreover, subjects with HF were more likely to be smokers and to have diabetes, whereas supine SBP and DBP values were lower among HF patients than controls. Only a few HF patients were classified as NYHA class I. Study population characteristics are shown in Table 1 .
Table 1.
Baseline characteristics and haemodynamic variables during head‐up tilt test
| Characteristic | HF (n = 61) | Controls (n = 60) |
| Age (years) | 70.7 (11.0) | 59.8 (11.5) |
| Sex (% male) | 82.0 | 41.7 |
| Current smoker (%) | 15.0 a | 8.8 c |
| Diabetes (%) | 30.0 a | 15.0 |
| EF, percentage (md, IQR) | 34 (24.5) d | |
| NYHA class (%) | — | |
| I | 3.3 | |
| II | 44.3 | |
| III | 52.4 | |
| SBP (mmHg) | 125.2 (22.9) a | 141.5 (19.3) |
| DBP (mmHg) | 66.5 (12.5) | 78.5 (11.6) |
| Heart rate (beats per min) | 71.2 (12.2) | 70.5 (11.6) |
DBP, diastolic blood pressure; EF, ejection fraction; HF, heart failure; HR, heart rate; HUT, head up tilt; IQR, interquartile range; Md, median; NYHA, New York Heart Association; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation.
Continuous variables are expressed as mean (standard deviation) unless other specified. Dichotomous data are expressed as percentages of total within each group.
n = 1 missing.
n = 2 missing.
n = 3.
n = 21 missing.
Changes in haemodynamic parameters and cerebral tissue oxygen saturation during head‐up tilt
The mean SctO2 was lower in HF patients compared with controls both in the supine position and after 10 min of HUT (Table 2 and Figure 1A–B ). In both patients with HF and controls, the mean SctO2 decreased during HUT (P < 0.001), whereas DBP (P = 0.038 for HF patients; P < 0.001 for controls) and HR increased (P = 0.005 for HF patients; P < 0.001 for controls). SBP did not change, neither in HF patients (P = 0.794) nor in controls (P = 0.527), during HUT. The change in SctO2 from supine to 10 min of HUT was greater in HF patients compared with controls (P = 0.026) (Table 2 and Figure 2 ). The proportion of patients with SctO2 below 65% and 60%, respectively, after 10 min of HUT was higher among HF patients than controls (Table 2 ).
Table 2.
Haemodynamic parameters and cerebral tissue oxygen saturation during head‐up tilt test in HF patients and controls
| Parameter | HF (n = 61) | Controls (n = 60) | P value |
| SBP supine | 125.2 (22.9) | 141.5 (19.3) | <0.001 |
| DBP supine | 66.5 (12.5) | 78.5 (11.6) | <0.001 |
| HR supine | 71.2 (12.2) | 70.5 (11.6) | 0.720 |
| SBP 10 min HUT | 124.7 (24.1) a | 142.4 (20.3) | <0.001 |
| DBP 10 min HUT | 69.2 (14.0) a | 85.1 (14.0) | <0.001 |
| HR 10 min HUT | 74.1 (12.0) a | 77.1 (12.5) | 0.184 |
| SctO2 supine before HUT | 67.0 (5.0) | 71.1 (3.5) | <0.001 |
| SctO2 10 min HUT | 63.9 (4.5) | 68.8 (3.1) | <0.001 |
| Delta SBP 10 min | 0.6 (16.9) b | −0.93 (11.4) | 0.570 |
| Delta HR 10 min | −3.2 (8.5) a | −6.7 (6.7) | 0.013 |
| Delta SctO2 10 min HUT | 3.1 (2.1) | 2.2 (2.0) | 0.026 |
| SctO2 10 min HUT < 65% | 59.0% | 8.3% | <0.001 |
| SctO2 10 min HUT < 60% | 14.8% | 0% | 0.002 |
DBP, diastolic blood pressure; HF, congestive heart failure; HR, heart rate; HUT, head up tilt; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation.
Continuous variables are expressed as mean (standard deviation), whereas dichotomous variables are displayed as proportions in %. P values are from independent samples t‐test for continuous data and Pearson's χ 2 test for dichotomous data. Delta SBP, HR, and SctO2 were defined as the difference in supine value to 10 min of HUT.
N = 1 missing.
N = 2 missing.
Figure 1.
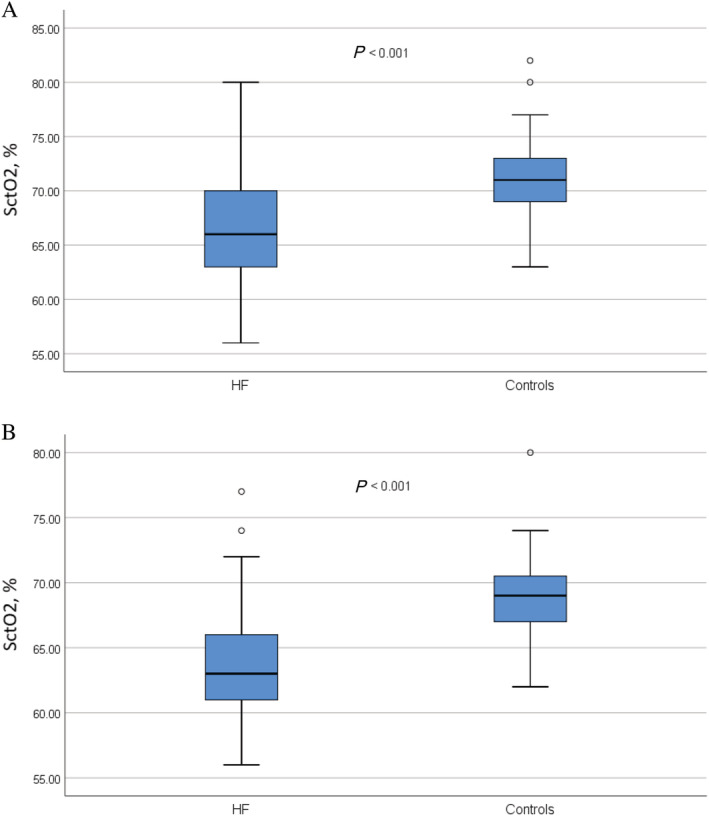
Cerebral tissue oxygen saturation (Y‐axis) shown as percentage points in HF patients and controls. Cerebral tissue oxygen saturation in HF patients and controls (A) in the supine position and (B) after 10 min of head‐up tilt test. P values denote the difference between mean values, using independent samples t‐test. HF, heart failure; HR, heart rate; HUT, head‐up tilt; SctO2, cerebral tissue oxygen saturation; SBP, systolic blood pressure.
Figure 2.
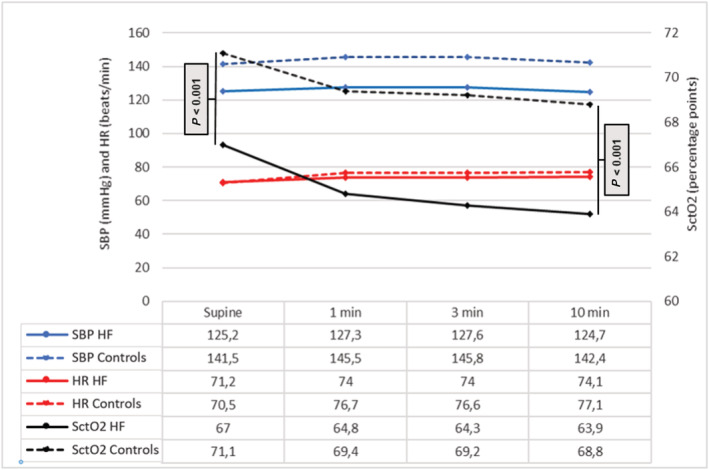
Changes in systolic blood pressure, heart rate, and cerebral tissue oxygen saturation during head‐up tilt testing. Mean cerebral tissue saturation (SctO2) in heart failure patients (solid black lines) and controls (dashed black lines) in relation to systolic blood pressure (SBP; in blue) and heart rate (HR; in red) during head‐up tilt test.
Cerebral tissue oxygen saturation in relation to haemodynamic variables
Heart failure was associated with lower SctO2 in supine position (−2.457 percentage points, P = 0.023) and after 10 min of HUT (−2.597 percentage points, P = 0.007) also after adjusting for age, sex, smoking, diabetes, supine SBP, and HR (Table 3 ). Among HF patients, age and a higher supine HR were associated with lower SctO2 in the supine position, whereas age, smoking, and lower SBP were associated with lower SctO2 after 10 min of HUT (Table 4 ). No associations were found between SctO2 and age, sex, smoking, diabetes, SBP, or HR in controls (Supporting Information, Table S1 ).
Table 3.
Relation between HF and cerebral tissue oxygen saturation in the multivariable adjusted models
| Model | Beta (unstandardized) | P value |
| Model 1 a | ||
| SctO2 supine | −2.773 | 0.004 |
| SctO2 10 min HUT | −3.605 | <0.001 |
| Delta SctO2 10 min | 0.832 | 0.079 |
| Model 2 b | ||
| SctO2 supine | −2.900 | 0.004 |
| SctO2 10 min HUT | −3.398 | <0.001 |
| Delta SctO2 10 min | 0.498 | 0.318 |
| Model 3 c | ||
| SctO2 supine | −2.457 | 0.023 |
| SctO2 10 min HUT | −2.597 | 0.007 |
| Delta SctO2 10 min | 0.596 | 0.251 |
SctO2, cerebral tissue oxygen saturation.
Multivariable‐adjusted linear regression models including heart failure (independent variable) and SctO2 (dependant variable).
Adjusted for age and sex.
Adjusted for age, sex, smoking, and diabetes
Adjusted for age, sex, smoking, diabetes, systolic blood pressure, and heart rate in supine position or after 10 min of HUT or delta systolic blood pressure and delta heart rate after 10 min of HUT.
Table 4.
Association between cerebral tissue oxygen saturation and the clinical profile in heart failure patients
| Dependent variable | Independent variable | B | P value |
| SctO2 supine | Age | −0.168 | 0.003 |
| SctO2 supine | Sex | 1.351 | 0.419 |
| SctO2 supine | Current smoker | −3.170 | 0.079 |
| SctO2 supine | Diabetes | −1.389 | 0.327 |
| SctO2 supine | SBP supine | 0.036 | 0.200 |
| SctO2 supine | HR supine | −0.143 | 0.005 |
| SctO2 10 min HUT | Age | −0.165 | 0.001 |
| SctO2 10 min HUT | Sex | 1.431 | 0.342 |
| SctO2 10 min HUT | Current smoker | −3.261 | 0.045 |
| SctO2 10 min HUT | Diabetes | −0.722 | 0.574 |
| SctO2 10 min HUT | SBP 10 min HUT | −0.061 | 0.011 |
| SctO2 10 min HUT | HR 10 min HUT | −0.085 | 0.083 |
HF, heart failure, HR, heart rate; HUT, head up tilt; SBP, systolic blood pressure; SctO2, cerebral tissue oxygen saturation.
Univariable linear regression in HF patients including SctO2 (dependent variable) and age, sex, smoking, diabetes, SBP, and HR (independent variables).
Discussion
In this study, we have shown that patients with compensated HF have lower SctO2 both in supine position and after 10 min of HUT compared with haemodynamically normal controls without HF. Among HF patients, higher age and HR were associated with lower SctO2 in the supine position, whereas higher age, smoking, and lower systolic blood pressure were associated with a lower SctO2 after 10 min of HUT. However, the proportion of subjects with an abnormal SctO2 value below 65% and 60%, respectively, was greater in HF patients compared with controls. Of interest, no subject in the control group had a SctO2 below 60%, whereas 15% of HF patients demonstrated a SctO2 value <60% after 10 min of orthostatic provocation, a value which is equivalent with concomitant orthostatic symptoms and/or syncope in many cases during HUT. 16
The lower SctO2 among HF patients compared with controls, both at rest and during orthostatic provocation, supports the hypothesis of increased susceptibility to cerebral hypoperfusion during orthostatic stress in HF. 9 In contrast to the current study, NIRS performed on elderly patients with diastolic dysfunction during 10 min of standing revealed that HF patients exhibited a smaller decrease in SctO2 compared with healthy elderly subjects. 20 The fact that the latter study required withdrawal of furosemide and captopril prior to tests, which may benefit the haemodynamic response to orthostasis, 21 may explain the conflicting results. In addition, the latter study included patients with normal left ventricular EF 20 as opposed to the current study which included patients with both preserved, mid‐range, and reduced EF.
It has been previously indicated that HF patients experience lower CBF 9 during upright posture compared with controls. These results are in line with those from the current study, indicating cerebral hypoperfusion during orthostatic stress compared with controls. The fact that CBF and cerebral oxygenation seem to be reduced in HF compared with controls, even after adjusting for haemodynamic measurements, merits some consideration. Although the mechanisms involved in CBF reduction in HF remain unclear, they may be related to a low CO 9 , 22 or vasoconstriction of the cerebral vasculature induced by higher activity of brain sympathetic nervous system and the renin–angiotensin–aldosterone system. 23 , 24 Furthermore, common HF comorbidities such as atrial fibrillation, obesity, diabetes, and sleep apnoea have also been associated with a reduction in cerebral perfusion, 25 , 26 , 27 , 28 which might worsen CBF regulation in patients with HF.
Cerebral autoregulation and vasomotor reactivity play an important role in maintaining CBF. In healthy subjects, cerebral autoregulation preserves constant CBF during fluctuations in systemic blood pressure. 29 It has been suggested that vasomotor reactivity, defined as the ability of cerebral vessels adequately to dilate or constrict in response to changes in CO2 levels in blood and surrounding tissue, 30 if reduced to a certain degree, may result in dysfunctional cerebral autoregulation. 31 Previous research indicates that HF patients have impaired vasomotor reactivity, 32 which in turn may suggest impaired cerebral autoregulation and failure to maintain CBF and tissue oxygenation.
As previously mentioned, the reduced CO in HF is also thought to play an important role behind cerebral hypoperfusion. Because CO is a result of HR and stroke volume, 33 cerebral hypoperfusion may be influenced by an impaired HR increase during HUT. Although there was a greater increase in HR among controls compared with HF patients, SctO2 seemed to decrease in HF regardless of the HR response to orthostatic stress. In contrast to controls, the HF patients had several medications with potential effects on the haemodynamic response, including beta‐blockers, angiotensin‐converting enzyme inhibitors, diuretics, and aldosterone antagonists. However, a previous study showed that HF patients had an abnormal haemodynamic response to orthostasis compared with patients treated for hypertension, despite similar medications. Hence, this difference was considered to be a result of cardiac dysfunction rather than medical therapy. 10
During orthostatic provocation, there is an activation of the sympathetic nervous system 34 . In HF where sympathetic drive is high, 3 physiological mechanisms are mobilized to the maximum to maintain homeostasis, and the autonomic nervous system has no reserve to respond adequately to upright posture. 10 Impaired HR and blood pressure variability has been found during orthostatic stress in HF patients compared with hypertensive patients and controls. 10 Thus, autonomic dysfunction may offer a possible explanation for the abnormal cerebral oximetry response to HUT in HF patients. Interestingly, a study on heart transplant recipients showed that cerebral oxygenation–perfusion, assessed by frontal near‐infrared spectroscopy, was reduced during exercise and recovery compared with age‐matched healthy controls. 35 This might support the role of other factors than CO such as autonomic dysfunction.
As a further support of an autonomic cause of the reduced cerebral oxygenation in HF patients, previous research has found that HF is associated with loss of tissue and neural injury in specific brain areas with the damage especially affecting structures that regulate autonomic action, such as the right insular and ventral medial prefrontal cortices, as well as the hypothalamus and hippocampus. 5 , 36 Areas of grey matter loss may contribute to inappropriate autonomic regulation in HF, 5 which in turn may affect physiological adaptations to upright posture. The SBP decrease did not differ between HF and controls in the current study. However, the SBP among HF patients was significantly associated with lower SctO2 after 10 min of HUT, but not in the supine position. This suggests that the interplay between the autonomic nervous system, the haemodynamic response, cerebral perfusion, and cerebral oxygenation is complex and may be interconnected at several levels.
It is well known from previous studies that impaired cognitive function is an independent risk factor for mortality in HF. Chronically reduced levels of SctO2 and impaired cerebral autoregulation during orthostatic challenge as shown in this study might serve as an important link in the pathophysiology of these findings. 37
Thus, the consequences of a lower SctO2 in HF patients both in the short and long term should be further explored.
Limitations
Our study has a number of important limitations. First, the control group consisted of patients investigated for syncope or orthostatic intolerance and were not recruited from the population. However, all controls had a normal haemodynamic response to passive HUT and had no known heart disease. Secondly, because HARVEST‐Malmö patients were offered to perform HUT, there is a possible risk of selection bias in this study, where one might assume that healthier HF patients are more prone to participate in such a study. On the other hand, from a preventive view, the current results may be more relevant than if only severely affected patients were included. Thirdly, medications with impact on the autonomic nervous system and haemodynamic response were widely used in the HF group but not in controls, which may have influenced the results. Finally, even though we tried to include patients of similar ages, the HF patients were older and more often male than the control subjects. It has previously been reported that SctO2 in the barrel cortex of healthy mice, decreases with age. 38 Also, age‐related arterial stiffness has been shown to reduce CBF, 39 , 40 which could have an impact on the results. However, the results in the present study were still statistically significant after adjusting for age in the linear regression models; thus, HF patients seem to have lower cerebral saturation regardless of age.
Conclusions
Patients with compensated HF have reduced levels of SctO2 in the supine and in standing position, compared with subjects without HF. The lower SctO2 is independent of differences in haemodynamic parameters. The consequences of a lower SctO2 in HF patients, both in the short and long term, should be further explored.
Conflict of interest
P.W. reports personal fees from Chiesi Pharma outside the submitted work. A.F. reports personal fees from Medtronic Inc. and Biotronik, and patent royalties from Thermo Fisher Scientific outside the submitted work. E.B. has been employed by AstraZeneca after completion of this study.
Funding
This study was supported by grants from the Swedish Heart and Lung Foundation, The Swedish Heart and Lung Association, The Medical Faculty of Lund University, ALF funds, Skåne University Hospital Funds, The Crafoord Foundation, Ernhold Lundströms Research Foundation, Region Skåne, Hulda and Conrad Mossfelt Foundation, Anna‐Lisa and Sven Eric Lundgrens Foundation for Medical Research, The Kockska foundation, and the Wallenberg Centre for Molecular Medicine.
Supporting information
Table S1. Association between cerebral tissue oxygen saturation and the clinical profile in controls
Acknowledgements
We thank the research nurses Hjördis Jernhed and Dina Chatziapostolou for valuable contributions. We would also like to thank the staff at the echocardiographic laboratory and at the Department Clinical Physiology and Nuclear Medicine at Skåne University Hospital, Malmö. The Knut and Alice Wallenberg foundation is acknowledged for generous support.
Kharraziha, I. , Holm, H. , Magnusson, M. , Wollmer, P. , Molvin, J. , Jujic, A. , Fedorowski, A. , Bachus, E. , and Hamrefors, V. (2021) Impaired cerebral oxygenation in heart failure patients at rest and during head‐up tilt testing. ESC Heart Failure, 8: 586–594. 10.1002/ehf2.13128.
Isabella Kharraziha and Hannes Holm shared first authorship.
Erasmus Bachus and Viktor Hamrefors shared senior authorship.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members , Document Reviewers . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld JA, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. ACC/AHA/HFSA focused Update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 3. Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986; 73: 615–621. [DOI] [PubMed] [Google Scholar]
- 4. Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol 2015; 178: 12–23. [DOI] [PubMed] [Google Scholar]
- 5. Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci 2011; 307: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond CA, Blades NJ, Chaudhry SI, Dodson JA, Longstreth WT Jr, Heckbert SR, Psaty BM, Arnold AM, Dublin S, Sitlani CM, Gardin JM. Long‐term cognitive decline after newly diagnosed heart failure. Circ Heart Fail 2018; 11: e004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol (1985) 2003; 94: 833–848. [DOI] [PubMed] [Google Scholar]
- 8. Cornwell WK 3rd, Levine BD. Patients with heart failure with reduced ejection fraction have exaggerated reductions in cerebral blood flow during upright posture. JACC Heart Fail 2015; 3: 176–179. [DOI] [PubMed] [Google Scholar]
- 9. Fraser KS, Heckman GA, McKelvie RS, Harkness K, Middleton LE, Hughson RL. Cerebral hypoperfusion is exaggerated with an upright posture in heart failure: impact of depressed cardiac output. JACC Heart Fail 2015; 3: 168–175. [DOI] [PubMed] [Google Scholar]
- 10. Bronzwaer AGT, Bogert LWJ, Westerhof BE, Piek JJ, Daemen M, van Lieshout JJ. Abnormal haemodynamic postural response in patients with chronic heart failure. ESC Heart Fail 2017; 4: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serber SL, Rinsky B, Kumar R, Macey PM, Fonarow GC, Harper RM. Cerebral blood flow velocity and vasomotor reactivity during autonomic challenges in heart failure. Nurs Res 2014; 63: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fantini S, Sassaroli A, Tgavalekos KT, Kornbluth J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016; 3: 031411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christensson A, Grubb A, Molvin J, Holm H, Gransbo K, Tasevska‐Dinevska G, Bachus E, Jujic A, Magnusson M. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population—the HARVEST study. Scand J Clin Lab Invest 2016; 76: 568–574. [DOI] [PubMed] [Google Scholar]
- 14. Fedorowski A, Burri P, Struck J, Juul‐Moller S, Melander O. Novel cardiovascular biomarkers in unexplained syncopal attacks: the SYSTEMA cohort. J Intern Med 2013; 273: 359–367. [DOI] [PubMed] [Google Scholar]
- 15. Fitzpatrick AP, Theodorakis G, Vardas P, Sutton R. Methodology of head‐up tilt testing in patients with unexplained syncope. J Am Coll Cardiol 1991; 17: 125–130. [DOI] [PubMed] [Google Scholar]
- 16. Bachus E, Holm H, Hamrefors V, Melander O, Sutton R, Magnusson M, Fedorowski A. Monitoring of cerebral oximetry during head‐up tilt test in adults with history of syncope and orthostatic intolerance. Europace 2018; 20: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 17. Kharraziha I, Holm H, Bachus E, Ricci F, Sutton R, Fedorowski A, Hamrefors V. Cerebral oximetry in syncope and syndromes of orthostatic intolerance. Front Cardiovasc Med 2019; 6: 171–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scott JP, Hoffman GM. Near‐infrared spectroscopy: exposing the dark (venous) side of the circulation. Paediatr Anaesth 2014; 24: 74–88. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 20. Mehagnoul‐Schipper DJ, Vloet LC, Colier WN, Hoefnagels WH, Verheugt FW, Jansen RW. Cerebral oxygenation responses to standing in elderly patients with predominantly diastolic dysfunction. Clin Physiol Funct Imaging 2003; 23: 92–97. [DOI] [PubMed] [Google Scholar]
- 21. van Kraaij DJ, Jansen RW, Bouwels LH, Gribnau FW, Hoefnagels WH. Furosemide withdrawal in elderly heart failure patients with preserved left ventricular systolic function. Am J Cardiol 2000; 85: 1461–1466. [DOI] [PubMed] [Google Scholar]
- 22. Loncar G, Bozic B, Lepic T, Dimkovic S, Prodanovic N, Radojicic Z, Cvorovic V, Markovic N, Brajovic M, Despotovic N, Putnikovic B, Popovic‐Brkic V. Relationship of reduced cerebral blood flow and heart failure severity in elderly males. Aging Male 2011; 14: 59–65. [DOI] [PubMed] [Google Scholar]
- 23. Francis GS. The relationship of the sympathetic nervous system and the renin‐angiotensin system in congestive heart failure. Am Heart J 1989; 118: 642–648. [DOI] [PubMed] [Google Scholar]
- 24. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 1992; 20: 248–254. [DOI] [PubMed] [Google Scholar]
- 25. Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, Oh JM, Gallagher CL, Koscik RL, Jonaitis EM, Hermann BP, LaRue A, Rowley HA, Asthana S, Sager MA, Bendlin BB. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity 2013; 21: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alosco ML, Spitznagel MB, Sweet LH, Josephson R, Hughes J, Gunstad J. Atrial fibrillation exacerbates cognitive dysfunction and cerebral perfusion in heart failure. Pacing Clin Electrophysiol 2015; 38: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, Cavallerano J, Lipsitz LA. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 2006; 29: 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yadav SK, Kumar R, Macey PM, Richardson HL, Wang DJ, Woo MA, Harper RM. Regional cerebral blood flow alterations in obstructive sleep apnea. Neurosci Lett 2013; 555: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tameem A, Krovvidi H. Cerebral physiology. Contin Educ Anaesth Crit Care Pain 2013; 13: 113–118. [Google Scholar]
- 30. Franklin KA. Cerebral haemodynamics in obstructive sleep apnoea and Cheyne–Stokes respiration. Sleep Med Rev 2002; 6: 429–441. [DOI] [PubMed] [Google Scholar]
- 31. Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2‐induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 1988; 19: 963–969. [DOI] [PubMed] [Google Scholar]
- 32. Georgiadis D, Sievert M, Cencetti S, Uhlmann F, Krivokuca M, Zierz S, Werdan K. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J 2000; 21: 407–413. [DOI] [PubMed] [Google Scholar]
- 33. Vincent JL. Understanding cardiac output. Crit Care 2008; 12: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheshire WP, Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res 2019; 29: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gayda M, Desjardins A, Lapierre G, Dupuy O, Fraser S, Bherer L, Juneau M, White M, Gremeaux V, Labelle V, Nigam A. Cerebral hemodynamics during exercise and recovery in heart transplant recipients. Can J Cardiol 2016; 32: 539–546. [DOI] [PubMed] [Google Scholar]
- 36. Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol 2003; 95: 677–684. [DOI] [PubMed] [Google Scholar]
- 37. Zuccalà G, Pedone C, Cesari M, onder G, Pahor M, Marzetti E, Lo Monaco MR, Cocchi A, Carbonin P, Bernabei R. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med 2003; 115: 97–103. [DOI] [PubMed] [Google Scholar]
- 38. Moeini M, Lu X, Avti PK, Damseh R, Bélanger S, Picard F, Boas D, Kakkar A, Lesage F. Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci Rep 2018; 8: 8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jefferson AL, Cambronero FE, Liu D, Moore EE, Neal JE, Terry JG, Nair S, Pechman KR, Rane S, Davis LT, Gifford KA, Hohman TJ, Bell SP, Wang TJ, Beckman JA, Carr JJ. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018; 138: 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muhire G, Iulita MF, Vallerand D, Youwakim J, Gratuze M, Petry FR, Planel E, Ferland G, Girouard H. Arterial stiffness due to carotid calcification disrupts cerebral blood flow regulation and leads to cognitive deficits. J Am Heart Assoc 2019; 8: e011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between cerebral tissue oxygen saturation and the clinical profile in controls


