Abstract
Aims
Percutaneous mitral valve repair (PMVR) has emerged as standard treatment in selected patients with clinically relevant mitral regurgitation (MR) and increased surgical risk. We aimed to evaluate the safety and clinical outcomes in nonagenarians undergoing PMVR.
Methods and results
Altogether, 493 patients with severe MR who were treated with PMVR were included in this open‐label prospective study and followed up for 2 years. We treated 25 patients with PMVR aged 90 years or above, 185 patients aged 80–89 years, and 283 patients aged <80 years. PMVR in nonagenarians was safe and did not differ from PMVR in younger patients in terms of safety endpoints. Device success did not differ among the groups (100% in nonagenarians, 95.7% in octogenarians, and 95.1% in septuagenarians, P = 0.100). Unadjusted 2 year mortality was 28% in nonagenarians, 32.4% in octogenarians, and 19.8% in septuagenarians (P = 0.008). Kaplan–Meier curves confirmed similar 2 year survival in the nonagenarian and octogenarian groups (P = 0.657). In the multivariate analysis, age [hazard ratio (HR) 1.031, 95% confidence interval (CI) 1.002–1.060, P = 0.034], higher post‐procedural transmitral valve gradients (HR 1.187, 95% CI 1.104–1.277, P = 0.001), and post‐procedural acute kidney injury (HR 2.360, 95% CI 1.431–3.893, P = 0.001) were independent predictors of 2 year mortality. Altogether, 89.4% of the nonagenarians, 85.9% of the octogenarians, and 86.4% of the septuagenarians had MR grade of 2+ or less at 1 year after PMVR (P = 0.910). New York Heart Association functional class improved in the vast majority of patients, irrespective of age (P = 0.129). After 1 year, 9.5% of the nonagenarians, 22.3% of the octogenarians, and 25.2% of the septuagenarians (each P = 0.001 compared with baseline) suffered from New York Heart Association Functional Class III or IV. The rate of heart failure rehospitalization in the first 12 months after PMVR did not differ among the groups (16% in the nonagenarians, 16.7% in the octogenarians, and 17.7% in the septuagenarians) (P = 0.954). Quality of life assessed by the Minnesota Living with Heart Failure Questionnaire before and at 1 year after PMVR improved in all age groups (P = 0.001).
Conclusions
Percutaneous mitral valve repair in carefully selected nonagenarians is feasible and safe with intermediate‐term beneficial effects comparable with those in younger patients.
Keywords: Mitral regurgitation, MitraClip, Octogenarians, Nonagenarians, Septuagenarians
Introduction
The number of nonagenarians is rising dramatically because of increased life expectancy of the population in developed countries with improvements in healthcare systems. Contemporary therapy leads to improved survival in patients with heart diseases. 1 Mitral regurgitation (MR) is one of the most common valvular heart diseases worldwide. Its prevalence increases with age, overrating the prevalence of aortic valve diseases. 2 , 3 Percutaneous mitral valve repair (PMVR) has emerged as a standard treatment in selected patients with clinically relevant MR and increased surgical risk related to the type of MR. 4 , 5
Nonagenarians are often under‐represented in clinical trials. In a recent study, Elbadawi et al. demonstrated that PMVR in nonagenarians was as safe as that in younger patients. However, the authors focused only on the in‐hospital outcome. 6 Data regarding the efficacy of PMVR in nonagenarians and its long‐term clinical outcomes are missing. In the nonagenarian patient population, primary care physicians and cardiologists agonise over the decision dilemma of conservative vs. interventional treatment in an attempt to assess risks, costs, and benefits. PMVR is an expensive procedure, and despite being safe, it might be associated with more complications in this extreme age group. On the other hand, treating patients conservatively might result in a poor quality of life. Therefore, it is challenging to choose between the two treatment strategies for this patient population.
In the present study, we aimed to evaluate the safety and clinical intermediate‐term outcomes in nonagenarians undergoing PMVR.
Materials and methods
We screened 543 consecutive patients with symptomatic severe MR at our institution between January 2010 and March 2018 for treatment with a percutaneous MitraClip® device (Abbott Laboratories, Chicago, IL, USA). Severe MR was defined according to the European Society of Cardiology guidelines for the management of valvular heart disease. 4 All patients were considered to be at high surgical risk by an interdisciplinary heart team. Patients were characterized as suitable for PMVR with the MitraClip system based on the presence of optimal or conditionally suitable valve morphology. 7 Nonagenarians were carefully selected for PMVR with inclusion of patients with low frailty (≤4 on clinical frailty scale) according the validated Fried criteria. 8
Altogether, 493 patients (90.1% of the screened patients) were considered as suitable for PMVR and were included in this open‐label prospective study. The rate of acceptance of PMVR for the screened patients was similar between the groups. All data were included in a registry (www.ClinicalTrials.gov, NCT02033811).
Patient characteristics and baseline and procedural data were assessed with the use of registry, medical records, procedure protocols, and follow‐up data (12 and 24 months after the procedure). The clinical course was monitored by follow‐up examinations and phone calls to the referring cardiologists, patients' primary physicians, or the patients themselves. The registry was approved by the local ethics committee of Heinrich Heine University, and the study was performed in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent for the procedure and for the collection and processing of their anonymous data. PMVR was performed using the MitraClip device. The procedure has been described previously in detail. 9
Clinical endpoints of this study were defined according to the Mitral Valve Academic Research Consortium. 10
The secondary endpoints of this study were improvement of functional status according to New York Heart Association (NYHA) functional classification, rate of heart failure rehospitalization, and the Minnesota Living with Heart Failure Questionnaire assessed at 1 year and mortality assessed at 1 and 2 years after PMVR.
Data were expressed as median (inter‐quartile range). The D'Agostino and Pearson omnibus normality test was used to assess the normal distribution of parameters. Patient characteristics were compared using Kruskal–Wallis test respectively analysis of variance for continuous data and two‐tailed Fisher's exact test respectively χ 2 test for categorical data.
Cox regression analysis was used to identify variables associated with mortality after PMVR. Variables with a P‐value <0.1 in the univariate analysis and variables known or thought to be associated with mortality after PMVR were included in the multivariable model. Because of the small number of events in the nonagenarian age group, the regression analysis was not group dependent but included patients from all three age groups.
Propensity score matching was used to compare the groups in terms of survival and to reduce confounding factors due to imbalances in baseline characteristics. We used these variables as potential cofounders that were significantly different among the three groups: gender, logistic EuroSCORE, Charlson Comorbidity Index, aetiology of MR, baseline left ventricular ejection fraction, and glomerular filtration rate (GFR). We created 1:1 matched groups using the nearest‐neighbour matching without replacement with a calliper of 0.2.
Statistical significance was set at P‐values <0.05. Statistical analysis was performed using SPSS® Statistics Version 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism® Version 7.0 (GraphPad Software, San Diego, CA, USA).
Results
We treated 493 patients with PMVR including 25 nonagenarians (aged 90 years or above), 185 octogenarians (aged 80–89 years), and 283 septuagenarians (aged under 80 years). Baseline patient characteristics are summarized in Table 1 . Functional MR (FMR) was more common than degenerative MR (56% in nonagenarians, 61% in octogenarians, and 71% in septuagenarians) (P = 0.053). The proportion of atrial FMR to ventricular FMR was higher in the nonagenarian age group [3 out of 14 (21.4%) FMR patients] when compared with octogenarians [12 out of 113 (10.6%) FMR patients] and septuagenarians [10 out of 200 (5%) FMR patients] (P = 0.028).
Table 1.
Patients' characteristics
| 90 years+ | 80–89 years | <80 years | P‐value | |
|---|---|---|---|---|
| n = 25 | n = 185 | n = 283 | ||
| Clinical characteristics | ||||
| Age (years) | 91.0 (90, 92) | 83.0 (81, 85) | 73.5 (67, 77) | 0.001* |
| Gender (F/M), n (%) | 15/10 (60/40) | 91/94 (49/51) | 99/184 (35/65) | 0.015* |
| Extracardial vascular disease, n (%) | 4 (16.0) | 46 (24.9) | 57 (20.1) | 0.298 |
| Diabetes mellitus, n (%) | 5 (20.0) | 57 (30.8) | 94 (33.2) | 0.377 |
| COPD, n (%) | 2 (8.0) | 35 (19.0) | 62 (21.9) | 0.221 |
| Prior stroke, n (%) | 1 (4.0) | 13 (7.0) | 18 (6.3) | 0.839 |
| Previous cardiac surgery, n (%) | 2 (8.8) | 32 (17.3) | 85 (30.0) | 0.001* |
| Atrial fibrillation, n (%) | 19 (76.0) | 128 (69.2) | 191 (67.5) | 0.662 |
| Logistic EuroSCORE | 20.0 (17.0, 31.5) | 23.0 (13.9, 35.0) | 19.0 (9.5, 30.1) | 0.070 |
| STS risk score (%) | 6.7 (3.7, 9.8) | 8.5 (5.6, 10.6) | 8.2 (7.5, 11.1) | 0.128 |
| Charlson Comorbidity Index | 5.5 (5.0, 6.0) | 6.0 (5.0, 8.0) | 5.0 (4.0, 7) | 0.001* |
| NYHA III/IV, n (%) | 25 (100) | 157 (84.9) | 247 (87.3) | 0.301 |
| Echocardiographic/haemodynamic data | ||||
| DMR/FMR, n (%) | 11/14 (44/56) | 72/113 (39/61) | 83/200 (29/71) | 0.053 |
| LVEDD (mm) | 54 (49, 59.5) | 55 (49, 61) | 58 (49, 65) | 0.258 |
| LA diameter (mm) | 40 (36, 44.5) | 41 (34, 46) | 39 (33, 45) | 0.110 |
| LVEF (%) | 45 (40, 50) | 42 (35, 50) | 40 (30, 49) | 0.020* |
| LVEF > 50%, n (%) | 11 (44) | 75 (40.5) | 79 (27.9) | 0.001* |
| LVEF 40–50%, n (%) | 10 (40) | 32 (17.3) | 54 (19.1) | |
| LVEF < 40%, n (%) | 4 (16) | 78 (42.2) | 150 (53.0) | |
| TAPSE (mm) | 17 (15, 20) | 17 (15, 21) | 17 (15, 20) | 0.850 |
| Moderate/severe TR, n (%) | 16 (64%) | 102 (55.1) | 144 (50.8) | 0.357 |
| Cardiac index (mL/min/m2) | 2.0 (1.8, 2.3) | 2.0 (1.7, 2.3) | 2.0 (1.7, 2.3) | 0.975 |
| PAPs (mmHg) | 46 (40, 56) | 53 (43, 66) | 51 (41, 62) | 0.120 |
| Laboratory assessment | ||||
| Haemoglobin (g/dL) | 12 (11.0, 12.9) | 11.7 (10.5, 12.8) | 12.2 (10.7, 13.6) | 0.290 |
| Estimated GFR (mL/min) | 38.0 (30, 50.0) | 44 (35, 60) | 52 (37, 67) | 0.002* |
| NT‐proBNP (pg/mL) | 3068 (1991, 6182) | 2521 (1399, 5010) | 2515 (1381, 4691) | 0.139 |
COPD, chronic obstructive pulmonary disease; DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation; GFR, glomerular filtration rate; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PAPs, pulmonary artery systolic pressure; STS, Society of Thoracic Surgeons scores for the risk of death within 30 days after mitral valve repair; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Values are n (%) or median (inter‐quartile range).
P ≤ 0.05 among the groups.
Nonagenarian patients who were eligible for PMVR had better left ventricular function than octogenarian or septuagenarian patients. Renal function was lower in the nonagenarian age group [GFR: 38.0 (30, 50.0) mL/min] when compared with octogenarians [GFR: 44 (35, 60) mL/min] and septuagenarians [GFR: 52 (37, 67) mL/min] (P = 0.002) (Table 1 ).
Percutaneous mitral valve repair in nonagenarians did not differ from PMVR in younger patients in terms of safety endpoints. Particularly, the rate of minor vascular complications and occurrence of acute renal failure did not differ among the groups (Table 2 ). The 30 day mortality rate was 4.0% in the nonagenarian group, 2.7% in the octogenarian group, and 1.8% in the septuagenarian group (P = 0.661). Device success did not differ among the groups of age. Device success was 100% in the nonagenarian group, 95.7% in the octogenarian group (no MitraClip device was implanted in eight patients), and 95.1% in the septuagenarian group (no MitraClip device was implanted in 14 patients) (P = 0.100). The reasons for device failure included inability to grasp the leaflets, inability to adequately reduce MR, and inadequate mitral valve orifice area. No difference was observed in post‐procedural transmitral valve gradient among the groups (Table 2 ).
Table 2.
Procedural outcome
| 90 years+ | 80–89 years | <80 years | P‐value | |
|---|---|---|---|---|
| n = 25 | n = 185 | n = 283 | ||
| Anaesthesia (GA/DS), n/n | 4/21 | 31/154 | 49/234 | 0.978 |
| Conversion to GA, n (%) | 1 (4) | 4 (2.2) | 5 (1.8) | 0.740 |
| Conversion to surgery, n (%) | 0 (0) | 2 (1.1) | 3 (1.1) | 0.983 |
| Periprocedural mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Minor vascular complication, n (%) | 3 (12) | 15 (8.1) | 18 (6.4) | 0.506 |
| Major vascular complication, n (%) | 0 (0) | 7 (3.8) | 7 (2.5) | 0.416 |
| Myocardial infarction, n (%) | 0 (0) | 1 (0.05) | 0 (0) | 1 |
| Pneumonia, n (%) | 2 (8.0) | 8 (4.3) | 13 (4.6) | 0.713 |
| Acute kidney failure, n (%) | 4 (16.0) | 29 (15.7) | 26 (9.2) | 0.087 |
| Stroke 30 days, n (%) | 1 (4.0) | 3 (1.6) | 2 (0.7) | 0.290 |
| 30 day mortality, n (%) | 1 (4.0) | 5 (2.7) | 5 (1.8) | 0.661 |
| Device success, n (%) | 25 (100) | 177 (95.7) | 269 (95.1) | 0.100 |
| Post‐procedural transmitral valve gradient (mmHg) | 3 (2.75, 4) | 4 (2.8, 4.6) | 3.6 (3, 4.8) | 0.244 |
| Length of stay in the ICU (days) | 2.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.714 |
| Post‐procedural length of hospital stay (days) | 7 (5, 10) | 5 (3, 9) | 5 (3, 8) | 0.697 |
| Total length of hospital stay (days) | 11 (7, 14) | 7 (5, 13) | 7 (5, 11) | 0.537 |
DS, deep sedation; GA, general anaesthesia; ICU, intensive care unit.
Values are n (%) or median (inter‐quartile range).
The post‐procedural length of hospital stay [nonagenarians: 7 (5, 10) days, octogenarians: 5 (3, 9) days, or septuagenarians: 5 [3, 8] days] (P = 0.785) as well as the total length of hospital stay [nonagenarians: 11 (7, 14) days), octogenarians: 7 (5, 13) days, or septuagenarians: 7 (5, 11) days] (P = 0.537) were not different among the groups (Table 2 ).
The unadjusted 1 year mortality rate was 16% (4 out of 25) in the nonagenarian group, 23.8% (44 out of 185) in the octogenarian group (P = 0.383), and 13.1% (37 out of 283) in the septuagenarian group (P = 0.011). The unadjusted 2 year mortality was 28% (7 out of 25) in the nonagenarian group, 32.4% (60 out of 185) in the octogenarian group, and 19.8% (56 out of 283) in the septuagenarian group (P = 0.008). Kaplan–Meier curves and the log‐rank (Mantel–Cox) test confirmed similar 2 year survival in the nonagenarian and octogenarian groups (P = 0.657). Patients aged <80 years exhibited higher 2 year survival rates when compared with patients aged 80 years or older (P = 0.002) (Figure 1 ). In the propensity score matched cohort, post‐procedural outcome as well as survival did not differ among the groups (Supporting Information, Tables S1 and S2 and Figure S1 ).
Figure 1.
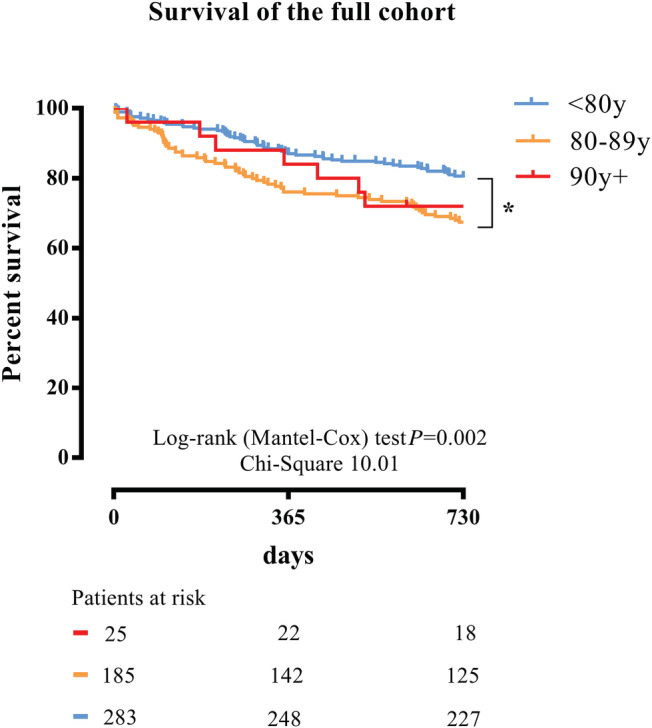
Survival after percutaneous mitral valve repair. Kaplan–Meier survival curves after percutaneous mitral valve repair stratified by age for 2 year all‐cause mortality. Two year mortality in the patients aged <80 years was lower than that in patients aged 80–89 years old and patients aged >90 years old (P = 0.003).
To identify the predictors of 2 year mortality, we performed a Cox regression analysis including parameters expected to be relevant in patients from all three age groups (Table 3 ). In the multivariate analysis, age [hazard ratio (HR) 1.031, 95% confidence interval (CI) 1.002–1.060, P = 0.034], higher post‐procedural transmitral valve gradients (HR 1.187, 95% CI 1.104–1.277, P = 0.001), and the occurrence of a post‐procedural acute kidney injury (HR 2.360, 95% CI 1.431–3.893, P = 0.001) were independent predictors of 2 year mortality in a model that also included haemoglobin, baseline kidney function, Charlson Comorbidity Index score, and the logistic EuroSCORE (Table 3 ).
Table 3.
Cox regression analysis for mortality in patients undergoing PMVR
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Baseline variable | ||||||
| Age | 1.055 | 1.033–1.078 | 0.001 | 1.031 | 1.002–1.060 | 0.034 |
| Left ventricular function | 1.010 | 0.995–1.025 | 0.194 | |||
| Right ventricular function | 0.982 | 0.932–1.034 | 0.483 | |||
| LVEDD | 0.994 | 0.977–1.011 | 0.472 | |||
| Tricuspid regurgitation | 1.006 | 0.814–1.244 | 0.953 | |||
| Diabetes mellitus | 1.250 | 0.884–1.767 | 0.208 | |||
| COPD | 1.236 | 0.845–1.808 | 0.274 | |||
| Haemoglobin | 0.090 | 0.849–1.012 | 0.090 | 1.015 | 0.988–1.043 | 0.291 |
| Atrial fibrillation | 1.318 | 0.920–1.889 | 0.132 | |||
| Charlson Comorbidity Index | 1.117 | 1.016–1.228 | 0.023 | 1.074 | 0.930–1.240 | 0.328 |
| Log EuroSCORE | 1.010 | 0.999–1.021 | 0.071 | 0.994 | 0.978–1.010 | 0.442 |
| GFR | 0.989 | 0.981–0.997 | 0.010 | 0.994 | 0.983–1.006 | 0.325 |
| Post‐procedural variable | ||||||
| Post‐procedural transmitral valve gradient | 1.160 | 1.086–1.240 | 0.001 | 1.187 | 1.104–1.277 | 0.001 |
| Post‐procedural AKI | 2.648 | 1.786–3.926 | 0.001 | 2.360 | 1.431–3.893 | 0.001 |
AKI, acute kidney injury; CI, confidence interval; COPD, chronic obstructive lung disease; GFR, glomerular filtration rate; HR, hazard ratio; LVEDD, left ventricular diastolic diameter; PMVR, percutaneous mitral valve repair.
Before undergoing PMVR, baseline MR grade was 3 or worse in 100% of the nonagenarian patients (25 out of 25), in 86.4% of the octogenarian patients (153 of 177 patients with successful PMVR), and in 88.9% of the septuagenarian patients (230 out of 269 patients with successful PMVR) (Figure 2 ). After the procedure, MR Grade 3 or worse was observed in 4.2% of the nonagenarians (1 out of 24), 8.6% of the octogenarians (15 out of 175), and 8.2% of the septuagenarians (22 out of 267) (P = 0.759). After 12 months, echocardiographic evaluation showed that MR Grade 3 or worse was present in 10.6% (2 out of 19) of the nonagenarians, 14.1% (18 out of 127) of the octogenarians, and 13.6% (30 out of 221) of the septuagenarians (P = 0.910).
Figure 2.
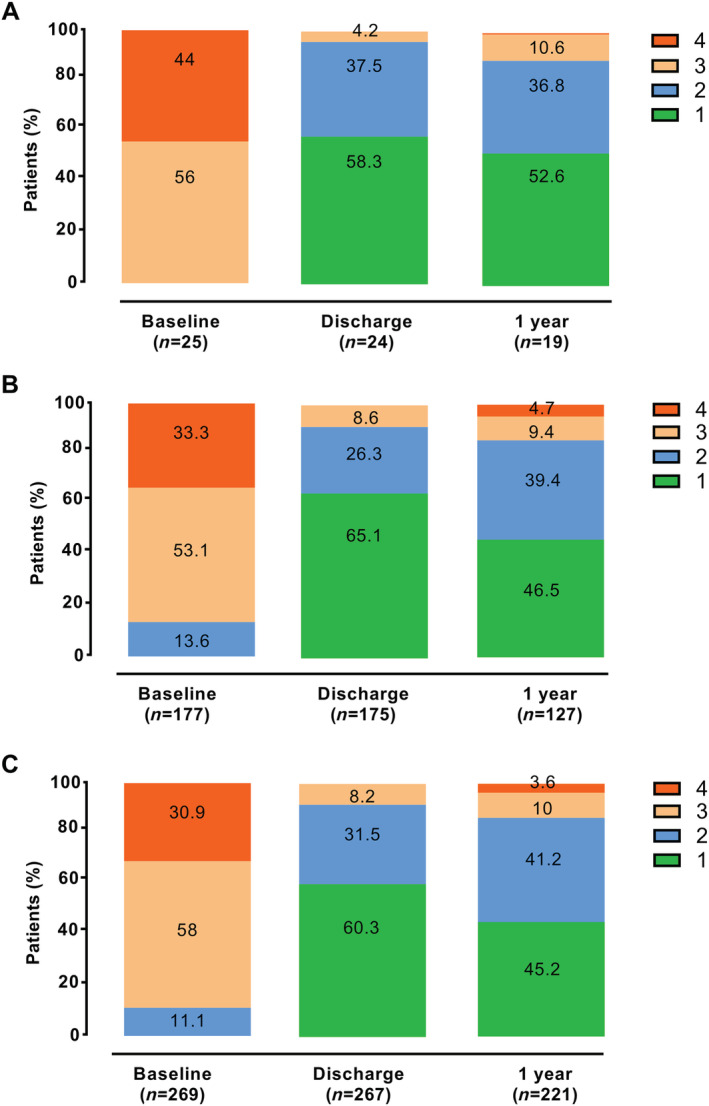
Mitral regurgitation after percutaneous mitral valve repair. Severity of mitral regurgitation in patients with an implanted MitraClip device from (A) the nonagenarian group, (B) the octogenarian group, and (C) the septuagenarian group at baseline, before discharge (post‐procedural), and at 1 year follow‐up.
New York Heart Association functional class improved in the vast majority of patients, irrespective of the age group. At baseline, 100% of the nonagenarians, 84.9% of the octogenarians, and 87.3% of the septuagenarians suffered from NYHA functional Class III or IV (P = 0.285). One year after PMVR, only 9.5% of the nonagenarians, 22.3% of the octogenarians, and 25.2% of the septuagenarians (each P = 0.001 compared with baseline) suffered from NYHA Functional Class III or IV (Figure 3A ) with no difference among the groups (P = 0.129).
Figure 3.
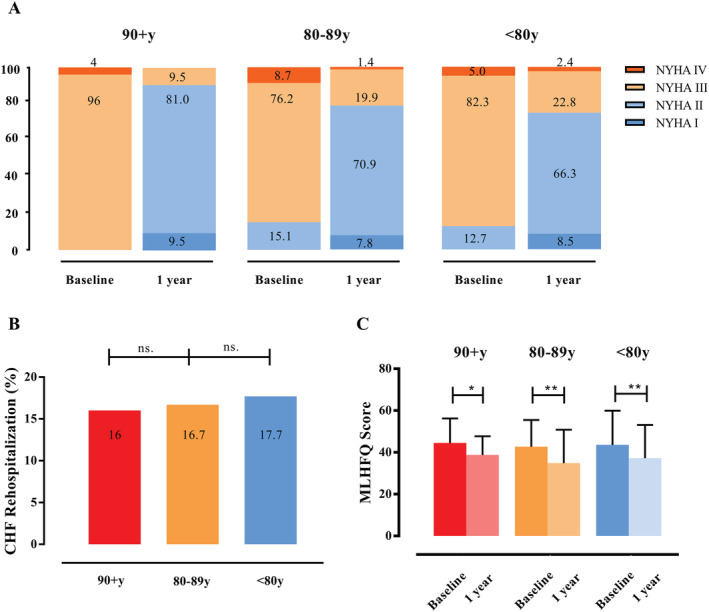
Symptoms and functional capacity after percutaneous mitral valve repair (PMVR). (A) The New York Heart Association (NYHA) functional class showed improvement at 1 year after PMVR when compared with the baseline values in all groups. (B) The rate of chronic heart failure (CHF) rehospitalization during the first year after PMVR did not differ among the groups. (C) Life quality assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) at 1 year after PMVR improved in all groups. *P ≤ 0.05 and **P ≤ 0.001 when compared with baseline.
The rate of heart failure rehospitalization in the first 12 month after PMVR did not differ among the groups: it was 16% (4 patients) in the nonagenarians, 16.7% (31 patients) in the octogenarians, and 17.7% (50 patients) in the septuagenarians (P = 0.954) (Figure 3B ).
Quality of life assessed by the Minnesota Living with Heart Failure Questionnaire before and at 1 year after PMVR improved from 45 (40, 52) to 40 (35, 46) (P = 0.021) in the nonagenarians, from 43 (34, 51) to 33 (23, 44) (P = 0.001) in the octogenarians, and from 45 (33, 55) to 36 (25, 46) in the septuagenarians (P = 0.001) (Figure 3C ).
Discussion
The findings of the presented study support the suggestion that PMVR in nonagenarians with severe MR is as feasible as that in younger patients, with similar low risk of complications and excellent short‐term and intermediate‐term outcomes.
In the present study, the clinical profile of the nonagenarian patients selected for PMVR was healthier compared with younger patients in terms of co‐morbidities and frailty. Nonagenarians had better left ventricular function and fewer co‐morbidities than octogenarians (expressed by a lower co‐morbidity index), even though age itself is a parameter of this score. The proportion of atrial FMR was higher in the nonagenarian age group when compared with the younger groups. The presence of atrial FMR might be characterized by a presumably more favourable clinical course as compared with patients with FMR due to left ventricular dysfunction. 11 Renal function was worse in nonagenarians than in younger patients. Ageing is associated with changes in the renal structure such as decreased cortical volume, nephrosclerosis, or a decline in the number of nephrons. 12 These changes are accompanied by a reduction of the measured GFR even in the absence of age‐related co‐morbidities. Evidence suggests that chronic kidney disease has been associated with poor long‐term outcomes including increased mortality and hospitalization among patients who underwent PMVR. 13 , 14
In addition to co‐morbidities, functional capacity and frailty of the patients have to be considered by the Heart Team for assessing and recommending individual therapy. Over 80% of the nonagenarians who live in households or non‐institutional groups and virtually all (98%) of those residing in institutional group quarters such as nursing homes have some type of disability. 1 Frailty is common in elderly patients and in patients with co‐morbidities and is associated with an adverse prognosis. 15 Frailty was observed in 46% of the patients undergoing PMVR according to the Fried criteria. Thus, it is a common condition in patients with PMVR and requires further attention. However, it was observed that PMVR can be performed with equal efficacy and is associated with at least similar short‐term functional improvement in frail patients. 15 We did not systematically calculate the frailty scores in our study. However, only nonagenarians with a good functional state were selected for PMVR to avoid delayed reconvalescence and to minimize complication associated with hospitalization complications. In addition, the expected functional benefit might be higher in patients with sufficient daily mobility.
Increasing clinical experience using the MitraClip system has already provided successful results in patients who were not considered suitable candidates for surgery. Reportedly, the procedural mortality rate was less than that predicted for surgical treatment, and the 30 day mortality rate was not different from that in patients receiving standard care. 16 In the present study, 30 day mortality rate in the nonagenarian group was not higher than that in younger patients.
The complication rate is usually low in PMVR procedures. 16 , 17 , 18 We observed no difference in major vascular complications, myocardial infarction, pneumonia, or stroke between the nonagenarians and the younger age groups. We also observed a trend towards acute kidney failure in the older age groups, possibly due to worse kidney function at baseline. These findings are consistent with those from previous studies that demonstrated similar in‐hospital mortality and complication rates between nonagenarians and younger patients. 6 , 19 These studies focused on safety endpoints and in‐hospital outcome but did not demonstrate data regarding technical and device success or regarding clinical follow‐up in this specific group. PMVR might be more stressful in the nonagenarians than in younger patients, which was reflected in longer length of hospital stay in nonagenarians. The reasons for this prolonged hospitalization might include a high‐risk baseline status due to age alone or in combination with lower renal function or post‐interventional delirium. However, the majority of the patients were discharged normally without the need for specific home health care. In 21 out of 25 nonagenarians in our study, PMVR was performed using conscious sedation instead of general anaesthesia. We have previously shown that PMVR performed using conscious sedation is as safe and effective as PMVR performed using general anaesthesia. 9 In another study, PMVR using conscious sedation reduced the length of stay in the intensive care unit without any negative effects on the safety and efficacy when compared with general anaesthesia. 20 Furthermore, in patients with extremely advanced age, the risk of general anaesthesia might be even higher than that in younger patients. We can only assume that the use of conscious sedation instead of general anaesthesia in the majority of our PMVR cases might have minimized complications. The present study did not demonstrate safety rates for the use of general anaesthesia for PMVR in these old patients. However, previous studies that analysed safety and in‐hospital outcome of PMVR, which was mainly performed under general anaesthesia, demonstrated similar in‐hospital mortality and complication rates between nonagenarians and younger patients. 6 , 19
We observed that successful procedural outcomes were reflected in the high procedural success rates even in nonagenarians undergoing PMVR (100% in the nonagenarians, 96% in the octogenarians, and 95% in the septuagenarians). One year after PMVR, 89.4% of the nonagenarians exhibited MR grades of 2+ or less. These results are consistent with the reduction in MR observed in younger patients. In addition to the reduction in MR, intermediate‐term improvement in NYHA functional class was observed. Moreover, rate of heart failure hospitalizations in the first 12 months was 15.4% in the nonagenarian group, which was similar to the rates in younger age groups from our study. The rate of heart failure hospitalizations was also similar to the rates reported in other studies, such as the EVEREST II High Risk Registry (16%) 21 and the TRAMI registry (16.9%). 17 The intermediate‐term survival of nonagenarians in our study was not worse than the survival of younger patients, suggesting considerable beneficial functional effects of the procedure in nonagenarians.
Study limitations
The present study was a single‐centre study involving a small number of individuals aged >90 years, which may have limited the statistical power of the analysis. Nonagenarians included in our study were a highly selected patient group. However, we cannot provide data about how many nonagenarians with clinically relevant MR can be considered suitable for PMVR, as the selection process had often occurred at the outpatient level before referral to our centre. The grading of MR post‐PMVR is challenging, and standard criteria for quantification of MR after PMVR are not well validated. Changes in quality of life assessment are subject to bias as other factors might have changed and affected quality of life in the course of 1 year.
Conclusions
The present study provides in a small population encouraging evidence regarding the feasibility and safety of PMVR in carefully selected nonagenarians, with beneficial effects comparable with those observed in younger patients. The chronological age alone should not deprive patients of PMVR. The physiological age including co‐morbidities and frailty should be considered in the shared decision‐making approach to determine a patient's candidacy for this procedure. Accurate selection may lead to a successful outcome in terms of palliation of heart failure symptoms and improvement in the quality of life.
Conflict of interest
None declared.
Author contributions
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supporting information
Figure S1. Survival after PMVR in propensity score matched groups
Propensity score matching was used to compare the groups in terms of survival and to reduce confounding factors due to imbalances in baseline characteristics. Kaplan–Meier survival curves after PMVR stratified by age for 2‐year all‐cause mortality. Two‐year mortality did not differ among the groups (each n = 24) (P = 0.611).
PMVR, Percutaneous mitral valve repair
Table S1. Patients' characteristics after propensity‐score matching. Values are n (%) or median (interquartile range). * indicates P ≤ 0.05 among the groups
Table S2. Procedural outcome after propensity‐score matching. Values are n (%) or median (interquartile range). * indicates P ≤ 0.05 among the groups
Acknowledgement
Open access funding enabled and organized by Projekt DEAL.
Christidi, A. , Haschemi, J. , Spieker, M. , Bönner, F. , Kelm, M. , Westenfeld, R. , and Horn, P. (2021) Two year outcome in nonagenarians undergoing percutaneous mitral valve repair. ESC Heart Failure, 8: 577–585. 10.1002/ehf2.13127.
References
- 1. He W, Muenchrath MN. 90+ in the United States: 2006–2008. American Community Survey Reports (ACS–17), US Census Bureau. Washington, DC: US Government Printing Office 2011.
- 2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet 2006; 368: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 3. Dziadzko V, Clavel MA, Dziadzko M, Medina‐Inojosa JR, Michelena H, Maalouf J, Nkomo V, Thapa P, Enriquez‐Sarano M. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet 2018; 391: 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017; 70: 252–289. [DOI] [PubMed] [Google Scholar]
- 6. Elbadawi A, Elgendy IY, Megaly M, Saad M, Garcia S, Abbott JD, Aronow H, Gafoor S, Jneid H, Sorajja P. Temporal trends and outcomes of transcatheter mitral valve repair among nonagenarians. JACC Cardiovasc Interv 2020; 13: 1385–1387. [DOI] [PubMed] [Google Scholar]
- 7. Boekstegers P, Hausleiter J, Baldus S, von Bardeleben RS, Beucher H, Butter C, Franzen O, Hoffmann R, Ince H, Kuck KH, Rudolph V, Schäfer U, Schillinger W, Wunderlich N, Germany Society of Cardiology Working Group on Interventional Cardiology Focus Group on Interventional Mitral Valve Therapy . Percutaneous interventional mitral regurgitation treatment using the Mitra‐Clip system. Clin Res Cardiol 2014; 103: 85–96. [DOI] [PubMed] [Google Scholar]
- 8. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 9. Horn P, Hellhammer K, Minier M, Stenzel MA, Veulemans V, Rassaf T, Luedike P, Pohl J, Balzer J, Zeus T, Kelm M, Westenfeld R. Deep sedation Vs. general anesthesia in 232 patients undergoing percutaneous mitral valve repair using the MitraClip® system. Catheter Cardiovasc Interv 2017; 90: 1212–1219. [DOI] [PubMed] [Google Scholar]
- 10. Stone GW, Adams DH, Abraham WT, Kappetein AP, Genereux P, Vranckx P, Mehran R, Kuck K‐H, Leon MB, Piazza N, Head SJ, Filippatos G, Vahanian AS, Mitral Valve Academic Research Consortium (MVARC) . Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 2015; 66: 308–321. [DOI] [PubMed] [Google Scholar]
- 11. Kim K, Kitai T, Kaji S, Pak M, Toyota T, Sasaki Y, Ehara N, Kobori A, Kinoshita M, Furukawa Y. Outcomes and predictors of cardiac events in medically treated patients with atrial functional mitral regurgitation. Int J Cardiol 2020; 316: 195–202. [DOI] [PubMed] [Google Scholar]
- 12. Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol 2017; 28: 2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spieker M, Hellhammer K, Katsianos S, Wiora J, Zeus T, Horn P, Kelm M, Westenfeld R. Effect of acute kidney injury after percutaneous mitral valve repair on outcome. Am J Cardiol 2018; 122: 316–322. [DOI] [PubMed] [Google Scholar]
- 14. Lo KB, Dayanand S, Ram P, Dayanand P, Slipczuk LN, Figueredo VM, Rangaswami J. Interrelationship between kidney function and percutaneous mitral valve interventions: a comprehensive review. Curr Cardiol Rev 2019; 15: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metze C, Matzik AS, Scherner M, Korber MI, Michels G, Baldus S, Rudolph V, Pfister R. Impact of frailty on outcomes in patients undergoing percutaneous mitral valve repair. JACC Cardiovasc Interv 2017; 10: 1920–1929. [DOI] [PubMed] [Google Scholar]
- 16. Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, Bajwa T, Herrmann HC, Lasala J, Maddux JT, Tuzcu M, Kapadia S, Trento A, Siegel RJ, Foster E, Glower D, Mauri L, Kar S, EVEREST II Investigators . Acute and 12‐month results with catheter‐based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge‐to‐Edge Repair) High Risk Study. J Am Coll Cardiol 2012; 59: 130–139. [DOI] [PubMed] [Google Scholar]
- 17. Kalbacher D, Schafer U, Bardeleben RS, Eggebrecht H, Sievert H, Nickenig G, Butter C, May AE, Bekeredjian R, Ouarrak T, Kuck KH. Long‐term outcome, survival and predictors of mortality after MitraClip therapy: Results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int J Cardiol 2019; 277: 35–41. [DOI] [PubMed] [Google Scholar]
- 18. Maisano F, Franzen O, Baldus S, Schafer U, Hausleiter J, Butter C, Ussia GP, Sievert H, Richardt G, Widder JD, Moccetti T. Percutaneous mitral valve interventions in the real world: early and 1‐year results from the ACCESS‐EU, a prospective, multicenter, nonrandomized post‐approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013; 62: 1052–1061. [DOI] [PubMed] [Google Scholar]
- 19. von Bardeleben RS, Hobohm L, Kreidel F, Ostad MA, Schulz E, Konstantinides S, Lankeit M, Feldman T, Münzel T, Keller K. Incidence and in‐hospital safety outcomes of patients undergoing percutaneous mitral valve edge‐to‐edge repair using MitraClip: five‐year German national patient sample including 13,575 implants. EuroIntervention 2019; 14: 1725–1732. [DOI] [PubMed] [Google Scholar]
- 20. de Waha S, Seeburger J, Ender J, Desch S, Eitel I, Reinhardt A, Pöss J, Fuernau G, Noack T, Merk DR, Schuler G. Deep sedation versus general anesthesia in percutaneous edge‐to‐edge mitral valve reconstruction using the MitraClip system. Clin Res Cardiol 2015; 105: 535–543. [DOI] [PubMed] [Google Scholar]
- 21. Kar S, Feldman T, Qasim A, Trento A, Kapadia S, Pedersen W, Lim DS, Kipperman R, Smalling RW, Bajwa T, Hermann HC, Hermiller JB, Lasala JM, Reisman M, Glower D, Mauri L, Whitlow P, EVEREST II Investigators . Five‐year outcomes of transcatheter reduction of significant mitral regurgitation in high‐surgical‐risk patients. Heart 2019; 105: 1622–1628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Survival after PMVR in propensity score matched groups
Propensity score matching was used to compare the groups in terms of survival and to reduce confounding factors due to imbalances in baseline characteristics. Kaplan–Meier survival curves after PMVR stratified by age for 2‐year all‐cause mortality. Two‐year mortality did not differ among the groups (each n = 24) (P = 0.611).
PMVR, Percutaneous mitral valve repair
Table S1. Patients' characteristics after propensity‐score matching. Values are n (%) or median (interquartile range). * indicates P ≤ 0.05 among the groups
Table S2. Procedural outcome after propensity‐score matching. Values are n (%) or median (interquartile range). * indicates P ≤ 0.05 among the groups


