Abstract
Aims
Many studies have explored the clinical characteristics of patients with coronavirus disease (COVID‐19), especially patients with cardiovascular disease. However, associated mechanisms and markers remain to be further investigated. This study aimed to investigate the effect of α‐hydroxybutyrate dehydrogenase (α‐HBDH) levels on disease progression and prognosis of patients with COVID‐19.
Methods and results
One thousand seven hundred and fifty‐one patients from the Leishenshan hospital in Wuhan were divided into elevated and normal groups by α‐HBDH level, and the clinical information between the two groups was compared retrospectively. The main outcome evaluation criteria included in‐hospital death and disease severity. Univariate and multivariate regression analyses, survival curves, logistic regression, and receiver operating characteristic curve models were performed to explore the relationship between elevated α‐HBDH and the two outcomes. Besides, curve fitting analyses were conducted to analyse the relationship between computed tomography score and survival. Among 1751 patients with confirmed COVID‐19, 15 patients (0.87%) died. The mean (SD) age of patients was 58 years in normal α‐HBDH group and 66 years in elevated α‐HBDH group (P < 0.001). The mortality during hospitalization was 0.26% (4 of 1559) for patients with normal α‐HBDH levels and 5.73% (11 of 192) for those with elevated α‐HBDH levels (P < 0.001). Multivariate Cox analysis confirmed an association between elevated α‐HBDH levels and higher risk of in‐hospital mortality [hazard ratio: 4.411, 95% confidence interval (95% CI), 1.127–17.260; P = 0.033]. Multivariate logistic regression for disease severity and α‐HBDH levels showed significant difference between both groups (odds ratio = 3.759; 95% CI, 1.895–7.455; P < 0.001). Kaplan–Meier curves also illustrated the survival difference between normal and elevated α‐HBDH patients (P < 0.001).
Conclusions
Our study found that serum α‐HBDH is an independent risk factor for in‐hospital mortality and disease severity among COVID‐19 patients. α‐HBDH assessment may aid clinicians in identifying high‐risk individuals among COVID‐19 patients.
Keywords: α‐HBDH, Coronavirus disease, Prognosis, Disease progression
Introduction
Coronavirus disease (COVID‐19) was first recognized in Wuhan in December 2019 and rapidly emerged as a global crisis. 1 , 2 As of 4 May 2020, more than 3 400 000 cases have been confirmed, and over 230 000 people have died worldwide. 3 The worldwide pandemic of COVID‐19, with the number of cases and deaths still climbing, is an unprecedented challenge to global health. 4 , 5
The Leishenshan Hospital is a 1600‐bed designated hospital to treat patients with COVID‐19 and is hosted by the Zhongnan Hospital. The well‐equipped hospital, which received newly diagnosed and hospitalized patients for critical care, was put into operation from 8 February 2020. A total of 2011 patients were admitted to this hospital until its closure on 15 April 2020. The patient data collected from these admissions were analysed to investigate the clinical features and validate valuable diagnostic indicators of COVID‐19.
Researchers are increasingly aware of the close association between cardiovascular events and poor outcomes COVID‐19 patients. 6 , 7 Acute cardiac injury occurs in approximately 8–12% of all patients. 8 Both acute cardiac injury during the course of the illness and pre‐existing cardiovascular disease are known to be associated with a shorter survival time. 9 , 10 α‐HBDH, a marker of cell death particularly reflecting renal, red blood cell, and myocardial damage, is an auxiliary marker of myocardial injury. 11 In the early days, it was found to have an increased specificity for the detection of myocardial damage, starting to increase 8–12 h after injury, reaching peak serum concentrations after 48–72 h and returning to baseline after 7–14 days. 12 In clinical practice, we observed that high α‐HBDH levels are associated with higher mortality in COVID‐19 patients. Herein, we investigated the effect of α‐HBDH levels on disease progression and prognosis of patients with COVID‐19.
Materials and methods
Study design and patients
A cohort of 1880 patients with laboratory confirmation of COVID‐19 admitted to the Leishenshan Hospital between 9 February and 18 March 2020 were enrolled in this study. Patient data were collected from electronic medical records and independently verified by three investigators (SW, SC, and YH) to confirm data accuracy. α‐HBDH samples were collected and tested by the Leishenshan hospital, using a continuous monitoring method with standard kits. One hundred and twenty‐nine patients without α‐HBDH were excluded, and we retrospectively analysed the clinical characteristics, laboratory findings, treatment, and outcomes of 1751 patients.
Definitions
Among the 1751 patients analysed, 192 had an elevated α‐HBDH (>199 U/L). Because no patients had low levels of α‐HBDH (<74 U/L), the cohort was analysed as two groups.
The primary outcome was in‐hospital death, followed by the highest disease severity. The illness status was defined as four levels based on the seventh edition of the Chinese management guideline for COVID‐19 published by the Chinese National Health Commission. 13 The comparative analysis of laboratory indicators between groups was based on their level at admission. The most serious level of illness during their hospitalization was also supplemented and listed separately. In identifying the highest disease severity as an outcome indicator, we distributed the 1751 patients to two categories: mild/common/severe and critical.
We adopted a semi‐quantitative score system for the evaluation of computed tomography (CT) images based on previous studies and the pulmonary pathological features of COVID‐19. According to international research standards, all chest CT images were evaluated independently by two radiologists, and in cases where there was a difference of opinion, consensus was arrived at by mutual discussion. Score 1 was the sum of the pathological type points as identified on CT scan. Each image feature including ground‐glass opacities, reticular or cordlike changes, consolidation, tracheal distortion, and pleural effusion were assigned one point. Score 2 was generated based on the lesion area. The bilateral lobes were divided into six parts as upper, middle, and lower lungs. Each lung area was scored based on the scope of lesions from 0 to 4; for every 25% increase in the proportion of lung involvement, the score was increased by one point. The total CT score was the sum of Scores 1 and 2.
Ethics approval and patient consent
The study was approved by the Research Ethics Commission of the Zhongnan Hospital of Wuhan University (approval number: 2020068). Because the study involved only perusal of patient records, the ethics committee waived the need for patient consent. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Statistical analysis
The characteristics of normal and elevated α‐HBDH group were compared. All the statistical analyses were performed using SPSS Version 22.0 (IBM Corp, Armonk, NY, USA). Categorical variables were presented as numbers and percentages, and tested by χ 2 test and Fisher's exact test, respectively. Continuous variables were presented as median (interquartile range), and tested by Mann–Whitney U test. Univariate and multivariate regression analyses and Kaplan–Meier survival analyses were used to explore whether α‐HBDH levels were associated with prognosis. The association between CT performance and survival time was assessed with curve fitting analysis. Two‐sided P values < 0.05 were regarded as statistically significant.
Results
Demographics, clinical information, and treatment
The median patient age in the elevated α‐HBDH group was greater than that in the normal α‐HBDH group (66 vs. 58 years; P < 0.001) (Table 1 ). Patients in the elevated α‐HBDH group were significantly more likely to have a history of underlying disease, received advanced airway management (high‐flow nasal cannula, tracheal intubation, or extracorporeal membrane oxygenation), and developed a critical illness on admission or during hospitalization. The mortality during hospitalization was 0.26% (4 of 1559) for patients with normal α‐HBDH levels, 5.73% (11 of 192) for those with elevated α‐HBDH levels (P < 0.001). In addition, more patients in the elevated α‐HBDH group had a higher CT score over 4 (72.97% vs. 53.91%; P = 0.009) (Table 1 ). Patients in the elevated α‐HBDH group were significantly more likely to have received anticoagulation treatment (32.81% vs. 3.91%) and corticosteroids (23.44% vs. 3.98%) and had a longer hospital stay (23 vs. 18 days) and higher in‐hospital mortality (5.73% vs. 0.26%) (Table 1 ).
Table 1.
Demographics, clinical information, and treatment of 1751 patients with COVID‐19
| Covariate | Level | All patients (n = 1751), n (%) | Normal α‐HBDH (n = 1559), n (%) | Elevated α‐HBDH (n = 192), n (%) | P value |
|---|---|---|---|---|---|
| Gender | 0.255 | ||||
| Female | 916 (52.31) | 823 (52.79) | 93 (48.44) | ||
| Male | 835 (47.69) | 736 (47.21) | 99 (51.56) | ||
| Age, median (IQR) | 59.00 (49.00–68.00) | 58.00 (48.00–67.00) | 66.00 (56.00–74.75) | <0.001 | |
| Any comorbidity | 523 (61.03) | 418 (59.21) | 105 (69.54) | 0.018 | |
| Cardiovascular diseases | 353 (41.19) | 287 (40.65) | 66 (43.71) | 0.488 | |
| Pulmonary diseases | 87 (10.74) | 68 (9.96) | 19 (14.96) | 0.094 | |
| Endocrine diseases | 135 (15.75) | 110 (15.58) | 25 (16.56) | 0.765 | |
| Malignancy | 59 (6.88) | 48 (6.80) | 11 (7.28) | 0.831 | |
| Digest system diseases | 45 (5.25) | 38 (5.38) | 7 (4.64) | 0.709 | |
| Neurological diseases | 54 (6.30) | 40 (5.67) | 14 (9.27) | 0.098 | |
| Initial symptoms, n (%) | |||||
| Fever or fatigue | 610 (79.02) | 504 (78.87) | 106 (79.70) | 0.831 | |
| Respiratory symptoms | 623 (80.71) | 509 (79.66) | 114 (85.71) | 0.107 | |
| Digestive symptoms | 80 (10.36) | 65 (10.17) | 15 (11.28) | 0.703 | |
| Neurological symptoms | 26 (3.37) | 19 (2.97) | 7 (5.26) | 0.286 | |
| Other | 25 (3.24) | 24 (3.76) | 1 (0.75) | 0.131 | |
| Drugs | |||||
| Antibiotic | 518 (29.58) | 430 (27.58) | 88 (45.83) | <0.001 | |
| Antiviral drugs | 852 (48.66) | 735 (47.15) | 117 (60.94) | <0.001 | |
| Antimalarial drugs | 134 (7.65) | 112 (7.18) | 22 (11.46) | 0.036 | |
| Anticoagulants | 124 (7.08) | 61 (3.91) | 63 (32.81) | <0.001 | |
| Corticosteroid | 107 (6.11) | 62 (3.98) | 45 (23.44) | <0.001 | |
| Vitamin C | 242 (13.82) | 209 (13.41) | 33 (17.19) | 0.152 | |
| Traditional Chinese medicine | 1508 (86.12) | 1346 (86.34) | 162 (84.38) | 0.458 | |
| Oxygen support | <0.001 | ||||
| Low‐flow nasal cannula | 256 (83.12) | 229 (88.42) | 27 (55.10) | ||
| Non‐invasive ventilation or high‐flow nasal cannula | 46 (14.94) | 29 (11.20) | 17 (34.69) | ||
| Invasive mechanical ventilation | 5 (1.62) | 1 (0.39) | 4 (8.16) | ||
| ECMO | 1 (0.32) | 0 (0) | 1 (2.04) | ||
| CT scores | 1–4 | 73 (38.62) | 53 (46.09) | 20 (27.03) | 0.009 |
| 5–7 | 116 (61.38) | 62 (53.91) | 54 (72.97) | ||
| Disease progression | <0.001 | ||||
| Stableness/hospitalization | 15 (0.87) | 6 (0.39) | 9 (4.92) | ||
| Improvement/recover | 1,699 (98.26) | 1,536 (99.35) | 163 (89.07) | ||
| Death | 15 (0.87) | 4 (0.26) | 11 (6.01) | ||
| Days in hospital, median (IQR) | 18 (13–24) | 18 (13–23) | 23 (15–30) | <0.001 | |
| Death | 15 (0.86) | 4 (0.26) | 11 (5.73) | <0.001 | |
| Severity on admission | |||||
| Mild | 784 (44.77) | 732 (46.95) | 52 (27.08) | <0.001 | |
| Common | 663 (37.86) | 592 (37.97) | 71 (36.98) | ||
| Severe | 280 (15.99) | 226 (14.50) | 54 (28.13) | ||
| Critical | 24 (1.37) | 9 (0.58) | 15 (7.81) | ||
| Severity at worst | |||||
| Mild/common | 908 (52.00) | 868 (55.78) | 40 (21.05) | <0.001 | |
| Severe | 792 (45.36) | 666 (42.80) | 126 (66.32) | ||
| Critical | 46 (2.63) | 22 (1.41) | 24 (12.63) |
α‐HBDH, α‐hydroxybutyrate dehydrogenase; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range.
Laboratory findings
In contrast to the normal α‐HBDH group, patients in the elevated α‐HBDH group had a much higher level of d‐dimer (1.31 vs. 0.34), interleukin‐6 (IL‐6) (6.66 vs. 1.50) lactate dehydrogenase (LDH) (294 vs. 179), and a lower level of lymphocyte count (1.16 vs. 1.64), erythrocyte count (3.90 vs. 4.13), and albumin (33.30 vs. 38.00). However, there were no significant differences observed between the groups for the prevalence of SARS‐CoV‐2 immunoglobulin M and immunoglobulin G (Table 2 ).
Table 2.
Laboratory test results of 1751 patients with COVID‐19
| Covariate | All patients (n = 1751) Median (IQR)/n (%) | Normal α‐HBDH (n = 1559) Median (IQR)/n (%) | Elevated α‐HBDH (n = 192) Median (IQR)/n (%) | P value | Reference range |
|---|---|---|---|---|---|
| Leucocyte count (×109/L) | 5.69 (4.71–6.92) | 5.64 (4.70–6.81) | 6.32 (4.91–8.14) | <0.001 | 3.50–9.50 |
| Neutrophil count (×109/L) | 3.28 (2.54–4.27) | 3.21 (2.51–4.12) | 4.07 (3.13–5.78) | <0.001 | 1.80–6.30 |
| Lymphocyte count (×109/L) | 1.60 (1.24–1.99) | 1.64 (1.31–2.01) | 1.16 (0.81–1.60) | <0.001 | 1.10–3.20 |
| Erythrocyte count (×1012/L) | 4.12 (3.76–4.49) | 4.13 (3.79–4.50) | 3.90 (3.46–4.38) | <0.001 | 4.30–5.80 |
| Monocyte count (×109/L) | 0.50 (0.40–0.63) | 0.50 (0.41–0.62) | 0.55 (0.40–0.73) | 0.029 | 0.10–0.60 |
| Haemoglobin (g/L) | 126.00 (115.00–137.0) | 126.00 (116.00–137.00) | 121.00 (107.00–134.75) | <0.001 | 130.00–175.00 |
| Platelet count (×109/L) | 228.00 (187.00–277.00) | 230.00 (190.00–276.00) | 217.50 (159.75–309.75) | 0.149 | 125.00–350.00 |
| Albumin (g/L) | 37.70 (35.00–40.00) | 38.00 (35.60–40.20) | 33.30 (30.70–36.90) | <0.001 | 40.00–55.00 |
| Alanine aminotransferase (U/L) | 23.00 (15.00–37.00) | 22.00 (14.00–36.00) | 29.00 (18.00–48.00) | <0.001 | 9.00–50.00 |
| Aspartate aminotransferase (U/L) | 20.00 (16.00–27.00) | 19.00 (15.00–25.00) | 28.00 (20.00–42.60) | <0.001 | 15.00–40.00 |
| Total bilirubin (μmol/L) | 9.10 (7.00–12.00) | 9.10 (7.00–11.90) | 9.40 (6.50–13.10) | 0.742 | 5.00–21.00 |
| LDH (U/L) | 184 (160–216) | 179 (157–205) | 294 (271–354) | <0.001 | 125–343 |
| α‐HBDH (U/L) | 141 (123–168) | 136 (121–156) | 233 (215–277) | <0.001 | 74–199 |
| Creatinine (μmol/L) | 64.30 (54.50–76.20) | 63.80 (54.00–75.40) | 66.00 (57.60–87.73) | <0.001 | 64.00–104.00 |
| Prothrombin time (s) | 11.30 (10.90–11.80) | 11.30 (10.90–11.70) | 11.70 (11.30–12.50) | <0.001 | 9.4–12.5 |
| INR | 0.97 (0.93–1.02) | 0.97 (0.93–1.01) | 1.01 (0.97–1.08) | <0.001 | 0.8–1.3 |
| APTT (s) | 27.20 (24.60–30.43) | 27.10 (24.40–30.20) | 28.80 (25.80–33.10) | <0.001 | 25.1–36.5 |
| Fibrinogen (g/L) | 2.95 (2.51–3.73) | 2.90 (2.47–3.58) | 3.87 (2.90–4.30) | <0.001 | 2.38–4.98 |
| Thrombin time (s) | 17.70 (17.70–18.50) | 17.70 (17.00–18.40) | 17.40 (16.70–18.60) | 0.026 | 10.3–16.6 |
| d‐dimer (g/L) | 0.38 (0.21–0.90) | 0.34 (0.20–0.78) | 1.31 (0.50–4.14) | <0.001 | 0–0.50 |
| Procalcitonin (ng/mL) | 0.04 (0.03–0.05) | 0.03 (0.02–0.05) | 0.07 (0.04–0.13) | <0.001 | <0.05 |
| Interleukin‐6 (pg/mL) | 1.50 (1.50–4.04) | 1.50 (1.50–3.415) | 6.66 (1.75–24.47) | <0.001 | 0–7.00 |
| SARS‐CoV‐19 IgM | 0.393 | ‐ | |||
| NO | 379 (64.57) | 332 (65.23) | 47 (60.26) | ||
| YES | 208 (35.43) | 177 (34.77) | 31 (39.74) | ||
| SARS‐CoV‐19 IgG | 0.512 | ||||
| NO | 49 (8.57) | 41 (8.27) | 8 (10.53) | ||
| YES | 523 (91.43) | 455 (91.73) | 68 (89.47) |
α‐HBDH, α‐hydroxybutyrate dehydrogenase; APTT, activated partial thromboplastin time; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase.
Regression analysis and Kaplan–Meier curve
Multivariate logistic regression for disease severity and α‐HBDH levels showed significant differences between the two groups (odds ratio, 3.759; 95% confidence interval, 1.895–7.455; P < 0.001) (Table 3 ). In addition, multivariate Cox analysis also validated the association between elevated α‐HBDH levels and higher risk of in‐hospital mortality (hazard ratio, 4.411; 95% confidence interval, 1.127–17.260; P < 0.033) (Table 4 ). Similar results were also visualized on the Kaplan–Meier curves (Figure 1 , P < 0.001). The adjustment factors included in the multivariate regression model were age, lymphocyte count, platelet count, d‐dimer, total bilirubin, and creatinine. We generated a receiver operating characteristic curve to assess the evaluation value of elevated α‐HBDH for the highest disease severity and found the area under curve to be 0.808 (P < 0.001) (Figure 2 ).
Table 3.
The risk of elevated α‐hydroxybutyrate dehydrogenase for the disease severity of COVID‐19
| Group | Logistic regression analysis | ||||
|---|---|---|---|---|---|
| OR | 95% CI | P value | |||
| Univariate analysis | Normal α‐HBDH | Ref | |||
| Elevated a‐HBDH | 10.081 | 5.531 | 18.374 | <0.001 | |
| Multivariate analysis a | Normal α‐HBDH | Ref | |||
| Elevated a‐HBDH | 3.759 | 1.895 | 7.455 | <0.001 | |
CI, confidence interval; OR, odds ratio.
Adjust for age, lymphocyte count, platelet count, d‐dimer, total bilirubin, and creatinine.
Table 4.
The risk of elevated α‐hydroxybutyrate dehydrogenase for the disease mortality of COVID‐19
| Group | Cox regression analysis | ||||
|---|---|---|---|---|---|
| HR | 95% CI | P value | |||
| Univariate analysis | Normal α‐HBDH | Ref | |||
| Elevated a‐HBDH | 20.902 | 6.637 | 65.820 | <0.001 | |
| Multivariate analysis a | Normal α‐HBDH | Ref | |||
| Elevated a‐HBDH | 4.411 | 1.127 | 17.260 | 0.033 | |
CI, confidence interval; HR, hazard ratio.
Adjust for age, lymphocyte count, platelet count, d‐dimer, total bilirubin, and creatinine.
Figure 1.
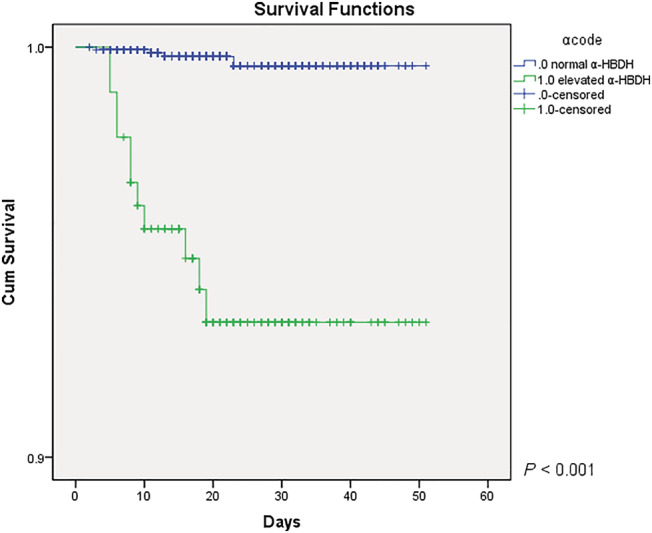
The Kaplan–Meier curves for α‐HBDH levels and survival. α‐HBDH, α‐hydroxybutyrate dehydrogenase.
Figure 2.
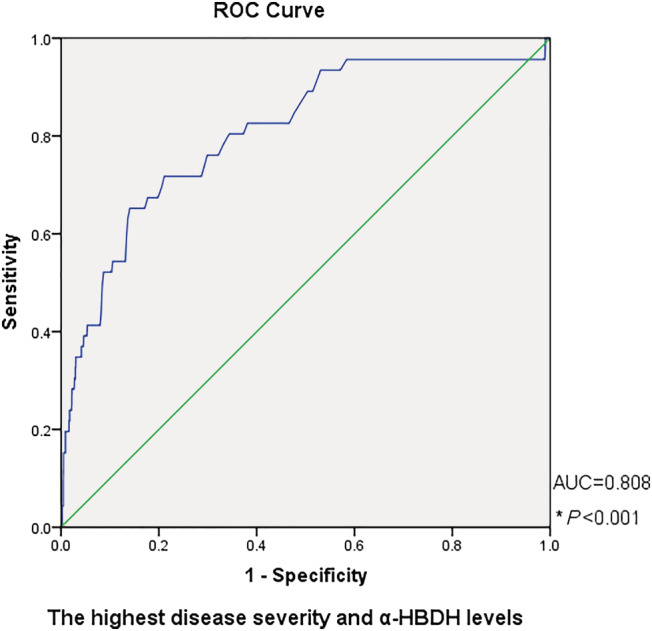
ROC curve for elevated α‐HBDH and the highest disease severity. α‐HBDH, α‐hydroxybutyrate dehydrogenase; AUC, area under the curve; ROC, receiver operating characteristic curve.
Curve fitting analysis for the evaluation of computed tomography images
Figure 3 shows the results of the curve fitting analysis for CT images and survival days. For all patients, Score 1, Score 2, and total score reached peak values of 2.5 at 20 days, 2.4 at 12 days, and 4.9 at 19 days, respectively (Figure 3 A, B, and C). For patients with normal α‐HBDH levels, Score 1 and total score reached peak values of 2.42 at 21 days and 4.65 at 18 days, respectively (Figure 3D,F ). However, for patients with evaluated level of α‐HBDH, Score 1, Score 2, and total score showed a descending trend (Figure 3G, H, and I ), consistent with Score 2 for normal α‐HBDH patients (Figure 3E ).
Figure 3.
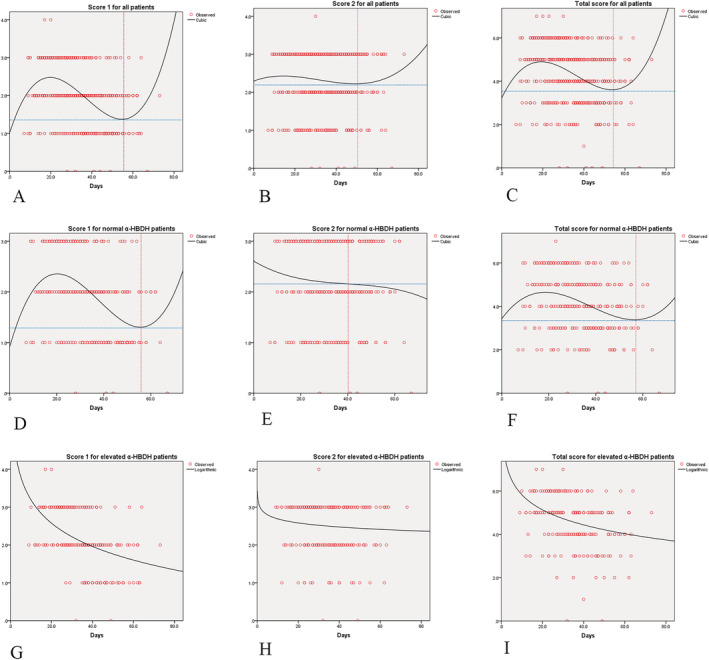
The curve fitting analysis for computed tomography images and survival days. α‐HBDH, α‐hydroxybutyrate dehydrogenase.
Discussion
This is the first and largest study so far to analyse the association between α‐HBDH and the clinical course and survival of COVID‐19 patients. We identified elevated α‐HBDH as a potential independent risk factor for in‐hospital mortality and disease severity in COVID‐19 patients.
The target organs in SARS‐Cov‐2 are lung and trachea. However, it is observed that the damage of organs other than these increases mortality as well. 14 Previous studies have shown that heart damage is closely related to poor outcomes, 2 , 15 mainly by two mechanisms: direct myocardial injury and systemic inflammation, which follow the binding of the virus to angiotensin‐converting enzyme 2 receptors in the heart and virus‐induced systemic inflammatory storm, respectively. 16 , 17
In cardiomyocytes, α‐HBDH is the same as aspartate aminotransferase, LDH, creatine kinase, and creatine kinase‐MB that participate in the formation of myocardial enzyme spectrum, and their levels reflect heart muscle injury and haemolysis. 18 , 19 , 20 Although the increases in LDH and α‐HBDH are relatively less specific than high‐sensitivity Troponin I (hs‐TNI) and creatine kinase‐MB for heart injury, they play a supportive role in the recognition of myocarditis or myocardial infarction. 21 Besides, α‐HBDH is implicated as a biomarker of insulin resistance, thrombosis, and cell death marker in renal and red blood cell damage. In the kidney, haemolysis may occur when attacked by the virus. In the blood system, the number of red blood cells decreases, and the incidence of thrombosis increases. In the heart, the attack of the virus damages the heart tissue. High levels of α‐HBDH, reflecting damage to one or more systems, lead to a worse outcome. The result of our study shows that patients in the elevated α‐HBDH group had higher d‐dimer (1.31:0.34, P < 0.001), LDH (294:179, P < 0.001), and fewer red blood cells (3.90:4.13, P < 0.001) compared with patients with normal α‐HBDH. Therefore, we hypothesized that high α‐HBDH levels of patients with poor prognosis are the result of multiple mechanisms.
Our results showed that high α‐HBDH on admission significantly correlated with higher mortality, which is consistent with previous reports. 21 COVID‐19 patients with higher α‐HBDH levels tended to be older, and more likely to have severe or critical disease, and require respiratory support. Age is an important factor affecting the prognosis. This has also been confirmed in other studies. 17 , 22 , 23 , 24 Older age is often accompanied by slowing down of cell metabolism, poor resistance to viruses, and more extensive damage to organs and tissues. α‐HBDH, as a marker of cell death, is obviously also closely related to age. In contrast, there was no significant difference in initial symptoms and comorbidity between the two groups. The initial symptoms of COVID‐19 are extensive and atypical, and the distribution of the underlying comorbidities showed that it would not affect the evaluation results of α‐HBDH on survival analysis.
Patients in the elevated α‐HBDH group had elevated white blood cell count, IL‐6, D‐dimer, LDH and reduced lymphocyte count, and albumin levels compared with patients with normal α‐HBDH. Changes in these indicators and the α‐HBDH levels may affect mutually, such as the malnutrition indicated by serum albumin levels; increased d‐dimers prompts the risk of thrombus and bleeding; increased IL‐6 indicates increased inflammation in the body; LDH levels are related to the degree of tissue damage. All these changes indicate a poor prognosis for patients. It also suggests that the increase in α‐HBDH levels is related to the abnormalities of various systems, but the specific mechanism still needs more further research. Among them, the difference in d‐dimer and IL‐6 levels between the two groups is greater. A previous pooled analysis clearly identified elevated d‐dimer levels as being associated with severity of COVID‐19. 25 IL‐6 was recommended as a marker for potential progression to critical illness. 26 , 27 In addition, a‐HBDH/LDH ratio of the elevated α‐HBDH group is 0.79 (233/294, Table 2 ). According to the test criterion, a ratio above 0.9 suggests myocardial lesion, under 0.6 suggests liver damage, while ratio between 0.63 and 0.81 is normal. We think this may be related to multiple organ damage caused by the virus, which involves heart, kidney, liver, immune system, and others. 28 The fitting curve for lung lesion type and area on imaging tended to rise followed by a fall in the total patient group as well as in the normal α‐HBDH group (Figure 3A, B, C, D, and F ). However, this trend was found to be declining in the elevated α‐HBDH group (Figure 3G, H, and I ). This may be because patients with elevated α‐HBDH tended to have severe clinical symptoms of pneumonia and were then transported to the hospital for comprehensive critical care.
In our study, a higher proportion of patients with elevated α‐HBDH received anticoagulation treatment and corticosteroids. There was no difference in the use of antiviral therapy and antibiotic therapy in the two groups. Drug treatment, especially the use of corticosteroids, may slow virus clearance owing to its immunosuppressive effect. 29 , 30 This may affect the disease course and biochemical indicators, including α‐HBDH. Further studies are needed to determine the effects of corticosteroids and anticoagulants on α‐HBDH in patients with COVID‐19.
There are some limitations in our study. First, this was a retrospective study, and the missing data and heterogeneity may contribute to bias. Second, we only evaluated the cardiac serum index and did not verify the actual cardiac changes on electrocardiogram or cardiac colour Doppler ultrasound. Third, the data defaults to the α‐HBDH level detected on admission and continuous monitoring had not been conducted. Finally, we did not correct for the role of interference with glucocorticoid, antiviral, and antibacterial drug treatment.
In conclusion, our study found that α‐HBDH levels may be an independent risk factor for in‐hospital mortality and disease severity among COVID‐19 patients. Early monitoring of α‐HBDH levels may have a role in identifying high risk individuals among COVID‐19 patients.
Conflict of interest
None declared.
Author contributions
Zeming Liu, Jinpeng Li, and Xiaohui Wu conceptualized the study. Sichao Chen, Rongfen Gao, Guang Zeng, Danyang Chen, Shipei Wang, Qianqian Li, Di Hu, and Man Li performed the data collection and processing. Wen Zeng, Liang Guo, and Xiaohui Wu interpreted the data. All authors contributed to the writing—review & editing of the study.
Liu, Z. , Li, J. , Li, M. , Chen, S. , Gao, R. , Zeng, G. , Chen, D. , Wang, S. , Li, Q. , Hu, D. , Zeng, W. , Guo, L. , and Wu, X. (2021) Elevated α‐hydroxybutyrate dehydrogenase as an independent prognostic factor for mortality in hospitalized patients with COVID‐19. ESC Heart Failure, 8: 644–651. 10.1002/ehf2.13151.
Zeming Liu, Jinpeng Li, Man Li, Sichao Chen, and Rongfen Gao are equal contributors.
Contributor Information
Wen Zeng, Email: 524578732@qq.com.
Liang Guo, Email: guoliangwhzn@163.com.
Xiaohui Wu, Email: wuxiaohui1971@sina.com.
References
- 1. Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus disease 2019 (COVID‐19): a perspective from China. Radiology 2020; 296: 200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. The world health organization coronavirus disease 2019 (COVID‐19) situation report −105 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200504‐covid‐19‐sitrep‐105.pdf?sfvrsn=4cdda8af_2. 2020‐05‐05
- 4. Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID‐19): emerging and future challenges for dental and oral medicine. J Dent Res 2020; 99: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The L. India under COVID‐19 lockdown. Lancet 2020; 395: 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID‐19): current status and future perspectives. Int J Antimicrob Agents 2020; 55: 105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bansal M. Cardiovascular disease and COVID‐19. Diabetes Metab Syndr 2020; 14: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu C, Hu X, Song J, Du C, Xu J, Yang D, Chen D, Zhong M, Jiang J, Xiong W, Lang K. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID‐19). medRxiv, 2020: p. 2020.02.26.20028589.
- 10. Foo R, Wang Y, Zimmermann WH, Backs J, Wang DW. Cardiovascular molecular mechanisms of disease with COVID‐19. J Mol Cell Cardiol 2020; 141: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S, Koppensteiner R, Kopp CW, Gremmel T. alpha‐Hydroxybutyrate dehydrogenase is associated with atherothrombotic events following infrainguinal angioplasty and stenting. Sci Rep 2019; 9: 18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kemp M, Donovan J, Higham H, Hooper J. Biochemical markers of myocardial injury. Br J Anaesth 2004; 93: 63–73. [DOI] [PubMed] [Google Scholar]
- 13. NHCotPsRo C . National Health Commission of the People's Republic of China. Guidelines for the Diagnosis and Treatment of COVID‐19 (Trial Version 7).
- 14. Yang X, Yu Y, Xu J, Shu H, Xia J', Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol 2020; 109: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J 2020; 41: 1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou F, Yu T, du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Laarse A, Hermens WT, Hollaar L, Jol M, Willems GM, Lemmers HEAS, Liem AH, Souverijn JHM, Oudhof JH, de Hooge J, Buis B, Arntzenius AC. Assessment of myocardial damage in patients with acute myocardial infarction by serial measurement of serum alpha‐hydroxybutyrate dehydrogenase levels. Am Heart J 1984; 107: 248–260. [DOI] [PubMed] [Google Scholar]
- 19. Orsonneau JL, Meflah K, Lustenberger P, Cornu G, Bernard S. Sensitisation and visualisation of biochemical measurements using the NAD/NADH system by means of Meldola blue. I Principle and application to the continuous flow measurement of LDH and alpha HBDH activities in serum. Clin Chim Acta 1982; 125: 177–184. [DOI] [PubMed] [Google Scholar]
- 20. Vasudevan G, Mercer DW, Varat MA. Lactic dehydrogenase isoenzyme determination in the diagnosis of acute myocardial infarction. Circulation 1978; 57: 1055–1057. [DOI] [PubMed] [Google Scholar]
- 21. Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, Guo F, Zhao H, Gao R. A comparative study on the clinical features of COVID‐19 pneumonia to other pneumonias. Clin Infect Dis 2020; 71: 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alfaraj SH, al‐Tawfiq JA, Assiri AY, Alzahrani NA, Alanazi AA, Memish ZA. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) infection: a cohort study. Travel Med Infect Dis 2019; 29: 48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis 2005; 41: S504–S512. [DOI] [PubMed] [Google Scholar]
- 24. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lippi G, Favaloro EJ. D‐dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost 2020; 120: 876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med 2020; 58: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Li J, Chen D, Gao R, Zeng W, Chen S, Huang Y, Huang J, Long W, Li M, Guo L, Wang X, Wu X. Dynamic interleukin‐6 level changes as a prognostic indicator in patients with COVID‐19. Front Pharmacol 2020; 11: 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID‐19 and multiorgan response. Curr Probl Cardiol 2020; 45: 100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang X, Mei Q, Yang T, Li L, Wang Y, Tong F, Geng S, Pan A. Low‐dose corticosteroid therapy does not delay viral clearance in patients with COVID‐19. J Infect 2020; 81: 147–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol 2020; 146: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]


