Abstract
Aims
Health insurance claims (HIC) databases in the Netherlands capture unselected patient populations, which makes them suitable for epidemiological research on sex differences. Based on a HIC database, we aimed to reveal sex differences in heart failure (HF) outcomes, with particular focus on co‐morbidities and medication.
Methods and results
The Achmea HIC database included 14 517 men and 11 259 (45%) women with a diagnosis treatment code for chronic HF by January 2015. We related their sex, co‐morbidities, and medication adherence (medication possession rate >0.8) with the primary endpoint (PE) of all‐cause mortality or HF admission during a median follow‐up of 3.3 years, using Cox regression. Median age of men and women was 72 and 76 years, respectively. Prevalence of co‐morbidities and use of disease‐modifying drugs was higher in men; however, medication adherence was similar. At the end of follow‐up, 35.1% men and 31.8% women had reached the PE. The adjusted hazard ratio for men was 1.25 (95% confidence interval: 1.19–1.30). A broad range of co‐morbidities was associated with the PE. Overall, these associations were stronger in women than in men, particularly for renal insufficiency, chronic obstructive pulmonary disease/asthma, and diabetes. Non‐adherence to disease‐modifying drugs was related with a higher incidence of the PE, with similar effects between sexes.
Conclusions
In a representative sample of the Dutch population, as captured in a HIC database, men with chronic HF had a 25% higher incidence of death or HF admission than women. The impact of co‐morbidities on the outcome was sex dependent, while medication adherence was not.
Keywords: Heart failure, Co‐morbidity, Medication adherence, Hospitalisation, Mortality, Big data
Introduction
Randomized controlled trials (RCTs) are broadly accepted as the golden standard to evaluate the efficacy and safety of pharmacological treatment. However, RCTs usually have strict inclusion and exclusion criteria, which makes their representativeness for clinical practice questionable. For example, in the cardiovascular domain, including heart failure (HF), RCT participants are selected from a predominantly (younger) male patient population, 1 whereas (elderly) women and those with more complex diseases are often underrepresented. 2 Hence, RCTs insufficiently cover the heterogeneity of the HF population, including the broad variety of socio‐economic factors, the presence of (multiple) co‐morbidities, and medication adherence among men and women. Consequently, clinical trial databases are generally less suitable for studying any sex‐specific effect of these factors on HF outcomes.
In the Netherlands, over 99% of the population has (basic) health insurance 3 as such insurance is mandatory by law. Thus, health insurance claims (HIC) databases in the Netherlands capture truly representative (random) samples of patient populations. The sample sizes are large, and data on co‐morbidities and medication claims are collected in a systematic way. 4 We used the HIC database of Zilveren Kruis Achmea, one of several health insurance companies, to describe the key characteristics of an unselected population of men and women with chronic heart failure (CHF) and to study the sex‐specific impact of co‐morbidities and medication on prognosis.
Methods
Study design and patient selection
A retrospective, observational study was carried out using anonymous HIC data of Zilveren Kruis Achmea, the largest insurance company in the Netherlands comprising about 5.1 million people (30%) of the Dutch population. 5 The database contained data from January 2012 to April 2018. The period until December 2014 was used for patient selection and determining characteristics of the study sample. Between January 2015 and April 2018, the outcomes of the selected patients were determined.
We identified 25 776 patients aged 18–85 years with a diagnosis treatment code for CHF and still alive by January 2015 (Figure 1 ). These patients had a CHF‐related claim according to the national diagnosis treatment classification system called ‘Diagnose Behandeling Combinatie’ (DBC), which is a combination of the International Classification of Diseases, 10th revision (ICD‐10) 6 and treatment applied. Additionally, they had used at least one prescription drug within the cardiovascular system (‘C’) based on World Health Organization Anatomical Therapeutic Chemical Classification index and Defined Daily Dose (WHO ATC/DDD) in the same period. 7
Figure 1.
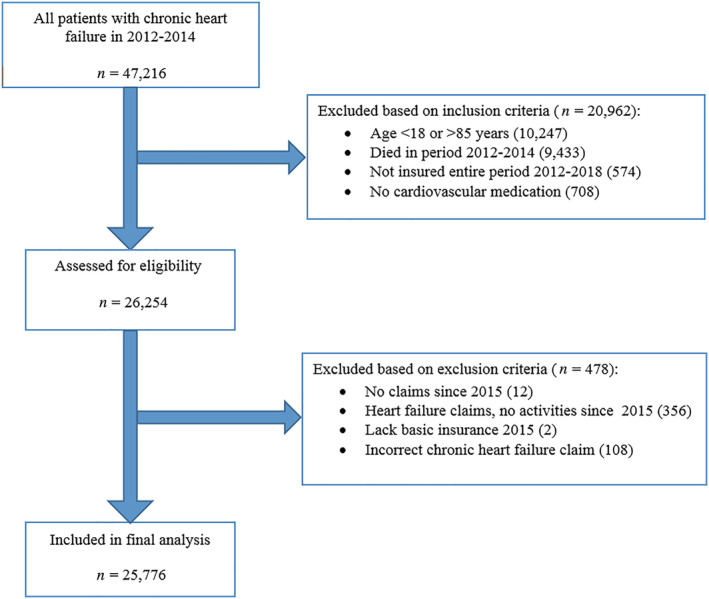
Flowchart of study population selection.
According to the European Society of Cardiology Heart Failure guidelines, 8 CHF patients should visit their treating physician at least once per year. To improve data quality, we therefore excluded patients who lacked any HF insurance claims after January 2015. Patients who switched to another insurance company between 2012 and 2018 were also excluded.
Co‐morbidity selection
Co‐morbidities were identified using a combination of the adapted diagnosis‐related group (DRG) classification and the pharmacy‐based cost group (FKG classification). In the Netherlands, DRG is an ICD‐10‐based system, used to determine health care costs in relation to specific diseases. The FKG is a classification method for medication type/dose in relation to chronic diseases, which is used in the national risk equalization model. It is used to adjust capitation payments by the health care insurer to the health care provider.
Medication use and adherence
Medication use and adherence was determined for the period 2012–2014. Extensive pharmacy data were available. The indication for prescribing a certain drug of selection is not known. Medication adherence was determined using the medication possession ratio (MPR). 9 This ratio was defined as the amount of pills corrected for different dosage schemes, the prescribed daily dose supplied, divided by the time (days) between two supply dates. Patients may switch drugs within the same class. Therefore, to obtain more reliable MPR estimates, medications were grouped into ATC classes. Consecutively, MPR was averaged over the total supply period per ATC group and categorized based on a threshold of 0.80, above which a patient was considered adherent to prescribed medication. 9
Outcomes and follow‐up
The primary endpoint (PE) was a composite of all‐cause mortality and hospitalisation for HF, based on the DBC system. Secondary endpoints were the two components of this composite. Biological sex differences in co‐morbidities and medication adherence in relation to the PE were of particular interest. Mortality was retrieved from the civil registry. HF admissions were not adjudicated by a committee.
For additional information on patient selection, definition of socio‐demographic factors, co‐morbidities, medication adherence, and outcomes, see Supporting Information, Appendix S1 .
Statistical analysis
Continuous variables are presented as median and interquartile range, and sex differences were evaluated using Mann–Whitney tests. Categorical variables are presented as counts and percentages, and sex differences were evaluated using χ 2‐tests.
The associations between sex, co‐morbidities, and medication adherence and the PE were assessed using Cox proportional hazard regression models, with adjustment for other predefined, clinically relevant baseline variables, including age, marital status, socio‐economic status, income level, and time since last CHF‐related outpatient/clinic visit. The proportional hazard assumption was satisfied for each variable. We investigated potential effect modification by sex via an interaction term in the Cox models.
For all tests, a P‐value < 0.05 was considered statistically significant. Data were analysed using R Statistical Software Version 3.4.2 (Vienna, Austria) and the "survival" package.
Results
Baseline characteristics and co‐morbidities
The analysis set included 14 517 (55%) men and 11 259 (45%) women. Women were significantly older (76 vs. 72 years). Baseline characteristics are described in Table 1 . More than a third of the patients (35%) had three or more co‐morbidities. Arrhythmia (41%), ischaemic heart disease (33%), diabetes mellitus type 1 or 2 (DM1/2) (25%), malignancy (25%), and chronic obstructive pulmonary disease (COPD)/asthma (17%) were most common. Men had a higher prevalence of these top five co‐morbidities. Women had a higher prevalence of valvular heart disease, hypertensive disease, thyroid dysfunction, and depression. Men and women had a similar prevalence of renal insufficiency (RI) and cerebrovascular disease.
Table 1.
Baseline characteristics
| Characteristics | All patients N = 25 776 | Men N = 14 517 | Women N = 11 259 | P‐value |
|---|---|---|---|---|
| Age (years), median (IQR) | 74 (66–80) | 72 (65–79) | 76 (67–81) | <0.001 |
| Sex, n (%) | ||||
| Men | 14 517 (56) | |||
| Women | 11 259 (44) | |||
| Marital status, n (%) | <0.001 | |||
| Married | 8697 (34) | 6007 (41) | 2690 (24) | |
| Unknown | 8428 (33) | 4616 (32) | 3812 (34) | |
| Widow/widower | 3802 (15) | 1211 (8) | 2591 (23) | |
| Never married | 3040 (12) | 1823 (13) | 1217 (11) | |
| Divorced | 1809 (7) | 860 (6) | 949 (8) | |
| SES score, median (IQR) | −0.37 (−1.17 to 0.47) | −0.15 (−1.17 to −0.35) | −0.21 (−1.26 to −0.40) | <0.001 |
| Income level, median (IQR) | 5.0 (2.0–7.0) | 5.0 (2.0–7.0) | 5.0 (2.0–7.0) | <0.001 |
| Duration since last visit a , n (%) | <0.001 | |||
| 0–6 months | 2993 (12) | 1617 (11) | 1376 (12) | |
| 6–12 months | 3327 (13) | 1822 (13) | 1505 (13) | |
| 1–2 years | 6975 (27) | 3827 (26) | 3148 (28) | |
| >2 years | 12 481 (48) | 7251 (50) | 5230 (46) | |
| Co‐morbidities b , n (%) | <0.001 | |||
| 0 | 2614 (10) | 1363 (9) | 1251 (11) | |
| 1 | 6552 (25) | 3562 (25) | 2990 (27) | |
| 2 | 7604 (30) | 4419 (30) | 3185 (28) | |
| ≥3 | 9006 (35) | 5173 (35) | 3833 (34) | |
| History of co‐morbidities b , n (%) | ||||
| Arrhythmia | 10 569 (41) | 6409 (44) | 4160 (37) | <0.001 |
| Ischaemic heart disease | 8445 (33) | 5045 (35) | 3400 (30) | <0.001 |
| Diabetes mellitus 1/2 | 6500 (25) | 3789 (26) | 2711 (24) | <0.001 |
| Malignancy | 6328 (25) | 3703 (26) | 2625 (23) | <0.001 |
| COPD/asthma | 4433 (17) | 2609 (18) | 1824 (16) | <0.001 |
| Hypercholesterolaemia | 3753 (15) | 2410 (17) | 1343 (12) | <0.001 |
| Valve disease | 3607 (14) | 1864 (13) | 1743 (15) | <0.001 |
| Renal insufficiency | 3110 (12) | 1757 (12) | 1353 (12) | 0.833 |
| Hypertensive disease | 2128 (8) | 1030 (7) | 1098 (10) | <0.001 |
| Cerebrovascular disease | 1941 (8) | 1100 (8) | 841 (7) | 0.745 |
| Thyroid dysfunction | 1792 (7) | 595 (4) | 1197 (11) | <0.001 |
| Depression | 1420 (6) | 570 (4) | 850 (8) | <0.001 |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SES, socio‐economic status.
Significant P‐values are in bold.
Duration since last chronic heart failure outpatient clinic visit or admission in period 2012–2014.
Only pre‐selected clinically relevant co‐morbidities.
Medication use and adherence
Baseline medication use is shown in Table 2 . The vast majority of patients used angiotensin‐converting enzyme‐inhibitors/angiotensin receptor blockers (ACE‐I/ARBs) (85%), beta‐blockers (83%), or diuretics (83%), in particular loop diuretics (71%) and mineralocorticoid receptor antagonists (MRAs) (40%). Up to a third used calcium blockers (33%), and digoxin was prescribed in 19% of patients. Almost a quarter of patients (23%) used oral nitrates (isosorbide). Cardiovascular medication use, in particular disease‐modifying drugs, was somewhat higher in men, except for calcium blockers, diuretics, and digoxin.
Table 2.
Medication use
| All patients N = 25 776 | Men N = 14 517 | Women N = 11 259 | P‐value | |
|---|---|---|---|---|
| Medication a , n (%) | ||||
| ACE‐inhibitors/ARBs | 21 846 (85) | 12 623 (87) | 9223 (82) | <0.001 |
| Beta‐blockers | 21 397 (83) | 12 267 (85) | 9130 (81) | <0.001 |
| Calcium blockers | 8446 (33) | 4522 (31) | 3924 (35) | <0.001 |
| Diuretics | 21 292 (83) | 11 654 (80) | 9638 (86) | <0.001 |
| Loop diuretics | 18 286 (71) | 10 056 (69) | 8230 (73) | <0.001 |
| Thiazide diuretics | 6685 (26) | 3220 (22) | 3465 (31) | <0.001 |
| Mineralocorticoid receptor antagonists | 10 434 (40) | 5956 (41) | 4478 (40) | 0.042 |
| Digoxin | 4980 (19) | 2723 (19) | 2257 (20) | 0.009 |
| Amiodarone | 2304 (9) | 1524 (10) | 780 (7) | <0.001 |
| Doxazosin | 963 (4) | 513 (4) | 450 (4) | 0.052 |
| Ivabradine | 636 (2) | 389 (3) | 247 (2) | 0.013 |
| Hydralazine | 119 (0) | 83 (1) | 36 (0) | 0.003 |
| Nitrates (isosorbide) | 5946 (23) | 3493 (24) | 2453 (22) | <0.001 |
| Anti‐coagulants | 14 482 (56) | 8493 (59) | 5989 (53) | <0.001 |
| Anti‐platelets | 13 422 (52) | 8094 (56) | 5328 (47) | <0.001 |
| Lipid‐lowering therapy | 17 221 (67) | 10 452 (72) | 6769 (60) | <0.001 |
| Glucose‐lowering therapy | 7828 (30) | 4498 (31) | 3330 (30) | 0.015 |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers.
Significant P‐values are in bold.
Medication use in period 2012–2014.
Baseline medication adherence based on MPR is presented in Table 3 . The adherence was largely similar in men and women in each ATC group. A (borderline) statistically significant difference between both sexes was observed for ACE‐I/ARBs, beta‐blockers, loop diuretics, and MRAs.
Table 3.
Medication adherence
| ATC group | Adherence | All patients a | Men a | Women a | P‐value |
|---|---|---|---|---|---|
| ACE‐I/ARBs, n (%) | n = 21 846 | n = 12 623 | n = 9223 | ||
| Adherent | 18 048 (82.6) | 10 482 (83.0) | 7566 (82.0) | 0.010 | |
| Non‐adherent | 1327 (6.1) | 782 (6.2) | 545 (5.9) | ||
| Unknown | 2471 (11.3) | 1359 (10.8) | 1112 (12.1) | ||
| Beta‐blockers, n (%) | n = 21 397 | n = 12 267 | n = 9130 | ||
| Adherent | 17 737 (82.9) | 10 158 (82.8) | 7579 (83.0) | 0.033 | |
| Non‐adherent | 1214 (5.7) | 736 (6.0) | 478 (5.2) | ||
| Unknown | 2446 (11.4) | 1373 (11.2) | 1073 (11.8) | ||
| Calcium blockers, n (%) | n = 8446 | n = 4522 | n = 4924 | ||
| Adherent | 5782 (68.5) | 3127 (69.2) | 2655 (67.7) | 0.258 | |
| Non‐adherent | 507 (6.0) | 273 (6.0) | 234 (6.0) | ||
| Unknown | 2157 (25.5) | 1122 (24.8) | 1035 (26.4) | ||
| Diuretics, n (%) | n = 21 292 | n = 11 654 | n = 9638 | ||
| Adherent | 15 489 (72.7) | 8519 (73.1) | 6970 (72.3) | 0.403 | |
| Non‐adherent | 1858 (8.7) | 996 (8.5) | 862 (8.9) | ||
| Unknown | 3945 (18.5) | 2139 (18.4) | 1806 (18.7) | ||
| Loop diuretics, n (%) | n = 18 286 | n = 10 056 | n = 8230 | ||
| Adherent | 11 966 (65.4) | 6653 (66.2) | 5313 (64.6) | 0.039 | |
| Non‐adherent | 2200 (12.0) | 1207 (12.0) | 993 (12.1) | ||
| Unknown | 4120 (22.5) | 2196 (21.8) | 1924 (23.4) | ||
| Thiazide diuretics, n (%) | n = 6685 | n = 3220 | n = 3465 | ||
| Adherent | 3823 (57.2) | 1837 (57.0) | 1986 (57.3) | 0.751 | |
| Non‐adherent | 403 (6.0) | 188 (5.8) | 215 (6.2) | ||
| Unknown | 2459 (36.8) | 1195 (37.1) | 1264 (36.5) | ||
| MRA, n (%) | n = 10 434 | n = 5956 | n = 4478 | ||
| Adherent | 7357 (70.5) | 4242 (71.2) | 3115 (69.6) | 0.051 | |
| Non‐adherent | 903 (8.7) | 483 (8.1) | 420 (9.4) | ||
| Unknown | 2174 (20.8) | 1231 (20.7) | 943 (21.1) | ||
| Amiodarone, n (%) | n = 2304 | n = 1524 | n = 780 | ||
| Adherent | 1427 (61.9) | 963 (63.2) | 464 (59.5) | 0.197 | |
| Non‐adherent | 159 (6.9) | 99 (6.5) | 60 (7.7) | ||
| Unknown | 718 (31.2) | 462 (30.3) | 256 (32.8) | ||
| Digoxin, n (%) | n = 4980 | n = 2723 | n = 2257 | ||
| Adherent | 3602 (72.3) | 1957 (71.9) | 1645 (72.9) | 0.636 | |
| Non‐adherent | 199 (4.0) | 114 (4.2) | 85 (3.8) | ||
| Unknown | 1179 (23.7) | 652 (23.9) | 527 (23.3) | ||
| Nitrates (isosorbide), n (%) | Refill rate | n = 5946 | n = 3493 | n = 2453 | |
| Adherent | 2977 (50.1) | 1712 (49.0) | 1265 (51.6) | 0.139 | |
| Non‐adherent | 252 (4.2) | 148 (4.2) | 104 (4.2) | ||
| Unknown | 2717 (45.7) | 1633 (46.8) | 1084 (45.7) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARBs, angiotensin receptor blockers; ATC, anatomical therapeutic chemical classification; MRA, mineralocorticoid receptor antagonist.
Significant P‐values are in bold.
Only patients who used medication within the specific ATC group and in period between 2012 and 2014.
Outcomes
Median follow‐up time was 3.3 years (interquartile range 2.2–3.3). The PE of all‐cause mortality or HF hospitalisation was reached in 8669 patients (34%) (Table 4 ). A total of 7152 patients (28%) died, and 3179 (12%) were hospitalized. Men had a higher incidence of the PE than women (35.1% vs. 31.8%). Adjusted hazard ratio (aHR) for the composite endpoint was 1.25 (95% confidence interval: 1.19–1.30, P < 0.001) for men. Furthermore, men had a higher overall mortality (29% vs. 26%, P < 0.001). Although men had higher mortality rates overall, no significant difference in mortality was found between men and women who had been admitted (54% vs. 51%, P = 0.101). Men were hospitalized more often for HF (60% of total admission count) compared with women (P < 0.001). Sixty‐six per cent had only one HF‐related admission during follow‐up. Average length of stay was 8.1 days for both men and women.
Table 4.
Heart failure outcomes
| HF outcomes | All patients N = 25 776 | Men N = 14 517 | Women N = 11 259 | P‐value |
|---|---|---|---|---|
| Mortality or HF hospitalisation a , n (%) | 8669 (33.6) | 5094 (35.1) | 3575 (31.8) | <0.001 |
| All‐cause mortality, n (%) | 7152 (27.7) | 4222 (29.1) | 2930 (26.0) | <0.001 |
| HF hospitalisation, n (%) | 3179 (12.3) | 1875 (12.9) | 1304 (11.6) | 0.001 |
| HF hospitalized n = 3179 | Men n = 1875 | Women n = 1304 | ||
| All‐cause mortality, n (%) | 1662 (52.3) | 1003 (53.5) | 659 (50.5) | 0.101 |
| HF hospital admissions b , n (%) | 5291 | 3166 | 2125 | <0.001 |
| 1 HF‐related admission | 2083 (65.5) | 1217 (64.9) | 866 (66.4) | |
| 2 HF‐related admissions | 624 (19.6) | 373 (19.9) | 251 (19.3) | |
| ≥3 HF‐related admissions | 472 (14.8) | 285 (15.2) | 187 (14.3) |
HF, heart failure.
Significant P‐values are in bold.
Composite endpoint.
Cumulative frequency of admissions.
Determinants of the primary endpoint
The patient's age and a broad range of co‐morbidities seemed to be predictive of the PE (Table 5 ). RI (aHR 1.49), COPD/asthma (aHR 1.46), DM1/2 (aHR 1.33), cerebrovascular disease (aHR 1.29), and malignancy (aHR 1.25) were the most significant determinants of increased risk. Interestingly, hypercholesterolaemia, hypertensive disease, and ischaemic heart disease were associated with a reduced risk. Non‐adherence to disease‐modifying drugs was significantly associated with increased risk, in particular ACE‐I/ARB (aHR 1.17) and MRA (aHR 1.20), but not beta‐blockers.
Table 5.
Determinants of all‐cause mortality or heart failure admission
| Sex‐specific estimates | |||||
|---|---|---|---|---|---|
| Characteristics | All patients | Men | Women | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | Interaction P‐value | ||
| Age, years | 1.04 (1.04–1.05) ‡ | 1.04 (1.04–1.05) ‡ | 1.04 (1.04–1.05) ‡ | 0.473 | |
| Sex, men | 1.25 (1.19–1.30) ‡ | — | — | — | |
| Marital status | |||||
| Married | Reference | Reference | Reference | ||
| Unknown | 0.83 (0.79–0.88) ‡ | 0.80 (0.75–0.86) ‡ | 0.85 (0.78–0.94) ‡ | 0.315 | |
| Widow/widower | 0.95 (0.89–1.01) | 0.87 (0.79–0.96) † | 0.96 (0.87–1.06) | 0.153 | |
| Never married | 1.20 (1.12–1.30) ‡ | 1.41 (1.29–1.54) ‡ | 0.90 (0.79–1.03) | <0.001 | |
| Divorced | 1.22 (1.12–1.33) ‡ | 1.44 (1.28–1.61) ‡ | 1.02 (0.89–1.16) | <0.001 | |
| Duration since last visit a | 0.95 (0.92–0.97) ‡ | 0.96 (0.93–0.99)* | 0.93 (0.89–0.96) ‡ | 0.135 | |
| Socio‐economic status | 0.99 (0.97–1.01) | 1.01 (0.99–1.04) | 0.96 (0.94–0.99) † | 0.005 | |
| Income level (0–10) | 0.97 (0.97–0.98) ‡ | 0.98 (0.97–0.99) ‡ | 0.97 (0.95–0.98) ‡ | 0.091 | |
| Co‐morbidities | |||||
| Arrhythmia | 0.96 (0.92–1.00) | 0.95 (0.89–1.00) ‡ | 0.98 (0.92–1.05) ‡ | 0.400 | |
| Cerebrovascular disease | 1.29 (1.20–1.39) ‡ | 1.27 (1.16–1.39) ‡ | 1.33 (1.19–1.48) ‡ | 0.564 | |
| COPD/asthma | 1.46 (1.39–1.54) ‡ | 1.39 (1.31–1.48) ‡ | 1.58 (1.47–1.71) | 0.011 | |
| Depression | 1.09 (0.99–1.19) | 1.05 (0.92–1.21) | 1.11 (0.98–1.25) ‡ | 0.587 | |
| Diabetes mellitus 1/2 | 1.33 (1.27–1.40) ‡ | 1.26 (1.19–1.34) ‡ | 1.44 (1.34–1.55) ‡ | 0.004 | |
| Hypercholesterolaemia | 0.78 (0.72–0.85) ‡ | 0.78 (0.71–0.87) ‡ | 0.77 (0.67–0.88) ‡ | 0.834 | |
| Hypertensive disease | 0.85 (0.78–0.92) ‡ | 0.83 (0.74–0.94) † | 0.86 (0.77–0.97)* | 0.695 | |
| Ischaemic heart disease | 0.90 (0.86–0.95) ‡ | 0.87 (0.81–0.92) ‡ | 0.96 (0.90–1.04) | 0.022 | |
| Malignancy | 1.25 (1.19–1.31) ‡ | 1.22 (1.15–1.30) ‡ | 1.29 (1.19–1.38) ‡ | 0.277 | |
| Renal insufficiency | 1.49 (1.41–1.58) ‡ | 1.41 (1.31–1.52) ‡ | 1.61 (1.48–1.75) ‡ | 0.018 | |
| Thyroid dysfunction | 1.01 (0.93–1.09) | 0.91 (0.79–1.03) | 1.07 (0.97–1.19) | 0.046 | |
| Valve disease | 1.05 (0.99–1.11) | 0.98 (0.90–1.06) | 1.14 (1.05–1.25) † | 0.008 | |
| Medication use and adherence | |||||
| ACE‐inhibitor/ARB | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.17 (1.06–1.29) † | 1.20 (1.06–1.35) † | 1.13 (0.97–1.31) | 0.537 | |
| Unknown | 1.18 (1.10–1.27) ‡ | 1.21 (1.10–1.32) ‡ | 1.14 (1.03–1.27)* | 0.426 | |
| Never used | 1.11 (1.05–1.19) ‡ | 1.16 (1.07–1.26) ‡ | 1.06 (0.97–1.16) | 0.144 | |
| Beta‐blockers | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.09 (0.98–1.20) | 1.17 (1.03–1.33)* | 0.97 (0.82–1.14) | 0.064 | |
| Unknown | 0.99 (0.92–1.07) | 1.02 (0.93–1.12) | 0.94 (0.84–1.06) | 0.282 | |
| Never used | 1.05 (0.99–1.12) | 1.07 (0.99–1.16) | 1.03 (0.94–1.13) | 0.531 | |
| Calcium blockers | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 0.98 (0.84–1.14) | 1.02 (0.83–1.26) | 0.92 (0.73–1.16) | 0.502 | |
| Unknown | 1.04 (0.96–1.13) | 1.11 (0.99–1.24) | 0.96 (0.85–1.08) | 0.076 | |
| Never used | 0.99 (0.94–1.04) | 1.06 (0.99–1.14) | 0.90 (0.83–0.97) ‡ | 0.001 | |
| Loop diuretics | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.00 (0.93–1.08) | 1.04 (0.94–1.14) | 0.95 (0.85–1.06) | 0.232 | |
| Unknown | 1.03 (0.97–1.09) | 0.99 (0.91–1.07) | 1.08 (0.99–1.18) | 0.132 | |
| Never used | 0.57 (0.53–0.61) ‡ | 0.57 (0.53–0.62) ‡ | 0.55 (0.50–0.62) ‡ | 0.607 | |
| Thiazide diuretics | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.07 (0.88–1.29) | 1.21 (0.93–1.58) | 0.94 (0.71–1.23) | 0.180 | |
| Unknown | 1.04 (0.95–1.13) | 1.01 (0.90–1.15) | 1.07 (0.94–1.21) | 0.567 | |
| Never used | 1.05 (0.98–1.12) | 1.08 (0.99–1.19) | 1.01 (0.92–1.11) | 0.319 | |
| MRAs | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.20 (1.09–1.33) ‡ | 1.16 (1.02–1.33)* | 1.26 (1.08–1.46) † | 0.455 | |
| Unknown | 1.00 (0.93–1.08) | 1.03 (0.93–1.13) | 0.97 (0.86–1.09) | 0.432 | |
| Never used | 0.77 (0.73–0.81) ‡ | 0.77 (0.72–0.82) ‡ | 0.76 (0.71–0.82) ‡ | 0.793 | |
| Amiodarone | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.06 (0.83–1.35) | 0.99 (0.73–1.35) | 1.18 (0.81–1.71) | 0.479 | |
| Unknown | 0.78 (0.68–0.90) ‡ | 0.80 (0.67–0.95)* | 0.75 (0.59–0.96)* | 0.663 | |
| Never used | 0.75 (0.69–0.81) ‡ | 0.76 (0.69–0.84) ‡ | 0.72 (0.62–0.82) ‡ | 0.495 | |
| Digoxin | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.00 (0.81–1.24) | 0.96 (0.71–1.28) | 1.06 (0.76–1.48) | 0.640 | |
| Unknown | 0.91 (0.82–1.01) | 0.87 (0.75–0.998)* | 0.97 (0.83–1.13) | 0.290 | |
| Never used | 0.89 (0.84–0.94) ‡ | 0.89 (0.83–0.96) † | 0.89 (0.81–0.96) † | 0.843 | |
| Nitrates (isosorbide) | |||||
| Adherent | Reference | Reference | Reference | ||
| Non‐adherent | 1.08 (0.89–1.32) | 0.98 (0.76–1.27) | 1.25 (0.94–1.68) | 0.215 | |
| Unknown | 0.88 (0.81–0.95) † | 0.85 (0.76–0.94) † | 0.93 (0.81–1.06) | 0.283 | |
| Never used | 0.80 (0.75–0.85) ‡ | 0.81 (0.75–0.88) ‡ | 0.79 (0.72–0.87) ‡ | 0.619 | |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; MRAs, mineralocorticoid receptor antagonists.
Significant interaction P‐values are in bold.
Duration in years since last chronic heart failure outpatient clinic visit or admission in period between 2012 and 2014.
Significant at P ≤ 0.05.
Significant at P ≤ 0.01.
Significant at P ≤ 0.001.
The relationship between the co‐morbidities and the PE was sex dependent. The sum of the regression coefficients of the co‐morbidities in the multivariable model in women (1.67) was larger than in men (0.65). In particular, RI, COPD/asthma, and DM1/2 had a stronger relationship with the PE in women. We found no significant difference between men and women in the prognostic value of medication (non‐)adherence.
Discussion
Main findings
In this analysis, based on a HIC database of >25 000 patients with CHF, we have shown that overall men demonstrated a worse prognosis compared with women. A broad range of co‐morbidities was significantly associated with increased risk of all‐cause mortality or HF admission. These associations were somewhat stronger in women than in men, in particular for RI, COPD/asthma, and diabetes, despite a higher prevalence in men. Men used more disease‐modifying drugs; however, adherence was similar between sexes. Non‐adherence to disease‐modifying drugs was related with a higher incidence of the PE, which again was similar between sexes.
Several large, national population‐based studies or registries on HF in Europe with >10 000 (10 190–88 195) patients have been published. 10 , 11 , 12 , 13 , 14 To our knowledge, in the Netherlands, only two clinical registries on CHF exist, both having assessed European Society of Cardiology guideline adherence to HF medication. 12 , 15 The average age of patients across these large population‐based databases was 72–78 years, which is similar to our study (74 years). The percentage of women was 40–55% compared with 44% in our study, which is considerably higher than RCTs on HF with a median of about 29%. 16 Considering the gender gap of ~25% between RCTs and the population at large reported for the US population, 16 one would expect close to 50% female representation in a registry or insurance dataset. In the CHAMP‐HF registry, the average age (66 vs. 74 years) and percentage of women (29% vs. 44%) were lower compared with our study. This difference can be explained by the inclusion of solely HF with reduced ejection fraction (HFrEF) outpatients in CHAMP‐HF. An analysis of the Get With The Guidelines‐Heart Failure (GWTG‐HF) registry 17 of 79 291 patients reported an average of 74–75 years and ~50% were women, which is relatively comparable with our study.
Co‐morbidities
In this discussion, we will focus solely on the most relevant co‐morbidities with the highest impact on outcomes in overall and gender survival analysis. Studies on co‐morbidities have shown that most patients with CHF have >2 or even >5 co‐morbidities. 18 Most patients in this dataset had ≥3 co‐morbidities, which is in line with the literature.
Across the previously described population‐based studies, prevalence of RI was 15–58%, COPD/asthma 15–32%, DM 25–43%, cerebrovascular disease 13–18%, and malignancy 5–21%. 10 , 11 , 13 , 14 , 17 , 19 In our study, this was 12%, 17%, 25%, 8%, and 25%, respectively. The higher rate for RI, COPD/asthma, and cerebrovascular disease in other studies is most likely inherent to the inclusion of mainly patients from outpatient clinics or after hospital discharge and a difference in classification method introducing some selection. In a cross‐sectional study of 122 630 CHF patients >65 of age, non‐cardiac co‐morbidities that had the highest risk for hospitalisations and overall mortality were COPD/bronchiectasis, renal failure, diabetes, depression, and other lower respiratory diseases. 20 We showed that COPD, renal failure, and diabetes indeed have a high impact on these outcomes; however, depression was not significantly associated in this study. Chronic kidney disease (CKD) is associated with worse prognosis in patients with CHF. 21 Analysis of the SwedeHF registry also showed a strong association of CKD with increased HF hospitalisation and all‐cause mortality. 14 Interestingly, despite an extensive coverage of all cerebrovascular diseases using DRG codes and in sharp contrast to the registries where usually only stroke is examined, in this analysis, the prevalence was lower due to less selection. Malignancy (covering all neoplasms), however, was higher in our study as expected. Malignancy and cardiovascular disease have shared risk factors and therefore frequently coincide. 22 Recently, Meijers et al. also demonstrated that HF can be considered a risk factor for incident cancer. 23
In contrast to the overall mortality, no significant difference in mortality was observed between men and women who had been admitted for HF during follow‐up. We believe this discrepancy is due to sub‐selection of patients. Older women with HF with preserved ejection fraction (HFpEF) might be overrepresented closing the gap between the two groups. Sex differences exist in characteristics, aetiology, co‐morbidities, and prognosis. These differences might be attributed to the HF aetiology, left ventricular ejection fraction (HFrEF vs. HFpEF), and New York Health Association classifications, which were not available in this dataset. Women have a higher incidence of HF at older age, have more HFpEF, and suffer more from obesity, diabetes, and hypertension, while man have a higher incidence at a younger age with more HFrEF due to ischaemic aetiology. 24 Furthermore, women generally live longer than men. In our study, women with RI, COPD/asthma, and diabetes had a worse prognosis, even though prevalence of RI was not significantly different, and COPD/asthma and diabetes were more common among men. In contrast, analysis of the SwedeHF registry showed that women were more likely to have CKD, with no sex difference on outcomes after adjustment. 14 COPD is more common in men than women and is in line with the work of Lawson et al., who showed a 15% higher risk of mortality in women than men. 25 Possible explanations are higher female age, pathophysiology, or delay in or poor response to treatment. Diabetes was more common among men in the SwedeHF registry, similar to ours. 14 However, the study of Marra et al. demonstrated that women had more diabetes than men. 24 Similar to our study, Johansson et al. showed that diabetes was a stronger predictor for mortality in women than men. 26 This might be due to less evidence‐based management in women leading to a poor control of glucose levels.
Medication use and adherence
Across the large population‐based studies, ACE‐I/ARBs use was 51–91%, beta‐blockers 52–90%, MRAs 12–56%, and loop diuretics 53–81%. 12 , 13 , 14 , 15 , 17 , 19 In our study, this was 85%, 83%, 40%, and 83%, respectively. Notably, there is a considerably large variation across studies, which might be related to differences in definition. Use of most of the aforementioned medications was particularly lower in the US registries, most likely due to lower guideline adherence. 17 , 19 Prescription rates in our study were comparable with the contemporary Dutch CHECK‐HF registry, a more clearly defined HF population, although we found lower rates for MRAs (40% vs. 56%) and loop diuretics (71% vs. 81%), which can be attributed to focus on HFrEF patients who are possibly more symptomatic (26%, New York Health Association III). 15
As expected, ACE‐I/ARBs and MRAs had a significant association with the outcome in this analysis. However, beta‐blockers were not significantly associated, which is contrary to what would have been expected based on prior knowledge. 8 We do not have a clear explanation for this phenomenon and hypothesize that it could be related to categorization of MPR, which leads to a loss of data and power. Moreover, women had a lower prescription rate of disease‐modifying drugs. However, adherence to medication and impact of adherence on outcome was not different between sexes. This could be in line with the work of Santema et al. who showed that women with HFrEF need lower doses (50% of recommended dose) of ACE‐I/ARB and beta‐blockers than men. 27 This emphasizes the need for a sufficiently powered prospective cohort study for sex‐stratified analysis on use, dosage, and adherence of common HF medication on outcomes, also distinguishing for left ventricular ejection fraction.
Strengths and limitations
Our study has several strengths. First, by using big data, a more representative, epidemiological overview compared with RCTs was given due to lower selection bias and a higher percentage of women. The results are more generalizable and apply to the CHF population at large in the Netherlands and possibly the rest of the EU. Second, extensive pharmacy data were available, and utilizing MPR with a cut‐off of 0.809 and prescribed daily dose, because daily defined dose is a poor estimator, 28 we reliably estimated medication adherence. This is the first step towards mapping patient compliance to medication. Last but not least, we compared our findings to multiple, large national registries, like the CHECK‐HF, to assess the validity of our findings. However, several limitations of this study should also be acknowledged using the checklist for retrospective database studies as a reference. 29
First of all, limitations specific to study design are that data wrangling is more difficult due to complex data with an incomplete view. The lack of detailed medical data, such as HF aetiology, left ventricular ejection fraction, New York Health Association class, smoking status, body mass index, and laboratory values, complicates inferences on the results. 29 Second, despite extensive adjustment for confounders in multivariable analysis, other (clinically) relevant confounders are lacking and residual confounding may be an issue. Regarding co‐morbidity selection, using the DRG/FKG method, a reasonable overview can be given. However, in most cases, the number of patients is susceptible to variation in criteria for diagnosis, and FKG data are prone to bias ‘healthier patients’. In our study, hypertensive disease, hypercholesterolaemia, and ischaemic heart disease showed to be protective of the outcome due to this reason. Therefore, caution is advised when interpreting these results. Third, due to the nature of data collection on insurance claims and the inherent delay that comes with it, not all data on hospitalisations might be present, leading to an incorrect representation. Furthermore, in HIC databases, coding can be subject to incorrect labelling (dyspnoea could be listed under CHF or COPD code), which can affect the validity of the results. This is however estimated at a maximum of 5%. Changes over time in codes can also lead to unreliable data, 29 but quality check did not reveal any relevant changes that could affect the study findings.
Conclusions
In a large, representative sample of the Dutch population, as captured in a HIC database, men with CHF had a 25% higher incidence of death or HF admission than women. The influence of co‐morbidities on the studied outcome was higher in women than in men, in particular for RI, COPD/asthma, and diabetes. No difference in sex for medication adherence and adherence in relation to the outcome was observed. These results underscore the merit of HIC databases as an addition to RCT data and demonstrate that additional research into sex differences in HF is warranted. To this end, the use and value of HIC databases should be further evaluated. Furthermore, treatment of HF should move towards a more patient‐tailored approach by sub‐classifying patients based on co‐morbidities and setting specific goals for these conditions that could potentially complicate HF treatment. More research is needed to determine these goals in (older) HF patients with multi‐morbidity and polypharmacy.
Disclaimer
The views presented here are those of the authors. The European Commission is not responsible for any use that may be made of the information it contains.
Conflict of interest
J.J.B. reports grants from Abbott, outside of the submitted work. All other authors have nothing to disclose.
Funding
This project was supported by the European Union's Horizon 2020 research and innovation programme (780495).
Supporting information
Data S1. Supplementary Appendix.
Acknowledgements
The authors would like to thank Achmea for providing access to the Achmea Health Database and the support from the whole Kenniscentrum team. All authors have read and approved the final version of the manuscript.
Gürgöze, M. T. , van der Galiën, O. P. , Limpens, M. A. M. , Roest, S. , Hoekstra, R. C. , IJpma, A. S. , Brugts, J. J. , Manintveld, O. C. , and Boersma, E. (2021) Impact of sex differences in co‐morbidities and medication adherence on outcome in 25 776 heart failure patients. ESC Heart Failure, 8: 63–73. 10.1002/ehf2.13113.
References
- 1. Niederseer D, Thaler CW, Niederseer M, Niebauer J. Mismatch between heart failure patients in clinical trials and the real world. Int J Cardiol 2013; 168: 1859–1865. [DOI] [PubMed] [Google Scholar]
- 2. Eisenberg E, Di Palo KE, Pina IL. Sex differences in heart failure. Clin Cardiol 2018; 41: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nederlandse Zorgautoriteit . Kerncijfers zorgverzekeraars. https://www.nza.nl/zorgsectoren/zorgverzekeraars/kerncijfers‐zorgverzekeraars (6 December 2019).
- 4. Du X, Khamitova A, Kyhlstedt M, Sun S, Sengoelge M. Utilisation of real‐world data from heart failure registries in OECD countries—a systematic review. Int J Cardiol Heart Vasc 2018; 19: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vektis Intelligence . Hoe zijn de marktaandelen van de zorgverzekeraars verdeeld? https://www.zorgprismapubliek.nl/producten/zorgverzekeringen/zorgverzekeringsmarkt/row‐2/os‐4/ (7 November 2019).
- 6. Prevention CfDCa . International Classification of Diseases, tenth revision. Clinical Modification (ICD‐10‐CM). 2016. [PubMed]
- 7. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for Anatomical Therapeutic Chemical (ATC) Classification Index and Defined Daily Doses (DDDs) assignment 2019. Oslo, Norway 2018.
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 9. Krueger K, Griese‐Mammen N, Schubert I, Kieble M, Botermann L, Laufs U, Kloft C, Schulz M. In search of a standard when analyzing medication adherence in patients with heart failure using claims data: a systematic review. Heart Fail Rev 2018; 23: 63–71. [DOI] [PubMed] [Google Scholar]
- 10. Brugts JJ, Linssen GCM, Hoes AW, Brunner‐La Rocca HP, Investigators C‐H . Real‐world heart failure management in 10,910 patients with chronic heart failure in the Netherlands: design and rationale of the Chronic Heart failure ESC guideline‐based Cardiology practice Quality project (CHECK‐HF) registry. Neth Heart J 2018; 26: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farre N, Vela E, Cleries M, Bustins M, Cainzos‐Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdu‐Rotellar JM, Comin‐Colet J. Real world heart failure epidemiology and outcome: a population‐based analysis of 88,195 patients. PLoS One 2017; 12: e0172745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kruik‐Kolloffel WJ, Linssen GCM, Kruik HJ, Movig KLL, Heintjes EM, van der Palen J. Effects of European Society of Cardiology guidelines on medication profiles after hospitalization for heart failure in 22,476 Dutch patients: from 2001 until 2015. Heart Fail Rev 2019; 24: 499–510. [DOI] [PubMed] [Google Scholar]
- 13. Maggioni AP, Orso F, Calabria S, Rossi E, Cinconze E, Baldasseroni S, Martini N, Observatory A. The real‐world evidence of heart failure: findings from 41 413 patients of the ARNO database. Eur J Heart Fail 2016; 18: 402–410. [DOI] [PubMed] [Google Scholar]
- 14. Stolfo D, Uijl A, Vedin O, Stromberg A, Faxen UL, Rosano GMC, Sinagra G, Dahlstrom U, Savarese G. Sex‐based differences in heart failure across the ejection fraction spectrum: phenotyping, and prognostic and therapeutic implications. JACC Heart Fail 2019; 7: 505–515. [DOI] [PubMed] [Google Scholar]
- 15. Brunner‐La Rocca HP, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma HJ, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, Brugts JJ, Investigators C‐H. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK‐HF registry. JACC Heart Fail. 2019; 7: 13–21. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen QD, Peters E, Wassef A, Desmarais P, Remillard‐Labrosse D, Tremblay‐Gravel M. Evolution of age and female representation in the most‐cited randomized controlled trials of cardiology of the last 20 years. Circ Cardiovasc Qual Outcomes 2018; 11: e004713. [DOI] [PubMed] [Google Scholar]
- 17. Greene SJ, DeVore AD, Sheng S, Fonarow GC, Butler J, Califf RM, Hernandez AF, Matsouaka RA, Samman Tahhan A, Thomas KL, Vaduganathan M, Yancy CW, Peterson ED, O'Connor CM, Mentz RJ. Representativeness of a heart failure trial by race and sex: results from ASCEND‐HF and GWTG‐HF. JACC Heart Fail 2019; 7: 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011; 124: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 20. Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003; 42: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 21. Hopper I, Kotecha D, Chin KL, Mentz RJ, von Lueder TG. Comorbidities in heart failure: are there gender differences? Curr Heart Fail Rep 2016; 13: 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016; 133: 1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon AR, van der Vegt B, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Sillje HHW, de Boer RA. Heart failure stimulates tumor growth by circulating factors. Circulation 2018; 138: 678–691. [DOI] [PubMed] [Google Scholar]
- 24. Marra AM, Salzano A, Arcopinto M, Piccioli L, Raparelli V. The impact of gender in cardiovascular medicine: lessons from the gender/sex‐issue in heart failure. Monaldi Arch Chest Dis 2018; 88: 988. [DOI] [PubMed] [Google Scholar]
- 25. Lawson CA, Mamas MA, Jones PW, Teece L, McCann G, Khunti K, Kadam UT. Association of medication intensity and stages of airflow limitation with the risk of hospitalization or death in patients with heart failure and chronic obstructive pulmonary disease. JAMA Netw Open 2018; 1: e185489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansson I, Dahlstrom U, Edner M, Nasman P, Ryden L, Norhammar A. Risk factors, treatment and prognosis in men and women with heart failure with and without diabetes. Heart 2015; 101: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 27. Santema BT, Ouwerkerk W, Tromp J, Sama IE, Ravera A, Regitz‐Zagrosek V, Hillege H, Samani NJ, Zannad F, Dickstein K, Lang CC, Cleland JG, Ter Maaten JM, Metra M, Anker SD, van der Harst P, Ng LL, van der Meer P, van Veldhuisen DJ, Meyer S, Lam CSP, Investigators A‐H , Voors AA. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 2019; 394: 1254–1263. [DOI] [PubMed] [Google Scholar]
- 28. Grimmsmann T, Himmel W. Discrepancies between prescribed and defined daily doses: a matter of patients or drug classes? Eur J Clin Pharmacol 2011; 67: 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, Martin BC, Stang P. A checklist for retrospective database studies—report of the ISPOR Task Force on Retrospective Databases. Value Health 2003. ‐Apr; 6: 90–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary Appendix.


