Abstract
目的
研究Fractalkine(CX3CL1,FKN)对脂多糖(LPS)诱导的RAW264.7巨噬细胞免疫应答的作用机制。
方法
体外培养RAW264.7细胞,使用慢病毒技术构建过表达FKN的稳定细胞珠。细胞分为8组:(1)空白对照组;(2)LPS组,细胞给予脂多糖(1 μg/mL,12 h);(3)ICG-001组,细胞给与Wnt/β-catenin信号通路抑制剂ICG-001(10 μmol/mL,48 h);(4)过表达FKN组;(5)ICG-001+LPS组,细胞给与ICG-001(10 μmol/mL预处理36 h)后,加入脂多糖(1 μg/mL,12 h);(6)过表达FKN+LPS组,过表达FKN细胞给与脂多糖(1 μg/mL,12 h);(7)过表达FKN+ICG-001组,过表达FKN细胞给予ICG-001(10 μmol/mL,48 h);(8)过表达FKN+ICG-001+LPS组,过表达FKN细胞给与ICG-001(10 μmol/mL预处理36 h)后,加入脂多糖(1 μg/mL,12 h);应用CCK-8细胞增殖实验检测ICG-001作用于巨噬细胞中的安全浓度;应用酶联免疫吸附(ELISA)实验检测巨噬细胞上清液中M1型极化因子TNF-α和IL-6的含量;应用蛋白质免疫印记(WB)实验检测巨噬细胞中FKN、Wnt/β-catenin通路关键因子Wnt-4和β-catenin、M1型极化因子iNOS、TNF-α和IL-6的蛋白表达水平;应用免疫荧光(IF)实验检测巨噬细胞中M1型极化因子IL-6蛋白的定位。
结果
过表达FKN的RAW264.7细胞中FKN的蛋白水平较空白对照组明显升高(P < 0.01)。CCK-8实验显示ICG- 001作用于RAW264.7细胞48 h的IC50为10 μmol/mL。与LPS组相比,ICG-001+LPS组上清液中TNF-α和IL-6的分泌量以及细胞内TNF-α、IL-6和iNOS蛋白含量均升高(P < 0.05),而细胞内FKN、Wnt-4和β-catenin蛋白含量均显著降低(P < 0.01);EXFKN+LPS组上清液中TNF-α和IL-6的分泌量以及细胞内TNF-α、IL-6和iNOS蛋白含量均显著降低(P < 0.01),而细胞内FKN、Wnt-4和β-catenin蛋白含量均显著升高(P < 0.01)。与LPS组相比,ICG-001+LPS组中IL-6在细胞质中的定位增强,而EXFKN+LPS组中IL-6在细胞质中的定位受到抑制。
结论
过表达FKN通过激活Wnt/β-catenin信号通路抑制脂多糖诱导的巨噬细胞M1型极化。
Keywords: 巨噬细胞, 极化, Fractalkine, Wnt/β-catenin信号通路
Abstract
Objective
To explore the mechanism by which fractalkine (CX3CL1; FKN) inhibits lipopolysaccharide (LPS)-induced immunological response in RAW264.7 cells.
Methods
A RAW264.7 cell model overexpressing FKN was established by transfection with the lentiviral vector CX3CL1. The effects of LPS, ICG-001 (a Wnt/β-catenin signaling pathway inhibitor), either alone or in combination, on M1 polarization of na?ve and FKN-overexpressing RAW264.7 cells were evaluated by detecting of intereukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) using ELISA. The protein expressions of the inflammatory factors (iNOS, TNF-α, and IL-6), FKN, Wnt-4, and β-catenin were detected by Western blotting. The subcellular localization of IL-6 in the cells was detected by immunofluorescence assay.
Results
The RAW264.7 cell model of FKN overexpression was successfully established. In na?ve RAW264.7 cells, treatment with both ICG-001 and LPS, as compared with LPS alone, significant promoted TNF-α and IL-6 secretions, increased intracellular levels of TNF-α, IL-6 and iNOS (P < 0.05), and reduced intracellular FKN, Wnt-4 and β-catenin levels (P < 0.01). In FKN-overexpressing RAW264.7 cells, LPS treatment significantly reduced the secretion of TNF-α and IL-6 and intracellular levels of TNF-α, IL-6 and iNOS (P < 0.01), increased intracellular FKN, Wnt-4 and β-catenin protein contents (P < 0.01), and inhibited IL-6 localization in the cytoplasm; compared with LPS, the combined treatment with ICG-001 and LPS obviously enhanced IL-6 localization in the cytoplasm of the cells.
Conclusion
FKN overexpression suppresses LPS-induced M1 type polarization of RAW264.7 cells by activating Wnt/β-catenin signaling pathway.
Keywords: macrophages, polarization, fractalkine, Wnt/ β-catenin signaling pathway
巨噬细胞M1型极化在持续性组织损伤期间的炎症反应中起到重要作用[1-2]。研究发现M1型巨噬细胞主要表现为吞噬病原体并分泌炎性因子TNF-α,IL-6,IL-1β,IL-23,IL-12和iNOS[3-4],是包括类风湿关节炎、炎症性肠病、败血症等炎性/免疫疾病进展的重要介质[5-7],而抑制M1型巨噬细胞的促炎效应则会恢复组织稳态,促进炎症转归[8-9]。因此深入探索抑制巨噬细胞M1型极化所涉及的分子信号机制可能为临床治疗炎性/免疫疾病提供新的治疗靶点。
经典的Wnt/β-catenin信号通路对细胞的生长、发育、迁移、周期进程和遗传稳定性至关重要[10-12]。最近研究报道激活Wnt/β-catenin信号通路会导致巨噬细胞M2型极化,促进巨噬细胞增殖浸润并加剧了纤维化疾病的进程和癌细胞的迁移[13-14]。而Wnt/β-catenin信号通路与M1巨噬细胞极化进程之间的关联尚不明朗。
趋化因子Fractalkine(CX3CL1, FKN)通过结合其特异性受体CX3CR1在机体发生炎症反应时作为黏附分子和化学引物粘附免疫细胞参与炎症反应进程[15-16]。最近研究发现抑制FKN通过调控巨噬细胞M1/M2型分化在包括动脉粥样硬化、类风湿关节炎、败血症、肌肉损伤等炎性/免疫疾病的病理进程中具有重要作用[17-20]。而激活FKN在巨噬细胞M1型极化进程中涉及的具体分子机制鲜有报道。课题组前期研究发现FKN关联Wnt/β-catenin信号通路参与了足细胞损伤的进展[21],因此本文深入探索激活FKN对脂多糖诱导的巨噬细胞M1型极化的作用以及该作用机制是否关联Wnt/β-catenin信号通路,对完善炎症环境中巨噬细胞极化失衡的分子机制以及寻找诊断和治疗靶点具有重要意义。
1. 材料和方法
1.1. 材料与试剂
小鼠巨噬细胞系RAW264.7细胞购自中国科学院昆明细胞库;过表达FKN的重组慢病毒载体Ubi-MCS-3FLAG-SV40-IRES-Puro-CX3CL1购自上海吉凯基因科技有限公司;DMEM培养基(Gibco);胎牛血清(FBS,Gibco);嘌呤霉素、脂多糖(Sigma);ICG-001(Selleck);CCK-8 kit(AbMole);TNF-α和IL-6酶联免疫(ELISA)试剂盒(Cusabio);兔抗iNOS、IL-6一抗(Affinity);兔抗FKN、β-catenin一抗、鼠抗TNF-α、Wnt-4、β-actin一抗(Abcam);山羊抗兔、山羊抗鼠IgG二抗(LI-COR);山羊抗兔IgG Fluor488结合二抗(Affinity)。
1.2. 细胞培养与转染后分组
RAW264.7细胞使用含10% FBS的DMEM培养基于37 ℃、含5% CO2的培养箱中传代培养。细胞按5× 104/mL的密度接种于6孔板中,待常规培养至30%时使用携带有过表达FKN的慢病毒载体按1×108 TU/mL(MOI=100)感染细胞14 h后用完全培养基进行换液,转染72 h后使用荧光倒置显微镜观察感染状况后使用4 μg/mL嘌呤霉素筛选得到稳定转染的细胞系,使用Western blotting法验证转染效果。RAW264.7细胞分为8组:(1)空白对照组;(2)LPS组,细胞给予脂多糖(1μg/mL,12 h);(3)ICG-001组,细胞给与Wnt/β-catenin信号通路抑制剂ICG-001(10 μmol/mL,48 h);(4)过表达FKN组;(5)ICG-001+LPS组,细胞给与ICG-001(10 μmol/mL预处理36 h)后,加入脂多糖(1 μg/mL,12 h);(6)过表达FKN+LPS组,过表达FKN细胞给与脂多糖(1 μg/mL,12 h);(7)过表达FKN+ICG-001组,过表达FKN细胞给予ICG-001(10 μmol/mL,48 h);(8)过表达FKN+ICG- 001+LPS组,过表达FKN细胞给与ICG-001(10 μmol/mL预处理36 h)后,加入脂多糖(1 μg/mL,12 h)。
1.3. 细胞活力测定实验
RAW264.7细胞按3×103/孔(在100 µL培养基中)接种于96孔板中。用ICG-001(5、10、15 μmol/L)处理24、28和72 h后,每孔加入8 μL的CCK-8试剂并在培养箱中培养1 h后使用TriStar LB 941多功能酶标仪测量450 nm处吸光度值。
1.4. ELISA实验
收集RAW264.7细胞培养上清液,于4 ℃、2500 r/min离心3 min,去除沉淀。按照ELISA试剂盒说明书中的步骤检测上清液中TNF-α和IL-6分泌量。
1.5. Western blotting实验
RAW264.7细胞去上清后用预冷PBS洗涤3次,吸弃上清后加入RIPA裂解液冰上裂解30 min,收集细胞并12 000 r/min离心5 min后收取上清液,BCA法测定蛋白浓度。取5×蛋白上样缓冲液按1:4配置后100 ℃煮沸5 min使蛋白质变性。SDS-PAGE凝胶进行电泳,250 mA电流60 min湿转至NC膜,加入β-actin、FKN、β-catenin、Wnt-4、iNOS、TNF-α和IL-6一抗孵育过夜。随后1×PBST洗膜3次,加入羊抗鼠和羊抗兔二抗孵育50 min,洗涤后采用双色红外激光成像系统(LI-COR)进行成像分析。
1.6. 免疫荧光实验
RAW264.7细胞去上清后用PBS洗涤3次,吸弃上清后加4%多聚甲醛固定细胞,随后加0.1%TritonX-100-PBS缓冲液透化20 min,使用4% BSA-PBS缓冲液封闭30 min后加入IL-6一抗孵育过夜。洗涤3次后加入羊抗兔IgG Fluor488结合荧光二抗避光孵育50 min,随后添加5 µg/mL的DAPI染液避光10 min,在激光共聚焦显微镜下拍照观察。
1.7. 统计学分析
实验结果以均数±标准差表示。所有数据采用SPSS23.0进行统计学分析。实验数据均进行正态性和方差齐性分析,符合方差齐性的采用单因素方差分析,不符合方差齐性的采用非参数法分析;P < 0.05表示差异有统计学意义。
2. 结果
2.1. 转染细胞结果鉴定
转染组RAW264.7细胞内可见红色荧光蛋白,转染率约为95%。与空白对照组相比,转染组FKN的蛋白含量明显高于正常组(P < 0.01),证实转染成功(图 1)。
1.
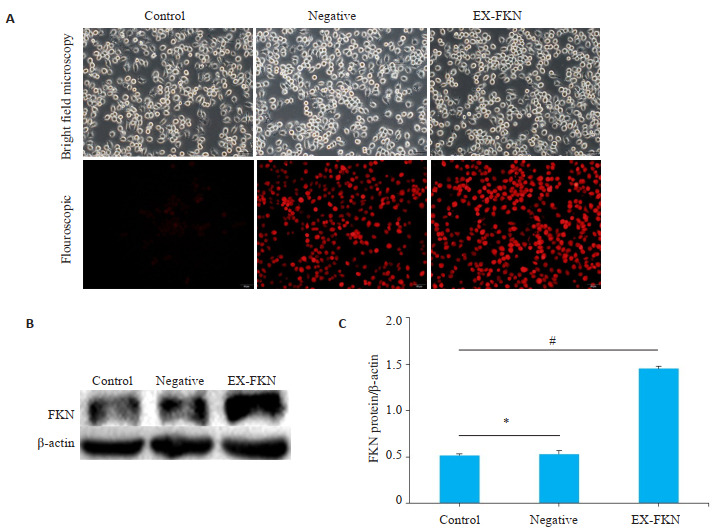
转染后RAW264.7细胞中FKN蛋白的表达
Expression of FKN in transfected RAW264.7 cells. A: Red fluorescence protein were observed in control group, negative control group and EX-FKN group (Original magnification: × 20). B: Western blotting of FKN protein. C: Relative gray value of FKN protein. *P>0.05; #P < 0.01.
2.2. ICG-001抑制RAW264.7细胞的活力
ICG-001以5、10、15 μmol/mL按指定时间处理RAW264.7细胞后均会抑制细胞活力。ICG-001的半数抑制浓度(IC50)是在10 μmol/mL处理48 h后。在以下研究中ICG-001均使用10 μmol/mL的浓度处理细胞48 h(图 2)。
2.

CCK-8法检测ICG-001对RAW264.7细胞活力的影响
CCK-8 assay for assessing viability of RAW264.7 cells treated with ICG-001.
2.3. FKN抑制脂多糖诱导的RAW264.7细胞炎症因子TNF-α和IL-6的分泌
相对于LPS组,EX-FKN组、EX-FKN+LPS组、EXFKN+ICG-001组和EX-FKN+ICG-001+LPS细胞上清液中TNF-α和IL-6的分泌量均显著降低(P < 0.01);而ICG-001+LPS组细胞上清液中TNF-α和IL-6的分泌量均显著升高(P < 0.01,图 3)。
3.

ELISA法检测ICG-001与过表达FKN对脂多糖诱导的RAW264.7细胞炎症因子TNF-α和IL-6分泌的影响
ELISA for detecting the effects of ICG-001 and FKN overexpression on secretion of TNF-α (A) and IL-6 (B) in RAW264.7 cells induced by LPS. *P < 0.05; #P < 0.01 vs LPS group.
2.4. FKN通过活化Wnt/β-catenin信号通路抑制了脂多糖诱导的RAW264.7细胞炎症因子的表达
Western blot结果显示,与LPS组相比,EX-FKN+ LPS组中FKN、Wnt-4和β-catenin蛋白含量均显著升高(P < 0.01);而ICG-001+LPS组中FKN、Wnt-4和β-catenin蛋白均显著降低(P < 0.01)。另外,与LPS组对比,EXFKN+LPS组中TNF-α、IL-6和iNOS蛋白含量均显著降低(P < 0.01),而ICG-001+LPS组中TNF-α、IL-6和iNOS蛋白含量升高(P < 0.05,P < 0.01,P < 0.05,图 4)。
4.

ICG-001与过表达FKN对脂多糖诱导的RAW264.7细胞中FKN、β-catenin、炎症因子iNOS、TNF-α和IL-6蛋白含量的影响
Effects of ICG-001 and overexpression of FKN on the protein contents of FKN, Wnt-4, β-catenin, iNOS, TNF-α and IL-6 in RAW264.7 cells with LPS stimulation. A: Western blotting of FKN, Wnt-4, β-catenin, iNOS, TNF-α and IL-6 proteins. B: Relative gray values of FKN, Wnt-4, β-catenin, iNOS, TNF-α and IL-6 protein. *P < 0.05; #P < 0.01 vs LPS group. C: Localization of IL-6 protein in RAW264.7 cells in different groups. Scale bar=10 μm
3. 讨论
FKN作为一类经典的炎症趋化因子,在炎症/免疫疾病的进程中发挥重要作用,能够诱导免疫细胞在受损组织中快速积累[22-23]。当炎症发生时,膜结合型FKN被分解成可溶性形式,黏附趋化炎症因子快速聚集到受损组织中参与炎症反应[24]。课题组前期研究发现FKN在狼疮性肾炎患者的血清中表达升高,并与狼疮性肾炎的病情严重程度呈正相关[25]。Yano等[26]在类风湿关节炎患者单核细胞中检测到更高水平表达的FKN。而Foussat等[27]在HIV感染到AIDS的整个阶段中发现,增加FKN可以诱导的有效的组织细胞保护作用,延缓了细胞凋亡进程。目前对FKN在炎性/免疫疾病进程中的调控机制研究尚不够透彻。
巨噬细胞亚群M1型极化具有促炎效应,促进炎性/免疫疾病的发展进程。我们推测FKN可以通过抑制巨噬细胞M1极化从而抑制炎性/免疫疾病的进程。本研究中使用小鼠RAW264.7巨噬细胞作为研究对象,体外脂多糖诱导模拟巨噬细胞受到的炎性刺激。我们的结果显示,脂多糖能够诱导RAW264.7细胞朝向M1型分化,炎症因子iNOS、TNF-α和IL-6表达量显著升高,与文献中的报道一致[28-29]。而过表达FKN能够抑制RAW264.7细胞上清液中炎症因子TNF-α和IL-6的分泌以及细胞内iNOS、TNF-α和IL-6蛋白的表达,并能够抑制脂多糖导致的炎症因子IL-6在细胞质中的定位。我们的结果发现过表达FKN会抑制脂多糖诱导的RAW264.7细胞M1型极化,进一步证实了FKN调控巨噬细胞极化进程。
Wnt/β-catenin信号通路是由配体蛋白Wnt和膜蛋白受体结合激发的一组多下游通道的信号转导途径,是调控细胞生长、增殖、分化的关键途径[30-31]。研究表明,Wnt/β-catenin信号的异常传导通过调控巨噬细胞的吞噬作用在免疫炎性疾病的恶性进展过程中起到十分重要的作用[32-35]。增强Wnt/β-catenin信号通路的活性会通过转录调节因子Yap/Taz途径诱导巨噬细胞M2型极化,促进肾纤维化的进展[36];而β-catenin蛋白缺失会延缓博来霉素诱导的小鼠纤维化病情进程[37]。然而,FKN与wnt/β-catenin之间的调控及其与巨噬细胞极化的作用关系,尚缺乏研究。我们的结果发现,FKN和Wnt/ β-catenin信号通路存在正向调控的关系。抑制Wnt/β-catenin信号通路会导致RAW264.7细胞炎症因子iNOS、TNF-α和IL-6表达量升高并增强了脂多糖的作用。FKN过表达可以增强Wnt/β-catenin信号通路的活性,抑制脂多糖诱导的RAW264.7细胞炎症因子iNOS、TNF-α和IL-6的表达,逆转了脂多糖导致的RAW264.7细胞M1型极化。分析其可能原因,虽然FKN关联Wnt/β-catenin信号通路,但过表达FKN也可能调控其他信号通路,表现出与单独使用脂多糖相反的细胞极化表型,具体机制需进一步研究证实。
综上所述,本文证实过表达FKN激活Wnt/β-catenin信号通路,调控炎症因子iNOS、TNF-α和IL-6表达及分泌作用,进而抑制了脂多糖诱导的RAW264.7细胞的M1型极化进程。本研究为明确FKN-Wnt/β-catenin信号轴在巨噬细胞极化失衡导致的相关免疫炎症反应中涉及到的分子信号机制奠定了基础,继续探究FKN-Wnt/β-catenin信号轴在体内免疫机制中的调控作用可能会免疫炎性疾病的诊断和治疗提供新的靶点。
Biography
巩奇明,在读硕士研究生,E-mail: 15610398015@163.com
Funding Statement
国家自然科学基金(H1008 81860296);广西自然科学基金项目(2017GXNSFDA198005和2018GXNSFAA281038);广西研究生创新计划项目(YCSW2020236)
Supported by National Natural Science Foundation of China (H1008 81860296)
Contributor Information
巩 奇明 (Qiming GONG), Email: 15610398015@163.com.
尤 燕舞 (Yanwu YOU), Email: youyanwu@163.com.
References
- 1.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89(4):557–63. doi: 10.1189/jlb.0710409. [Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization[J]. J Leukoc Biol, 2011, 89(4): 557-63.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funes SC, Rios M, Escobar-Vera J, et al. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–95. doi: 10.1111/imm.12910. [Funes SC, Rios M, Escobar-Vera J, et al. Implications of macrophage polarization in autoimmunity[J]. Immunology, 2018, 154(2): 186-95.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong XY, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21(5):941–54. doi: 10.1111/jcmm.13034. [Kong XY, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury[J]. J Cell Mol Med, 2017, 21(5): 941-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukui S, Iwamoto N, Takatani A, et al. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol. 2017;8:1958. doi: 10.3389/fimmu.2017.01958. [Fukui S, Iwamoto N, Takatani A, et al. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis[J]. Front Immunol, 2017, 8: 1958.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21(6):1297–305. doi: 10.1097/MIB.0000000000000384. [Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD[J]. Inflamm Bowel Dis, 2015, 21(6): 1297-305.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–7. doi: 10.1084/jem.20030896. [Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation[J]. J Exp Med, 2003, 198(12): 1951-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. doi: 10.1172/JCI59643. [Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas[J]. J Clin Invest, 2012, 122(3): 787-95.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo D, Guo YM, Cheng YY, et al. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging. 2017;9(10):2069–82. doi: 10.18632/aging.101302. [Luo D, Guo YM, Cheng YY, et al. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways[J]. Aging, 2017, 9(10): 2069-82.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–97. doi: 10.1172/JCI44490. [Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice[J]. J Clin Invest, 2011, 121(3): 985-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan WJ, BothwellL ALM. Canonical and non-canonical wnt signaling in immune cells. Trends Immunol. 2018;39(10):830–47. doi: 10.1016/j.it.2018.08.006. [Chan WJ, BothwellL ALM. Canonical and non-canonical wnt signaling in immune cells[J]. Trends Immunol, 2018, 39(10): 830-47.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99. doi: 10.1016/j.cell.2017.05.016. [Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities[J]. Cell, 2017, 169(6): 985-99.] [DOI] [PubMed] [Google Scholar]
- 12.Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145(11):dev146589. doi: 10.1242/dev.146589. [Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis[J]. Development, 2018, 145(11): dev146589.] [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Ren J, Gui Y, et al. Wnt/β-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. 2018;29(1):182–93. doi: 10.1681/ASN.2017040391. [Feng Y, Ren J, Gui Y, et al. Wnt/β-catenin-promoted macrophage alternative activation contributes to kidney fibrosis[J]. J Am Soc Nephrol, 2018, 29(1): 182-93.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Ye YC, Chen Y, et al. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9(8):793. doi: 10.1038/s41419-018-0818-0. [Yang Y, Ye YC, Chen Y, et al. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors[J]. Cell Death Dis, 2018, 9(8): 793.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julia V, STAUMONT SD, DOMBROWICZ D. Role of fractalkine/ CX3CL1 and its receptor CX3CR1 in allergic diseases. Med Sci (Paris) 2016;32(3):260–6. doi: 10.1051/medsci/20163203010. [Julia V, STAUMONT SD, DOMBROWICZ D. Role of fractalkine/ CX3CL1 and its receptor CX3CR1 in allergic diseases[J]. Med Sci (Paris), 2016, 32(3): 260-6.] [DOI] [PubMed] [Google Scholar]
- 16.Ostuni MA, Hermand P, Saindoy E, et al. CX3CL1 Homo-oligomerization drives cell-to-cell adherence. Sci Rep. 2020;10(1):9069. doi: 10.1038/s41598-020-65988-w. [Ostuni MA, Hermand P, Saindoy E, et al. CX3CL1 Homo-oligomerization drives cell-to-cell adherence[J]. Sci Rep, 2020, 10 (1): 9069.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold L, Perrin H, de Chanville CB, et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat Commun. 2015;6:8972. doi: 10.1038/ncomms9972. [Arnold L, Perrin H, de Chanville CB, et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production[J]. Nat Commun, 2015, 6: 8972.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizoguchi M, Ishida Y, Nosaka M, et al. Prevention of lipopo-lysaccharide-induced preterm labor by the lack of CX3CL1-CX3CR1 interaction in mice. PLoS One. 2018;13(11):e0207085. doi: 10.1371/journal.pone.0207085. [Mizoguchi M, Ishida Y, Nosaka M, et al. Prevention of lipopo-lysaccharide-induced preterm labor by the lack of CX3CL1-CX3CR1 interaction in mice[J]. PLoS One, 2018, 13(11): e0207085.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanki T. Treatment for rheumatoid arthritis by chemokine blockade. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39(3):172–80. doi: 10.2177/jsci.39.172. [Nanki T. Treatment for rheumatoid arthritis by chemokine blockade[J]. Nihon Rinsho Meneki Gakkai Kaishi, 2016, 39(3): 172-80.] [DOI] [PubMed] [Google Scholar]
- 20.Riopel M, Vassallo M, Ehinger E, et al. CX3CL1-Fc treatment prevents atherosclerosis in Ldlr KO mice. Mol Metab. 2019;20:89–101. doi: 10.1016/j.molmet.2018.11.011. [Riopel M, Vassallo M, Ehinger E, et al. CX3CL1-Fc treatment prevents atherosclerosis in Ldlr KO mice[J]. Mol Metab, 2019, 20: 89-101.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senouthai S, Wang JJ, Fu DD, et al. Fractalkine is involved in lipopolysaccharide-induced podocyte injury through the wnt/β-catenin pathway in an acute kidney injury mouse model. Inflammation. 2019;42(4):1287–300. doi: 10.1007/s10753-019-00988-1. [Senouthai S, Wang JJ, Fu DD, et al. Fractalkine is involved in lipopolysaccharide-induced podocyte injury through the wnt/β-catenin pathway in an acute kidney injury mouse model[J]. Inflammation, 2019, 42(4): 1287-300.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conroy MJ, Lysaght J. CX3CL1 signaling in the tumor microen-vironment. Adv Exp Med Biol. 2020;1231:1–12. doi: 10.1007/978-3-030-36667-4_1. [Conroy MJ, Lysaght J. CX3CL1 signaling in the tumor microen-vironment[J]. Adv Exp Med Biol, 2020, 1231: 1-12.] [DOI] [PubMed] [Google Scholar]
- 23.Zheng YY, Corrêa-Silva S, de Souza EC, et al. Macrophage profile and homing into breast milk in response to ongoing respiratory infections in the nursing infant. Cytokine. 2020;129:155045. doi: 10.1016/j.cyto.2020.155045. [Zheng YY, Corrêa-Silva S, de Souza EC, et al. Macrophage profile and homing into breast milk in response to ongoing respiratory infections in the nursing infant[J]. Cytokine, 2020, 129: 155045.] [DOI] [PubMed] [Google Scholar]
- 24.Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168(12):6173–80. doi: 10.4049/jimmunol.168.12.6173. [Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression[J]. J Immunol, 2002, 168(12): 6173-80.] [DOI] [PubMed] [Google Scholar]
- 25.You YW, Qin YQ, Lin X, et al. Upregulated fractalkine levels in Chinese patients with lupus nephritis. Cytokine. 2018;104:23–8. doi: 10.1016/j.cyto.2018.01.027. [You YW, Qin YQ, Lin X, et al. Upregulated fractalkine levels in Chinese patients with lupus nephritis[J]. Cytokine, 2018, 104: 23-8.] [DOI] [PubMed] [Google Scholar]
- 26.Yano R, Yamamura M, Sunahori K, et al. Recruitment of CD16 + monocytes into synovial tissues is mediated by fractalkine and CX3CR1 in rheumatoid arthritis patients. Acta Med Okayama. 2007;61(2):89–98. doi: 10.18926/AMO/32882. [Yano R, Yamamura M, Sunahori K, et al. Recruitment of CD16 + monocytes into synovial tissues is mediated by fractalkine and CX3CR1 in rheumatoid arthritis patients[J]. Acta Med Okayama, 2007, 61(2): 89-98.] [DOI] [PubMed] [Google Scholar]
- 27.Foussat A, Bouchet DL, Berrebi D, et al. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1 –infected patients. Blood. 2001;98(6):1678–86. doi: 10.1182/blood.v98.6.1678. [Foussat A, Bouchet DL, Berrebi D, et al. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1 –infected patients[J]. Blood, 2001, 98(6): 1678-86.] [DOI] [PubMed] [Google Scholar]
- 28.刘 璇, 罗 宇维, 孙 尔维. 坏死细胞诱导并促进炎症反应. 南方医科大学学报. 2009;29(4):659–62. [刘璇, 罗宇维, 孙尔维.坏死细胞诱导并促进炎症反应[J].南方医科大学学报, 2009, 29(4): 659-62.] [PubMed] [Google Scholar]
- 29.Chae BS. Pretreatment of low-dose and super-low-dose LPS on the production of in vitro LPS-induced inflammatory mediators. Toxicol Res. 2018;34(1):65–73. doi: 10.5487/TR.2018.34.1.065. [Chae BS. Pretreatment of low-dose and super-low-dose LPS on the production of in vitro LPS-induced inflammatory mediators[J]. Toxicol Res, 2018, 34(1): 65-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosin-Roger J, Ortiz-Masià MD, Barrachina MD. Macrophages as an emerging source of wnt ligands: relevance in mucosal integrity. Front Immunol. 2019;10:2297. doi: 10.3389/fimmu.2019.02297. [Cosin-Roger J, Ortiz-Masià MD, Barrachina MD. Macrophages as an emerging source of wnt ligands: relevance in mucosal integrity[J]. Front Immunol, 2019, 10: 2297.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran FH, Zheng JJ. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017;26(4):650–61. doi: 10.1002/pro.3122. [Tran FH, Zheng JJ. Modulating the wnt signaling pathway with small molecules[J]. Protein Sci, 2017, 26(4): 650-61.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malsin ES, Kim S, Lam AP, et al. Macrophages as a source and recipient of wnt signals. Front Immunol. 2019;10:1813. doi: 10.3389/fimmu.2019.01813. [Malsin ES, Kim S, Lam AP, et al. Macrophages as a source and recipient of wnt signals[J]. Front Immunol, 2019, 10: 1813.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Au DT, Migliorini M, Strickland DK, et al. Macrophage LRP1 promotes diet-induced hepatic inflammation and metabolic dysfunction by modulating wnt signaling. Mediat Inflamm. 2018;2018:1–15. doi: 10.1155/2018/7902841. [Au DT, Migliorini M, Strickland DK, et al. Macrophage LRP1 promotes diet-induced hepatic inflammation and metabolic dysfunction by modulating wnt signaling[J]. Mediat Inflamm, 2018, 2018: 1-15.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsseeb M, Pirzada RH, Ain QU, et al. Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics. Cells. 2019;8(11):1380. doi: 10.3390/cells8111380. [Hsseeb M, Pirzada RH, Ain QU, et al. Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics[J]. Cells, 2019, 8(11):1380.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao Y, Zheng QQ, Wang W, et al. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget. 2016;7(41):67674–84. doi: 10.18632/oncotarget.11874. [Shao Y, Zheng QQ, Wang W, et al. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases[J]. Oncotarget, 2016, 7(41): 67674-84.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y, Liang Y, Zhu XW, et al. The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem. 2018;293(50):19290–302. doi: 10.1074/jbc.RA118.005457. [Feng Y, Liang Y, Zhu XW, et al. The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz[J]. J Biol Chem, 2018, 293(50): 19290-302.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sennello JA, Misharin AV, Flozak AS, et al. Lrp5/β-catenin signaling controls lung macrophage differentiation and inhibits resolution of fibrosis. Am J Respir Cell Mol Biol. 2017;56(2):191–201. doi: 10.1165/rcmb.2016-0147OC. [Sennello JA, Misharin AV, Flozak AS, et al. Lrp5/β-catenin signaling controls lung macrophage differentiation and inhibits resolution of fibrosis[J]. Am J Respir Cell Mol Biol, 2017, 56(2): 191-201.] [DOI] [PMC free article] [PubMed] [Google Scholar]


