Abstract
目的
探讨乳酸杆菌GR-1 & RC-14对妊娠晚期生殖道B族链球菌(GBS)阳性患者GBS定植、妊娠结局及阴道菌群微生态的影响。
方法
选取2019年3月~2019年11月于南方医科大学珠江医院门诊行妊娠晚期GBS检测且结果阳性患者符合纳排标准155例,剔除乳酸杆菌干预 < 2周或产后复查32例,根据有无乳酸杆菌干预,分为干预组(60例)、无干预组(63例); 干预组60例根据再次检测GBS结果分为转阴组(18例)、持续阳性组(42例); 计算GBS转阴率,比较各分组妊娠结局; 从干预组60例患者中随机选取配对乳酸杆菌干预前后阴道分泌物5对行扩增子测序及生物信息分析。
结果
干预组GBS转阴率为30%(18/60),无干预组GBS转阴率为23%(3/13);乳酸杆菌干预降低胎膜早破发生率(P < 0.05),并降低抗生素应用(P < 0.05)。阴道分泌物OTU分析提示乳酸杆菌干预后总序列数、GBS序列数减少及物种组成增加。乳酸杆菌干预后链球菌属物种丰度降低,差异有统计学意义(P < 0.05)。MetaStat提示乳酸杆菌干预后肠球菌、葡萄球菌属、链球菌属物种丰度均减少且差异有统计学意义(P < 0.05)。
结论
乳酸杆菌对妊娠晚期生殖道GBS定植、妊娠结局有一定的改善作用,乳酸杆菌降低链球菌属物种相对丰度及致病菌物种丰度,对阴道菌群微生态有一定改善作用。
Keywords: B族链球菌, 乳酸杆菌, 阴道菌群微生态
Abstract
Objective
To explore the effects of intervention with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on vaginal Group B Streptococcus (GBS) colonization, pregnancy outcome and vaginal microbiome in GBS-positive women in the third trimester of pregnancy.
Methods
This study were conducted among 155 women in the third trimester of pregnancy with positive results of GBS culture in the Outpatient Department of Zhujiang Hospital from March to November, 2019. After excluding 32 patients who received lactobacillus intervention for less than 2 weeks or underwent postpartum GBS retesting, the women were divided into oral probiotics intervention group (60 cases) and non-intervention group (63 cases). According to the results of GBS retesting, the 60 women in the intervention group were divided into GBS-negative group (18 cases) and persistent GBS-positive group (42 cases). At the end of the intervention, the rates of negative GBS culture result were calculated and the pregnancy outcomes were compared. From 5 women randomly selected from the intervention group, samples of vaginal secretions were collected before and after the intervention for amplicon sequencing and bioinformatics analysis.
Results
At the end of the intervention, the GBS-negative rate in the intervention group was 30% (18/60), as compared with 23% (3/13) in the non-intervention group. Probiotic intervention significantly reduced the incidence of premature rupture of membranes (P < 0.05) and reduced the use of antibiotics during pregnancy (P < 0.05). OTU analysis of the vaginal secretions suggested probiotic intervention decreased the total sequence number and GBS sequence number, increased the species composition, and significantly decreased GBS abundance (P < 0.05). Probiotics intervention also significantly decreased the species abundance of Enterococcus, Staphylococcus and Streptococcus in the vaginal flora (P < 0.05).
Conclusion
Intervention with oral probiotics can reduce vaginal GBS colonization in late pregnancy and improve the pregnancy outcome. Lactobacillus is capable of reducing the abundance of GBS and other pathogenic bacteria to improve the microbiome of vaginal flora.
Keywords: group B Streptococcus, Lactobacillus, vaginal flora microbiome
妊娠晚期生殖道B族链球菌(GBS)是一种定植于人体胃肠道及泌尿生殖道的兼性革兰阳性微生物,属于条件致病菌[1-2]。GBS感染在孕母多表现为无症状带菌感染,可与宫腔内感染、早产等有关; B族链球菌是新生儿感染的首位因素,新生儿早发型GBS疾病的首要高危因素孕母阴道及胃肠道的GBS定植,主要特征为败血症、新生儿肺炎等[3]。2019年美国妇产科医师学会委员会最新推荐所有的GBS患者应在临产或破膜后接受适当的抗生素预防,推荐至少4 h的肠外抗生素治疗[3]。由于妊娠期GBS感染对母儿的危害,缺少合适的抗生素治疗,将增加新生儿发生早发型GBS疾病的风险,广泛抗生素应用增加耐药的风险[4-5],亟需寻找有效途径降低GBS定植率,改善母儿预后,具有较强的临床意义和应用价值。
目前乳酸杆菌对妊娠晚期GBS阳性患者GBS定植的研究较少,一项随机对照研究对妊娠晚期GBS感染患者予以乳酸杆菌GR-1 & RC-14胶囊或安慰剂干预3周,干预组GBS转阴率42.9%,安慰剂组GBS转阴率18.0%,表明乳酸杆菌能降低GBS定植率,但是年龄、体质量、教育水平、产次、分娩孕周、新生儿体质量、新生儿转儿科和Apgar评分等临床资料无差异,应用GBS培养法定性表明乳酸杆菌干预降低GBS定植,但没有对乳酸杆菌降低GBS进一步定量验证及机制研究[6]。英国和加拿大分别有研究对早中孕期正常妊娠女性予以乳酸杆菌或安慰剂干预,表明乳酸杆菌对GBS定植的预防和阴道菌群微生态无影响,但是早中孕期健康女性的GBS定植预防作用并不直接体现乳酸杆菌对妊娠晚期GBS阳性患者的定植作用[7-8]。口服乳酸杆菌可定植于阴道中,恢复及维持女性正常阴道菌落[9-12]。因此乳酸杆菌对妊娠期GBS的定植及阴道菌群的影响尚不明确。所以我们需要进一步试验了解乳酸杆菌干预对GBS定植及阴道菌群影响,进一步了解乳酸杆菌在妊娠期应用的可能影响。
本研究对妊娠晚期GBS阳性患者予以乳酸杆菌干预2周,分析乳酸杆菌干预对GBS定植及妊娠结局的影响,并增加阴道分泌物扩增子测序,定量分析乳酸杆菌干预对GBS等阴道菌群的影响,通过观察菌群的表型变化,与临床妊娠结局相联系,进行相关初步机制的探索,乳酸杆菌干预有望成为妊娠期生殖道感染及维持母胎界面稳态的生态治疗新策略。
1. 资料和方法
1.1. 研究对象
2019年3月~2019年11月在南方医科大学珠江医院产检的孕周≥34周孕妇行GBS检测且结果阳性,并符合纳排标准的GBS感染患者共155例。纳入标准:在南方医科大学珠江医院产检并计划在我院分娩; 自愿完成生殖道GBS检测; GBS结果为阳性; 单胎妊娠晚期妇女。排除标准:双胎妊娠; 外院分娩; 宫内死胎或胎儿畸形引产者; 合并恶性肿瘤、多器官功能障碍及其他慢性消耗性需长期服用药物的疾病; 合并慢性消化系统疾病; 妊娠合并严重内外科疾病; 拒绝行GBS检测孕妇。本研究通过南方医科大学珠江医院伦理委员会审查,伦理审查批号为:2019-KY-052-01。所有患者签署知情同意书。
1.1.1. 分组
分组1:符合纳排标准孕妇155例,其中乳酸杆菌干预的GBS阳性患者共92例,剔除干预 < 2周或产后复查GBS患者32例,干预满2周且产前复查GBS患者60例,无乳酸杆菌干预患者63例,因此分为干预组(60例)、无干预组(63例)。分组2:干预组60例患者根据再次检测GBS结果分为转阴组(18例)、持续阳性组(42例)。分组3:干预组60例患者随机选取配对的5对乳酸杆菌干预前后阴道分泌物,分为乳酸杆菌干预前阴道分泌物(A组,5例)、乳酸杆菌干预后阴道分泌物(B组,5例)。
1.1.2. 干预措施
干预组干预措施为:口服或低于30 ℃温水冲服乳酸杆菌,1包/d,共2周; 干预菌株类型为鼠李糖乳酸杆菌GR-1 & 罗伊氏乳酸杆菌RC-14,菌株含量为3×1010 CFU/包,1.2 g/包,14包/盒,产地为丹麦原料,中国台湾包装。无干预组则无乳酸杆菌干预,自然妊娠的一个过程。
1.2. 方法
1.2.1. 标本采集及细菌检测
外生殖器局部无菌大棉签擦拭,采用GBS运送培养基配套的无菌拭子插入阴道内1/3处,沿阴道壁轻轻旋转获取标本,并将无菌拭子放置于无菌管内,密闭送检。行扩增子测序的阴道分泌物按前法留取置入Amies带活性炭凝胶培养基,编号并记录,-80 ℃冰箱保存。按照南方医科大学珠江医院检验科培养法检测GBS。
1.2.2. 扩增子测序
采用CTAB方法提取样本的基因组DNA,利用琼脂糖凝胶电泳检测DNA的纯度和浓度,取适量样品于离心管中,使用无菌水稀释至1 ng/μL,检测合格后送北京诺禾致源科技股份有限公司,应用New England Biolabs公司Illumina试剂盒建库,HiSeq上机测序。
1.2.3. 生物信息分析
1.3. 资料收集
收集孕产妇的以下信息:一般临床资料:包括年龄、身高、体质量; 复查GBS结果、抗生素治疗、复查时机、用药天数; 妊娠结局:包括抗生素治疗、胎盘炎、胎膜早破、羊水Ⅲ度、产时发热、剖宫产、催产、产后2 h出血量、新生儿窒息、新生儿转儿科、新生儿体质量、新生儿身长。诊断参照人民卫生出版社《妇产科学》(第9版)[15]。
1.4. 统计学处理
根据中国台湾研究试验结果,干预组和无干预组的GBS转阴率分别42.9%、18%,确定Ⅰ类错误α=0.05,检验效能1-β=0.90,应用PASS 11.0软件计算,每组需要69例。纳入研究干预组患者为92例、无干预组63例,但由于孕妇的局限性及中国国情的特殊性,实际自愿再次检测GBS的无干预组仅13例。
应用SPSS 20.0软件进行统计学分析,计量资料正态方差齐用均数±标准差表示,应用独立样本t检验;非正态分布用中位数(P25,P75)表示,采用非参数检验。计数资料组间比较采用卡方检验。组间比较采用Bonferroni校正。二分类Logistic回归分析妊娠结局及抗生素应用有统计学意义变量,P < 0.05为差异有统计学意义。
2. 结果
2.1. 乳酸杆菌干预对妊娠晚期GBS阳性患者GBS定植及妊娠结局的研究
2.1.1. 乳酸杆菌干预对GBS定植的影响
乳酸杆菌干预的GBS阳性患者共92例,干预两周并产前复查60例,60例患者中转阴18例,计算转阴率30%(18/60);无干预组63例患者自愿2周后再次检测GBS共13例,转阴3例,计算自然转阴率23%(3/13)。两组间转阴率差异无统计学意义(P >0.05)。
2.1.2. 干预组及无干预组一般资料和妊娠结局分析
干预组及无干预组两组间一般资料(年龄、身高、体质量)分布无差异(P >0.05);妊娠结局(产后2 h出血量、新生儿体质量、新生儿身长)两组间差异无统计学意义(P >0.05),但是新生儿血气组间差异有统计学意义(P < 0.05,表 1)。
1.
干预组及无干预组临床资料的非参数检验
Nonparametric tests of clinical data between intervention and non-intervention groups
| Clinical datas | Intervention group (n=60) | Non-intervention group (n=63) | P |
| The intervention group vs non-intervention group, *P < 0.05. | |||
| Age (year) | 29 (27, 32) | 29 (27, 33) | 0.919 |
| Height (cm) | 160 (158, 163) | 160 (158, 160) | 0.283 |
| Weight (kg) | 64 (60, 72) | 65 (56, 71) | 0.809 |
| 2 h postpartum hemorrhage (mL) | 150 (100, 200) | 150 (100, 200) | 0.283 |
| Neonatal blood gas (pH) | 7.31 (7.27, 7.34) | 7.27 (7.11, 7.34) | 0.024* |
| Neonatal weight (kg) | 3.14 (2.95, 3.45) | 3.27 (3.00, 3.49) | 0.809 |
| Length of newborn (cm) | 50 (49, 51) | 50 (49, 51) | 0.185 |
干预组胎膜早破发生率降低,差异有统计学意义(P < 0.05);胎盘炎、羊水Ⅲ度、剖宫产、催产、新生儿窒息、新生儿转儿科的临床资料组间差异无统计学意义(P >0.05,表 2)。两组妊娠结局的比较中,将P < 0.1的影响因素(新生儿血气、胎膜早破)纳入Logistic回归分析,乳酸杆菌干预降低胎膜早破的发生,差异有统计学意义(P < 0.05)。
2.
妊娠结局的卡方检验
Chi-square test of pregnancy outcomes
| Pregnancy outcomes | Intervention group (n=60) | Non-intervention group (n=63) | χ2 | P |
| The intervention group vs non-intervention group, *P < 0.05. | ||||
| Antibiotic application | 49(49/60) | 55(55/63) | 0.747 | 0.387 |
| Placentitis | 17(17/60) | 14(14/63) | 0.609 | 0.435 |
| PROM | 5(5/60) | 14(14/63) | 15.221 | 0.000* |
| Third degree of amniotic fluid | 7(7/60) | 3(3/60) | 1.962 | 0.161 |
| Caesarean section | 18(18/60) | 22(22/63) | 0.339 | 0.560 |
| Push induced labor | 21(21/60) | 15(15/63) | 1.859 | 0.173 |
| Neonatal asphyxia | 1(1/60) | 1(1/63) | 0.001 | 0.972 |
| Neonatal pediatrics | 8(8/60) | 9(9/63) | 0.023 | 0.878 |
2.1.3. 转阴组及持续阳性组妊娠结局分析
乳酸杆菌干预组重再次检测GBS的转阴组及持续阳性组产后2 h出血量、新生儿血气、新生儿体质量、新生儿身长非参数检验提示组间差异无统计学意义(P >0.05)。GBS转阴降低抗生素的应用,组间差异有统计学意义(P < 0.05)。胎盘炎、胎膜早破、羊水Ⅲ度、剖宫产、催产、新生儿窒息、新生儿转儿科的妊娠结局组间差异无统计学意义(P >0.05,表 3)。抗生素应用相关影响因素纳入Logistic回归分析提示GBS转阴是抗生素应用的保护因素(or= 0.015,P < 0.05)。
3.
转阴组及持续阳性组妊娠结局比较
Comparsion of pregnancy outcomes between GBS-negative and persistent GBS-positive groups
| Pregnancy outcomes | Negative group (n=18) | Positive group (n=42) | χ2 | P |
| The negative group vs positive group, *P < 0.05. | ||||
| Antibiotic application | 8 (8/18) | 41(41/42) | 20.376 | 0.000* |
| Placentitis | 7 (7/18) | 10(10/42) | 1.411 | 0.235 |
| PROM | 1 (1/18) | 4(4/42) | 0.000 | 1 |
| Third degree of amniotic fluid | 3 (3/18) | 4(4/42) | 0.123 | 0.726 |
| Caesarean section | 3 (3/18) | 15(15/42) | 1.364 | 0.243 |
| Push induced labor | 9 (9/18) | 12(12/42) | 0.305 | 0.581 |
| Neonatal asphyxia | 0 (0/18) | 1(1/42) | - | 1 |
| Neonatal pediatrics | 1 (1/18) | 7(7/42) | 0.556 | 0.456 |
2.2. 乳酸杆菌干预对妊娠晚期GBS阳性患者阴道菌群微生态的影响
2.2.1. OTU分析
干预前最终分析总序列数为400 653个,干预后最终分析总序列数为372 401个;干预前GBS种水平序列数为769个,干预后GBS种水平序列数为12个。干预前特有OTU个数为30个,干预后特有OTU个数为40个。乳酸杆菌干预后阴道总的序列数及GBS序列数减少,即乳酸杆菌干预后阴道总物种丰度及GBS物种丰度均减少,物种组成增加。
2.2.2. 物种相对丰度
乳酸杆菌干预后在门水平厚壁菌门、放线菌门相对丰度增加,变形菌门、拟杆菌门及软壁菌门相对丰度降低(图 1)。在属水平加德纳均属、乳酸杆菌属及阿托波菌属相对丰度增加,葡萄球菌属、链球菌属、普氏菌属、寄蝇科属、解脲支原体属、杆菌属和气球菌属相对丰度菌降低(图 2)。依次在门纲目科水平行组间相对丰度t检验,提示组间差异无统计学意义(P >0.05),但在属水平找出差异显著的链球菌属,乳酸杆菌干预后链球菌属相对丰度降低,差异有统计学意义(P < 0.05)。
1.
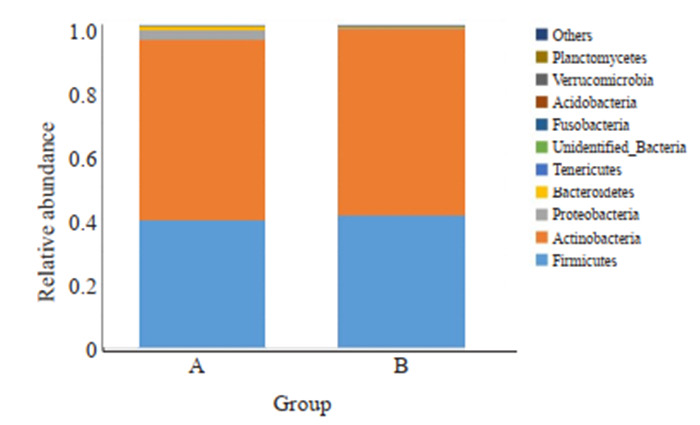
门水平组间相对丰度柱形图
Comparison of relative abundance at phylum level between the two groups.
2.
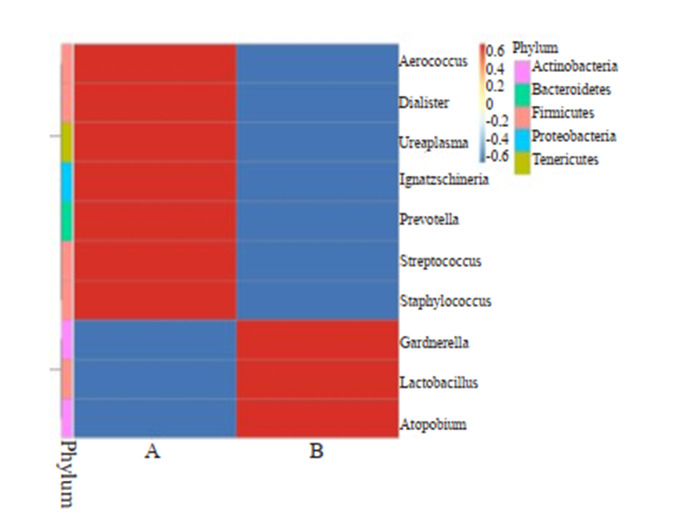
属水平物种丰度聚类热图
Heatmap of genus abundance clustering.
2.2.3. α多样性分析
乳酸杆菌干预前平均shannon指数为0.689,干预后平均shannon指数为0.629,组间差异无统计学意义(P =0.4206)。当测序条数趋近于37 786时,测序数据趋向于合理,测序物种包含大部分物种,乳酸杆菌干预前平均测序条数为80 131,干预后平均测序条数为74 480。
2.2.4. β多样性分析
应用Anosim分析发现,乳酸杆菌干预前后两组之间R=-0.085,P =0.783,R < 0,乳酸杆菌干预前后两组组内差异大于组间差异,P >0.05表示差异无统计学意义。
2.2.5. LEfSe分析
将LDA Score大于设定值2行LEfSe分析,寻找组间差异有具有统计学意义的物种共7个, 其中5个在乳酸杆菌干预前富集,依次为链球菌科、链球菌属、肠球菌属、肠球菌科、粪肠球菌;梭杆菌科、梭杆菌属在干预后富集(图 3)。
3.
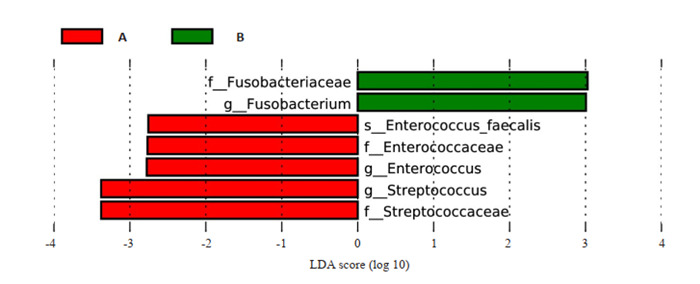
LEfSe分析
LEfSe analysis.
2.2.6. MetaStat分析
行MetaStat分析找出组间差异显著的物种(图 4),发现乳酸杆菌干预后的肠球菌属、葡萄球菌属、链球菌属物种丰度均减少,且组间差异有统计学意义(P < 0.05)。
4.
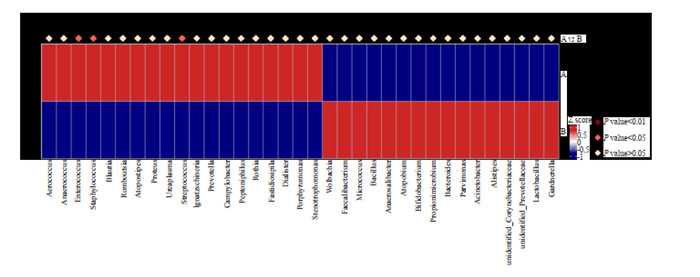
MetaStat物种丰度分布箱图
Boxdiagram of MetaStat in species abundance distribution.
3. 讨论
本研究干预组GBS转阴率为30%,无干预组GBS转阴率为23%,提示乳酸杆菌对GBS定植作用无影响,然而应用扩增子测序发现GBS物种相对丰度在乳酸杆菌干预后降低,表明乳酸杆菌干预对GBS定植作用不确定。目前乳酸杆菌对GBS定植的影响尚不明确,对比中国台湾研究中乳酸杆菌干预组GBS转阴率为42.9%,对照组GBS转阴率为18.0%。本研究乳酸杆菌干预组GBS转阴效果不如中国台湾研究中乳酸杆菌干预组GBS转阴效果,可能是由于中国台湾研究干预时长为3周,高于本研究干预时长2周,另外本研究中无干预组GBS转阴率高于中国台湾研究中安慰剂组GBS转阴率可能受样本量少影响[6]。本研究进一步增加扩增子测序分析定量验证乳酸杆菌干预对GBS定植的影响,通过阴道菌群的表型变化结合临床,探索乳酸杆菌降低GBS定植的可能表型机制。GBS可以降低乳酸杆菌的物种丰度,虽然没有直接表明乳酸杆菌干预对GBS物种丰度的影响,但是间接提示增加乳酸杆菌可在一定程度上影响GBS的定植[7-8]。对妊娠9~14周妇女予以口服乳酸杆菌GR-1&RC-14或安慰剂干预,妊娠18~20周再次检测乳酸杆菌、GBS物种丰度组间无区别,表明妊娠早中期口服乳酸杆菌并不预防妊娠期GBS感染的发生[7];对妊娠中期健康女性予以乳酸杆菌及安慰剂干预12周,发现妊娠中晚期乳酸杆菌干预对GBS定植无预防作用[8];对妊娠早期健康女性予以乳酸杆菌及安慰剂干预8周,发现乳酸杆菌干预对细菌性阴道炎的预防无作用[10],但没有对GBS进行定性及定量研究,所以并不能表明乳酸杆菌对GBS无预防作用。以上3个研究的研究对象为妊娠早中期健康女性,了解乳酸杆菌干预对阴道菌群的影响及对病原体的预防作用,本研究及中国台湾研究的研究对象为妊娠晚期的GBS阳性患者,了解乳酸杆菌干预对GBS的治疗效果。体外试验发现GBS可降低乳酸杆菌的相对丰度,间接表明GBS与乳酸杆菌的相关关系[16-17]。国内有研究认为妊娠晚期菌群多样性降低,菌群群落分布较集中,阴道菌群稳态高于妊娠早中期[18],但是美国有研究发现妊娠早期至妊娠晚期不同部位的菌群种类组成差异无意义[19],妊娠是一个动态变化的过程,阴道内菌群的变化受多种因素的影响,如机体的免疫状态。因此妊娠早中期与妊娠晚期予以乳酸杆菌干预结局不同可能是由于妊娠晚期阴道菌群趋向于稳定也可能由于机体免疫状态等相关。乳酸杆菌对GBS定植作用可与干预时长、样本量、不同妊娠时期、机体免疫状态等多因素相关,在今后的研究中进一步增加样本量了解乳酸杆菌GR-1&RC-14对妊娠晚期GBS阳性孕妇GBS定植的影响。
中国台湾研究乳酸杆菌干预降低GBS定植率,但是年龄、体质量、教育水平、产次、分娩孕周、新生儿体质量、新生儿转儿科和Apgar评分的临床资料无明显差异[6];本研究中也分析了乳酸杆菌干预组间年龄、体质量、分娩孕周、新生儿体质量、新生儿转儿科和Apgar评分无明显差异,另外增加了对身高、抗生素治疗、胎盘炎、胎膜早破、羊水Ⅲ度、产时发热、剖宫产、催产、产后2 h出血量、新生儿身长的临床资料分析。本研究表明乳酸杆菌干预在一定程度上降低胎膜早破的发生,并且GBS转阴在一定程度上降低抗生素的应用。生殖道感染是胎膜早破的主要因素,常见的病原体有厌氧菌、衣原体、B族链球菌和淋病奈瑟菌等[15]。本研究中乳酸杆菌干预降低GBS阳性患者胎膜早破发生率,可能是由于乳酸杆菌干预在一定程度上降低GBS、肠球菌属、葡萄球菌属及衣原体等病原体的物种相对丰度,从而减少病原体上行侵袭宫颈内口局部胎膜,保持胎膜张力平衡[15],使阴道菌群微生态保持平衡,使母胎界面保持稳态平衡,维持正常妊娠生理过程。GBS转阴后在一定程度上降低抗生素的应用,减少临床抗生素的应用,使医疗资源得到更好的再支配和应用,并在一定程度上减少抗生素耐药问题。同时非洲有研究表明乳酸杆菌联合抗生素治疗细菌性阴道炎增加治愈率,并减少抗生素的应用[20]。妊娠期口服乳酸杆菌是安全可行的,也是不良妊娠结局的生物治疗策略[7, 21-24]。体外试验表明乳酸杆菌可以改善和调节母儿免疫系统[23];乳酸杆菌干预可降低炎症反应[22],乳酸杆菌可降低血糖血脂指标,减少不良妊娠结局的发生[21, 24]。因此,乳酸杆菌GR-1&RC-14在一定程度上减少GBS等病原体定植,降低胎膜早破的发生率,减少抗生素的应用,在妊娠期应用是安全可行的,并进一步说明乳酸杆菌对妊娠结局的影响。
本研究中乳酸杆菌GR-1&RC-14干预降低GBS、肠球菌属及葡萄球菌属等病原体物种丰度,增加乳酸杆菌物种丰度,对阴道菌群微生态平衡有改善作用。妊娠期阴道菌群微生态是动态平衡的,阴道内各个菌群的作用不可或缺,且乳酸杆菌为阴道内主要组成群落,乳酸杆菌在阴道上皮可以通过分解阴道糖原为单糖,进一步产生乳酸来维持阴道微环境的平衡,保持一个较低的PH值(通常 < 4.5);乳酸杆菌参与生成过氧化氢(H2O2),可以防止其它条件致病菌的过度生长和外来病原体的入侵。因此,乳杆酸菌通过维持阴道内酸性pH和H2O2的浓度,发挥预防感染的作用,使阴道菌群微生态系统处于动态平衡[25-26]。妊娠期阴道菌群微生态群落可能影响下一代[27-28]。乳酸杆菌在生活中的应用有潜在能力,某些乳酸杆菌菌株类型可以帮助机体恢复内环境稳态,给予补充一定剂量时,有助于机体的健康状态。口服乳酸杆菌后乳酸杆菌可定植于阴道中,恢复及维持女性正常阴道菌落[8-11, 20, 24]。也有研究表明乳酸杆菌干预对阴道菌群无影响[7, 18, 23, 29]。本研究阴道菌群的分析进一步验证乳酸杆菌在妊娠期的应用价值及改善不良妊娠结局的影响,寻找乳酸杆菌干预对GBS定植的可能机制。
综上所述,乳酸杆菌GR-1&RC-14对妊娠晚期生殖道GBS的定植作用不明确,乳酸杆菌干预在一定程度上降低GBS阳性患者胎膜早破的发生,并且GBS转阴后在一定程度上降低抗生素应用。乳酸杆菌GR- 1&RC-14干预增加阴道内乳酸杆菌相对丰度,降低肠球菌、葡萄球菌属、链球菌属相对丰度,对阴道菌群微生态平衡有一定改善作用。
Biography
刘映玲,硕士,E-mail: yldd_liu@163.com
Funding Statement
国家重点研发计划《基于国产医疗设备的智慧产科解决方案及应用示范》(2019YFC0121904);广东省教育厅高水平大学建设经费南方医科大学临床研究启动项目(LC2016PY035)
Contributor Information
刘 映玲 (Yingling LIU), Email: yldd_liu@163.com.
潘 石蕾 (Shilei PAN), Email: 13602882918@163.com.
References
- 1.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31(Suppl 4):D7–D12. doi: 10.1016/j.vaccine.2013.01.009. [Le Doare K, Heath PT. An overview of global GBS epidemiology [J]. Vaccine, 2013, 31(Suppl 4): D7-D12.] [DOI] [PubMed] [Google Scholar]
- 2.Madrid L, Seale AC, Kohli-Lynch M, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S160–72. doi: 10.1093/cid/cix656. [Madrid L, Seale AC, Kohli-Lynch M, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses[J]. Clin Infect Dis, 2017, 65(suppl_2): S160-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion No. 782: Prevention of Group B Streptococcal Early-Onset Disease in Newborns[J]. Obstet Gynecol, 2019, 134(1): e19-40.
- 4.Huang M, Wang JH. Gram stain as a relapse predictor of bacterial vaginosis after metronidazole treatment. J Microbiol Immunol Infect. 2005;38(2):137–40. [Huang M, Wang JH. Gram stain as a relapse predictor of bacterial vaginosis after metronidazole treatment[J]. J Microbiol Immunol Infect, 2005, 38(2): 137-40.] [PubMed] [Google Scholar]
- 5.Schuchat A, Zywicki SS, Dinsmoor MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. 2000;105(1 Pt 1):21–6. doi: 10.1542/peds.105.1.21. [Schuchat A, Zywicki SS, Dinsmoor MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study[J]. Pediatrics, 2000, 105(1 Pt 1): 21-6.] [DOI] [PubMed] [Google Scholar]
- 6.Ho M, Chang YY, Chang WC, et al. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan J Obstet Gynecol. 2016;55(4):515–8. doi: 10.1016/j.tjog.2016.06.003. [Ho M, Chang YY, Chang WC, et al. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: a randomized controlled trial[J]. Taiwan J Obstet Gynecol, 2016, 55(4): 515-8.] [DOI] [PubMed] [Google Scholar]
- 7.Husain S, Allotey J, Drymoussi Z, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG. 2020;127(2):275–84. doi: 10.1111/1471-0528.15675. [Husain S, Allotey J, Drymoussi Z, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double-blind, placebo-controlled trial with microbiome analysis[J]. BJOG, 2020, 127(2): 275-84.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe M, Shah V, Freire-Lizama T, et al. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2019:1–8. doi: 10.1080/14767058.2019.1650907. [Sharpe M, Shah V, Freire-Lizama T, et al. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial[J]. J Matern Fetal Neonatal Med, 2019: 1-8.] [DOI] [PubMed] [Google Scholar]
- 9.Bertazzoni Minelli E, Benini A, Marzotto M, et al. Assessment of novel probiotic Lactobacillus casei strains for the production of functional dairy foods. Int Dairy J. 2004;14(8):723–36. [Bertazzoni Minelli E, Benini A, Marzotto M, et al. Assessment of novel probiotic Lactobacillus casei strains for the production of functional dairy foods[J]. Int Dairy J, 2004, 14(8): 723-36.] [Google Scholar]
- 10.Gille C, Böer B, Marschal M, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol. 2016;245(5):608.e1–608.e7. doi: 10.1016/j.ajog.2016.06.021. [Gille C, Böer B, Marschal M, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial[J]. Am J Obstet Gynecol, 2016, 215(5): 608.e1-608.e7.] [DOI] [PubMed] [Google Scholar]
- 11.Greenbaum S, Greenbaum G, Moran-Gilad J, et al. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol. 2019;220(4):324–35. doi: 10.1016/j.ajog.2018.11.1089. [Greenbaum S, Greenbaum G, Moran-Gilad J, et al. Ecological dynamics of the vaginal microbiome in relation to health and disease[J]. Am J Obstet Gynecol, 2019, 220(4): 324-35.] [DOI] [PubMed] [Google Scholar]
- 12.Laitinen K, Poussa T, Isolauri E, et al. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2009;101(11):1679–87. doi: 10.1017/S0007114508111461. [Laitinen K, Poussa T, Isolauri E, et al. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial[J]. Br J Nutr, 2009, 101 (11): 1679-87.] [DOI] [PubMed] [Google Scholar]
- 13.He YN, Huang Y, Zhang ZY, et al. Exploring profile and potential influencers of vaginal microbiome among asymptomatic pregnant Chinese women. PeerJ. 2019;7:e8172. doi: 10.7717/peerj.8172. [He YN, Huang Y, Zhang ZY, et al. Exploring profile and potential influencers of vaginal microbiome among asymptomatic pregnant Chinese women[J]. PeerJ, 2019, 7: e8172. DOI:10.7717/peerj.8172.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendz GL, of Medicine Sydney The University of Notre Dame Australia Oxford St Darlinghurst NSW Australia S School, Kaakoush NO, et al. New techniques to characterise the vaginal microbiome in pregnancy. AIMS Microbiol. 2016;2(1):55–68. [Mendz GL, of Medicine Sydney The University of Notre Dame Australia Oxford St Darlinghurst NSW Australia S School, Kaakoush NO, et al. New techniques to characterise the vaginal microbiome in pregnancy[J]. AIMS Microbiol, 2016, 2(1): 55-68.] [Google Scholar]
- 15.谢 幸, 孔 北华, 段 涛, et al. 妇产科学. 北京: 人民卫生出版社; 2018. pp. 154–6. [谢幸, 孔北华, 段涛, 等.妇产科学[M]. 9版, 北京:人民卫生出版社, 2018: 154-6] [Google Scholar]
- 16.Altoparlak U, Kadanali A, Kadanali S. Genital flora in pregnancy and its association with group B streptococcal colonization. Int J Gynaecol Obstet. 2004;87(3):245–6. doi: 10.1016/j.ijgo.2004.08.006. [Altoparlak U, Kadanali A, Kadanali S. Genital flora in pregnancy and its association with group B streptococcal colonization[J]. Int J Gynaecol Obstet, 2004, 87(3): 245-6.] [DOI] [PubMed] [Google Scholar]
- 17.Kubota T, Nojima M, Itoh S. Vaginal bacterial flora of pregnant women colonized with group B Streptococcus. J Infect Chemother. 2002;8(4):326–30. doi: 10.1007/s10156-002-0190-x. [Kubota T, Nojima M, Itoh S. Vaginal bacterial flora of pregnant women colonized with group B Streptococcus[J]. J Infect Chemother, 2002, 8(4): 326-30.] [DOI] [PubMed] [Google Scholar]
- 18.王艳.妊娠期阴道微生物群落的分布特点与GDM孕妇妊娠结局的相关性分析[D].广州: 南方医科大学, 2016.
- 19.DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci USA. 2015;112(35):11060–5. doi: 10.1073/pnas.1502875112. [DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy[J]. Proc Natl Acad Sci USA, 2015, 112(35): 11060-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450–4. doi: 10.1016/j.micinf.2006.01.003. [Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial[J]. Microbes Infect, 2006, 8(6): 1450-4.] [DOI] [PubMed] [Google Scholar]
- 21.Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 2017;117(6):804–13. doi: 10.1017/S0007114517000289. [Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial[J]. Br J Nutr, 2017, 117(6): 804-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui MJ, Qi C, Yang LP, et al. A pregnancy complication-dependent change in SIgA-targeted microbiota during third trimester. Food Funct. 2020;11(2):1513–24. doi: 10.1039/c9fo02919b. [Cui MJ, Qi C, Yang LP, et al. A pregnancy complication-dependent change in SIgA-targeted microbiota during third trimester[J]. Food Funct, 2020, 11(2): 1513-24.] [DOI] [PubMed] [Google Scholar]
- 23.Azagra-Boronat I, Tres A, Massot-Cladera M, et al. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota. Cells. 2020;9(3):E575. doi: 10.3390/cells9030575. [Azagra-Boronat I, Tres A, Massot-Cladera M, et al. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota[J]. Cells, 2020, 9(3): E575.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CN, Hou CY, Chan JYH, et al. Hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy. Nutrients. 2019;11(12):E2908. doi: 10.3390/nu11122908. [Hsu CN, Hou CY, Chan JYH, et al. Hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy[J]. Nutrients, 2019, 11(12): E2908.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller EA, Beasley DE, Dunn RR, et al. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front Microbiol. 2016;7:1936. doi: 10.3389/fmicb.2016.01936. [Miller EA, Beasley DE, Dunn RR, et al. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique[J]? Front Microbiol, 2016, 7: 1936.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirmonsef P, Hotton AL, Gilbert D, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS One. 2014;9(7):e102467. doi: 10.1371/journal.pone.0102467. [Mirmonsef P, Hotton AL, Gilbert D, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH[J]. PLoS One, 2014, 9(7): e102467.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Yoo SM, Sohn YH, et al. Predominant Lactobacillus species types of vaginal microbiota in pregnant Korean women: quantification of the five Lactobacillus species and two anaerobes. J Matern Fetal Neonatal Med. 2017;30(19):2329–33. doi: 10.1080/14767058.2016.1247799. [Kim JH, Yoo SM, Sohn YH, et al. Predominant Lactobacillus species types of vaginal microbiota in pregnant Korean women: quantification of the five Lactobacillus species and two anaerobes[J]. J Matern Fetal Neonatal Med, 2017, 30(19): 2329-33.] [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto A, Yamagishi Y, Miyamoto K, et al. Characterization of the vaginal microbiota of Japanese women. Anaerobe. 2018;54:172–7. doi: 10.1016/j.anaerobe.2018.10.001. [Matsumoto A, Yamagishi Y, Miyamoto K, et al. Characterization of the vaginal microbiota of Japanese women[J]. Anaerobe, 2018, 54: 172-7.] [DOI] [PubMed] [Google Scholar]
- 29.Yang SW, Reid G, Challis JRG, et al. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillusreuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients. 2020;12(2):E368. doi: 10.3390/nu12020368. [Yang SW, Reid G, Challis JRG, et al. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillusreuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women[J]. Nutrients, 2020, 12(2): E368.] [DOI] [PMC free article] [PubMed] [Google Scholar]


