Abstract
Simple Summary
The incidence of prostate cancer (PC) is statistically biased due to the increase in prostate-specific antigen (PSA) screening and the accuracy of national cancer registration systems. However, studies on latent PC provide less biased information. This comprehensive review included studies evaluating latent PC in several countries. The prevalence of latent PC has been stable since 1950 in Western countries, but it has increased over time in Asian countries. Latent PC in Asian men has increased in prevalence and is higher in grade. This increase occurred not only due to the increase in PSA screening, but also due to increasing adoption of a Westernized lifestyle. Racial differences between Caucasian and Asian men may also explain the tumor location of latent PC. The autopsy findings in patients with latent PC included a significant proportion of high grade and stage cancers, suggesting a need to reconsider the definition of clinically insignificant PC.
Abstract
The incidence of prostate cancer (PC) has been increasing in Asian countries, where it was previously low. Although the adoption of a Westernized lifestyle is a possible explanation, the incidence is statistically biased due to the increase in prostate-specific antigen (PSA) screening and the accuracy of national cancer registration systems. Studies on latent PC provide less biased information. This review included studies evaluating latent PC in several countries after excluding studies using random or single-section evaluations and those that did not mention section thickness. The findings showed that latent PC prevalence has been stable since 1950 in Western countries, but has increased over time in Asian countries. Latent PC in Asian men has increased in both prevalence and number of high-grade cases. Racial differences between Caucasian and Asian men may explain the tumor location of latent PC. In conclusion, the recent increase in latent PC in Asian men is consistent with an increase in clinical PC. Evidence suggests that this increase is caused not only by the increase in PSA screening, but also by the adoption of a more Westernized lifestyle. Autopsy findings suggest the need to reconsider the definition of clinically insignificant PC.
Keywords: latent cancer, prostate cancer, autopsy
1. Introduction
The incidence of prostate cancer (PC) has been increasing globally in recent years. It is the second most frequently diagnosed cancer and the fifth leading cause of cancer-related deaths among men worldwide [1]. The incidence of PC in recent decades has been heavily influenced by the emergence of prostate-specific antigen (PSA) testing. The availability of PSA testing from the middle to the late 1980s led to the intensive use of the test for screening, with a subsequent rapid increase in the incidence rate in Western countries. This trend has also been growing in Asian countries, where the incidence of PC was previously low [1,2]. The cause of this increase in Asian countries is thought to be multifactorial. Although the spread of PSA screening may be a major cause, changes in lifestyle due to more Westernized diets might be another [3,4]. The accuracy of national cancer registration systems may also influence the incidence, as national cancer registration has not been developed in some Asian countries. However, PC mortality has been decreasing in many Western countries, possibly linked to earlier diagnosis due to PSA screening and improved treatment. In contrast, PC mortality is increasing in several Asian and developing countries [1]. These reports may support the influence of changes in risk factors due to more Westernized lifestyles in such countries.
Studies on latent PC provide less biased information about PC incidence compared to studies on clinical PC. Latent PC is defined as PC that is first detected in autopsy without any clinical signs of PC during the patient’s lifetime. Since Mintz and Smith first reported latent PC in 13% of 100 autopsied cases in 1934 [5], many studies have been reported globally. A recent meta-analysis of 29 studies from 1948 to 2013 indicated that while the prevalence of latent PC significantly increases with age, there is no obvious time trend [6]. However, the time trend of latent PC prevalence might differ among countries. For example, a more recent study indicated that the prevalence of latent PC in Japanese men has been increasing [7]. In addition, a prospective study comparing latent PC in Asian and Caucasian men indicated that the prevalence in Asian men did not differ significantly from that in Caucasian men [8]. These results suggest that not only recent efforts for early detection, such as PSA screening, but also the change to Westernized diets and lifestyles may have influenced the increase in PC in Asian countries.
Information from studies on latent PC provides important insights from a different viewpoint. This review comprehensively discusses the results of latent PC studies in Western and Asian countries.
2. Potential Biases in Methodology in Latent PC Studies
As the methodology for latent PC studies has not yet been standardized and there are several biases between the studies, careful evaluation of their methods is required for precise interpretation. First, study populations, subject sources, and inclusion criteria differed among the studies. While most of the studies involved autopsies performed in hospitals, other studies assessed forensic autopsies. In addition, some studies have analyzed databases of institutional autopsy records or national or regional autopsy registries. However, a meta-analysis indicated that the source of subjects (population vs. hospital-based) was not significantly associated with the prevalence of latent PC [6]. Age was significantly associated with latent PC prevalence, which increased with each decade of age [6]. Thus, the inclusion and exclusion criteria of age and its distribution significantly influenced the prevalence of latent PC. Race is another major factor that affects the prevalence of latent PC. These results must be presented separately in studies that include various races. The methods of sample preparation also differed among the studies. The time elapsed from death to autopsy, step-sectioning versus random and/or single-section evaluation, and the interval between sections in step sectioning can also influence the prevalence of latent PC. The prevalence was reportedly higher in step-sectioned tissues than in randomly divided tissues [9], whereas there was no evidence that the section thickness or delay of autopsy affect PC prevalence [6]. However, information regarding the delay of autopsies is limited in most studies. The methods of diagnosis such as central review or not and use of immunohistochemical evaluation may also differ among studies, although a meta-analysis concluded that the use of immunohistochemistry was not associated with PC prevalence [6].
3. Prevalence of Latent PC in Western Countries
The most important topic in autopsy studies was the prevalence of latent PC. After the first report by Mintz and Smith in 1934, many such studies have been conducted in Western countries [5]. Studies evaluating latent PC by step-sectioning of the prostate in the US and Europe are listed in Table 1 [8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Studies using random or single-section evaluations and those that did not mention section thickness were excluded. Cohorts of different nationalities or races are listed separately even if they were reported within the same study. The prevalence of latent PC varied from 9.6% to 58.6% between the studies. The ranges and distributions of age also varied between the studies. For example, some studies included men under 20 years of age [25,29], while another study included only men older than 70 years [15]. Age influenced the prevalence of latent PC, as it was one of the most significant factors associated with prevalence [6,34]. A recent meta-analysis of 29 studies reported an estimated mean cancer prevalence at age < 30 years of 5% (95% confidence interval (CI): 3–8%), which increased nonlinearly to 59% (95% CI: 48–71%) by age > 79 years [6]. Race is another factor that affects prevalence. Six studies in the US reported the prevalence of latent PC in Caucasian and Black men separately [15,22,23,25,26,33], with reported prevalence rates of 25.9–58.6% and 19.4–43.3%, respectively. All four studies that conducted statistical analyses on the prevalence of latent PC in Caucasian and Black men concluded that racial differences did not exist. However, these results require careful interpretation, as age distributions may differ between these races, especially in forensic studies. A recent review of 19 studies including 6,024 autopsies suggested a racial difference in latent PC prevalence between Caucasian and Black men (35.7% vs. 50.5%), but did not conduct a statistical analysis [34].
Table 1.
Studies evaluating latent PC by step-sectioning of prostate in the US and Europe.
| Author | Year Published | Country/Ethnicity | Duration of Study | Study Population | No. of Cases | Age | No. of Cancers | % | Pathology Section Width (mm) | Ref. No. |
|---|---|---|---|---|---|---|---|---|---|---|
| Moore | 1935 | Austria | 1931–1932 | Hospital | 304 | Range, 21–90 | 52 | 16.7 | 4 | [10] |
| Andrews | 1949 | UK | NA | Hospital | 142 | Range, 40–79 | 17 | 12.0 | 4 | [11] |
| Edwards | 1953 | Canada | 1942–1945 | Hospital | 173 | Mean, 64.1 | 35 | 16.7 | 4 | [12] |
| Franks | 1954 | US | NA | Forensic | 220 | NA | 69 | 31.4 | 4 | [13] |
| Viitanen | 1958 | Finland | NA | Hospital | 100 | ≥50 | 22 | 22.0 | 5 | [14] |
| Halpert | 1965 | US/Black | NA | Hospital | 30 | Range, 70–79 | 13 | 43.3 | 4 | [15] |
| Halpert | 1965 | US/Caucasian | NA | Hospital | 70 | Range, 70–79 | 41 | 58.6 | 4 | [15] |
| Liavag | 1968 | Norway | NA | Hospital | 340 | ≥40 | 90 | 26.5 | 4 | [16] |
| Lundberg | 1970 | Sweden | 1967 | Hospital | 292 | NA | 116 | 39.7 | 5 | [17] |
| Harbitz | 1973 | Norway | 1967–1968 | Hospital | 172 | ≥40 | 54 | 31.4 | 4–6 | [18] |
| Akazaki | 1973 | US/men of Japanese ancestry | 1969–1972 | Hospital | 158 | ≥50 | 46 | 29.1 | 3 | [19] |
| Breslow | 1977 | Germany | NA | Hospital | 145 | Mean, 65 | 43 | 29.7 | 5 | [20] |
| Breslow | 1977 | Israel | NA | Hospital | 143 | Mean, 65 | 32 | 22.4 | 5 | [20] |
| Breslow | 1977 | Sweden | NA | Hospital | 306 | Mean, 65 | 123 | 40.2 | 5 | [20] |
| Hølund | 1980 | Denmark | 1971–1977 | Hospital | 223 | Range, 36–94 | 57 | 25.6 | 3 | [21] |
| Gulleyardo | 1980 | US/Black | NA | Hospital | 207 | NA | 65 | 31.4 | 3 | [22] |
| Gulleyardo | 1980 | US/Caucasian | NA | Hospital | 293 | NA | 85 | 29 | 3 | [22] |
| Yatani | 1982 | Colombia | 1967–1970 | Hospital | 182 | Mean, 64.4 | NA | 31.5 | 3 | [23] |
| Yatani | 1982 | US/Black | 1969–1978 | Hospital | 178 | Mean, 63.6 | NA | 36.9 | 3 | [23] |
| Yatani | 1982 | US/Caucasian | 1969–1978 | Hospital | 253 | Mean, 63.2 | NA | 34.6 | 3 | [23] |
| Yatani | 1982 | US/men of Japanese ancestry | 1969–1978 | Hospital | 417 | Mean, 70.1 | NA | 25.6 | 3 | [23] |
| Stemmermann | 1992 | US/men of Japanese ancestry | 1970–1990 | Hospital | 293 | Mean, 67.9 | 80 | 27.3 | 3 | [24] |
| Sakr | 1993 | US/Black | NA | Forensic | 98 | 10–50 | 19 | 19.4 | 3–4 | [25] |
| Sakr | 1993 | US/Caucasian | NA | Forensic | 54 | 10–50 | 14 | 25.9 | 3–4 | [25] |
| Brawn | 1996 | US/Black | NA | Hospital | 15 | ≥50 | 5 | 33.3 | 3 | [26] |
| Brawn | 1996 | US/Caucasian | NA | Hospital | 89 | ≥50 | 39 | 43.8 | 3 | [26] |
| Sanchez-Chapado | 2003 | Spain | NA | Forensic | 146 | Mean, 48.5 | 27 | 18.5 | 3-4 | [28] |
| Soos | 2005 | Hungary | NA | Hospital | 139 | 18–95 | 54 | 38.8 | 4 | [29] |
| Stamtiou | 2007 | Greece | 2002–2004 | Hospital | 212 | ≥30 | 40 | 18.8 | 4 | [30] |
| Haas | 2007 | US (92% Caucasian) | NA | Hospital | 164 | Median, 64 | 47 | 28.7 | 4 | [31] |
| Polat | 2009 | Turkey | NA | Hospital | 114 | Mean, 55 | 11 | 9.6 | 4 | [32] |
| Powell | 2010 | US/Black | 1993–2004 | Forensic | 630 | 20–79 | NA | 35.1 | 2.5 | [33] |
| Powell | 2010 | US/Caucasian | 1993–2004 | Forensic | 426 | 20–79 | NA | 48.1 | 2.5 | [33] |
| Zlotta | 2013 | Russia | 2008–2011 | Hospital | 220 | Mean, 62.5 | 82 | 37.3 | 4 | [8] |
NA: not available.
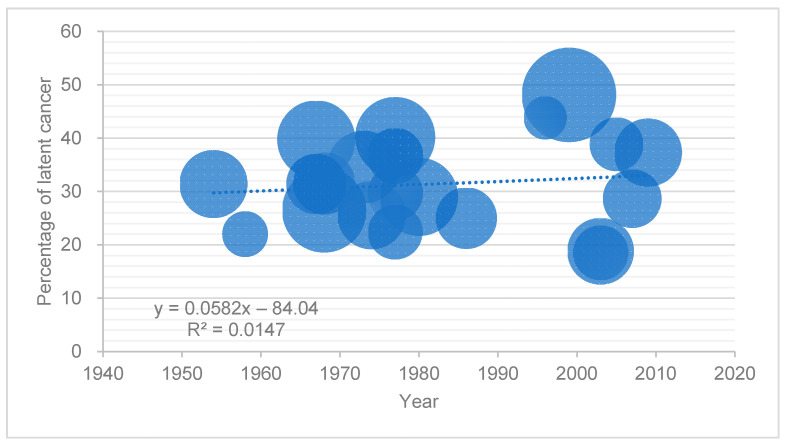
Figure 1 shows the prevalence of latent PC in studies of Caucasians in the US and Europe published after 1950 by year of publication. The size of each point was proportional to the number of men included in each study. The analytic linear approximation line of the datapoints indicated that the latent PC prevalence was stable over time. The spread of PSA screening programs is thought to have increased the diagnosis of insignificant PC and decreased the prevalence of latent PC. However, few studies have examined the changes in the prevalence of latent PC before and after the PSA era. A retrospective study using an autopsy record database from a single institution in the US reported that the prevalence of latent PC decreased three-fold with the widespread use of PSA screening [35]. In this study, the prevalence was 4.8% in men older than 40 years between 1955 and 1960, compared to 1.2% between 1991 and 2001. However, this study was limited by the lack of whole-mount sections to examine the prostate, which might lead to a lower prevalence compared to those in other autopsy studies using step-sectioning. However, the prevalence of latent PC in Japan has increased despite the spread of PSA screening, although the exposure rate of PSA testing in Asian countries is still lower than that in Western countries [2,7]. The trends in Asian countries are discussed in Section 4.
Figure 1.
Prevalence of latent PC in studies of Caucasians in the US and Europe.
The prevalence of latent PC in studies of US and European Caucasians published after 1950 by year of publication. The size of each point was proportional to the number of men included in each study.
4. The prevalence of Latent PC in Asian and Other Countries
Studies investigating latent PC are fewer in Asian countries than in Western countries. In 1937, Yotsuyanagi et al. first reported a 3% prevalence of latent PC in Japanese men in a domestic journal [36]. Among the literature published in international journals, in 1961, Karube first reported a latent PC prevalence of 10.9% in Japanese men older than 40 years by step-sectioning [36]. Studies on latent PC in Asia and Africa are listed in Table 2 [7,8,19,20,23,36,37,38,39,40,41,42,43,44]. Studies using random or single-section evaluations that were not published in English were excluded. Some results are part of a multinational study. Most studies in Asia were from Japan [7,8,19,23,36,38,39,40], with the exception of two studies from Singapore [20,37] and one each from China [41], Hong Kong [20], and Iran [42]. Reports from other regions include Jamaica in Latin America and Uganda in Africa as part of a multinational study in 1977 [20]. The prevalence of latent PC in Jamaica and Uganda was 32.7% and 24.0%, respectively, which were higher than those in Asian countries in the same reports (15.0% and 14.5% in Hong Kong and Singapore, respectively). In addition, the mean age of men in Uganda was 58.3 years, which was 5 years younger than of those in other countries (64.5 in Singapore, 63.4 in Hong Kong, and 63.2 in Jamaica). Updated data for prevalence in Latin America and Africa are required.
Table 2.
Studies evaluating latent PC by step-sectioning of prostate in Asian and other countries.
| Author | Year Published | Country/Ethnicity | Duration of Study | Study Population | No. of Cases | Age | No. of Cancers | % | Pathology Section Width (mm) | Ref. No. |
|---|---|---|---|---|---|---|---|---|---|---|
| Karube | 1961 | Japan | 1954–1959 | Hospital | 229 | ≥40 | 25 | 10.9 | 4–5 | [36] |
| Lee | 1972 | Singapore | NA | Hospital | 156 | Range, 42–87 | 13 | 8.3 | 4 | [37] |
| Akazaki | 1973 | Japan | 1969–1972 | Hospital | 239 | ≥50 | 47 | 19.7 | 3 | [19] |
| Bean | 1973 | Japan | 1961–1969 | Hospital | 213 | ≥50 | 58 | 27.2 | 5 | [38] |
| Breslow | 1977 | Hong Kong | NA | Hospital | 173 | Mean, 65 | 26 | 15.0 | 5 | [20] |
| Breslow | 1977 | Jamaica | NA | Hospital | 168 | Mean, 65 | 55 | 32.7 | 5 | [20] |
| Breslow | 1977 | Singapore | NA | Hospital | 242 | Mean, 65 | 35 | 14.5 | 5 | [20] |
| Breslow | 1977 | Uganda | NA | Hospital | 150 | Mean, 65 | 36 | 24.0 | 5 | [20] |
| Yatani | 1982 | Japan | 1965–1979 | Hospital | 576 | Mean, 67.7 | NA | 20.5 | 3 | [23] |
| Billis | 1986 | Brazil | NA | Hospital | 180 | Range, 40–88 | 45 | 25.0 | 3–5 | [27] |
| Yatani | 1988 | Japan | 1965–1979 | Hospital | 576 | Mean, 67.6 | NA | 22.5 | 3 | [39] |
| Yatani | 1988 | Japan | 1982–1986 | Hospital | 660 | Mean, 68.7 | NA | 34.6 | 3 | [39] |
| Takahashi | 1992 | Japan | NA | Hospital | 29 | ≥90 | 17 | 58.6 | 3–4 | [40] |
| Gu | 1994 | China | 1989–1992 | Hospital | 381 (including 60 RCP) | NA | 21 | 5.5 | 5 | [41] |
| Zare–Mirzaie | 2012 | Iran | 2008–2009 | Hospital | 149 | Mean, 64.5 | 14 | 9.4 | 4 | [42] |
| Zlotta | 2013 | Japan | 2008–2011 | Hospital | 100 | Mean, 68.5 | 35 | 35.0 | 4 | [8] |
| Kimura | 2016 | Japan | 1983–1987 | Hospital | 501 | Mean, 63.5 | 104 | 20.8 | 5 | [7] |
| Inaba | 2020 | Japan | 2009–2017 | Hospital | 182 | Median, 72 | 71 | 39.0 | 5 | [43] |
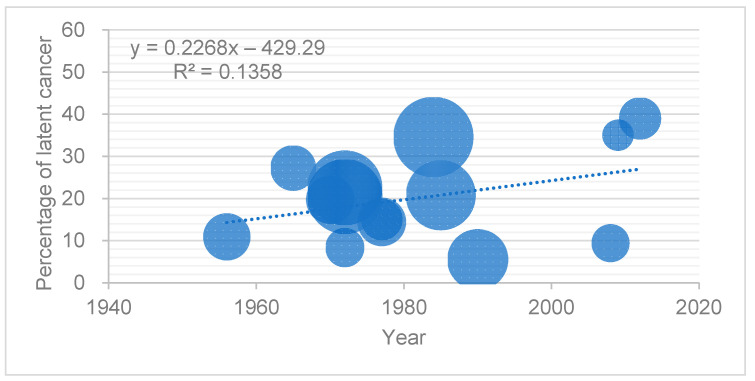
Figure 2 shows the prevalence of latent PC in studies of Asian countries published after 1950. Data from the study conducted by Takahashi et al. in Japan were excluded because they focused on men older than 90 years of age [40]. The size of each point is proportional to the number of men in the study. The analytic linear approximation line of the datapoints indicated that the prevalence of latent PC in Asia increased over time compared to that in the US and Europe (Figure 1). The prevalence was 8.3–27.2% from the 1960s to 1970s, 5.5–34.6% from the 1980s to the 1990s, and 9.4–39.0% after 2000. Two Japanese studies directly compared the time trends in latent PC within the same institutions. Yatani et al. compared the latent PC prevalence within the same institution between men from 1965 to 1979 and from 1982 to 1986, both in pre-PSA era periods. The prevalence increased significantly from 22.5% to 34.6% [39]. More recently, Kimura et al. compared the prevalence of latent PC between Japanese men in pre- and post-PSA eras. The prevalence in men was 20.8% in 1983–1987 and 43.3% in 2008–2013 [7]. Both studies indicated a significant increase in higher-grade and larger cancers. Yatani et al. reported a higher rate of infiltrative tumors in the cohort in 1965–1979 than in 1982–1986, at 9.8% and 17.8%, respectively [39]. Kimura et al. reported a significantly larger index cancer volume in men in 2008–2013 compared to that in 1983–1987 [7].
Figure 2.
Prevalence of latent PC in the studies on Asian populations. Prevalence of latent PC in studies of Asian countries published after 1950 by year of publication. The size of each point was proportional to the number of men in the study.
The increased prevalence of latent PC in Asian men is consistent with the increased prevalence of clinical PC [45]. A major explanation for the increase in latent PC in Asian countries may be lifestyle changes due to more Westernized diets. The incidence of clinical PC in US men of Japanese ancestry in 1973–1986 was between that of Caucasians in the US and Japanese men born in Japan within the same period, suggesting the influence of both genetic and lifestyle factors on PC incidence [46]. In contrast, a comparative study published in 1973 showed that the age-adjusted prevalence of latent PC did not differ significantly between Japanese men in Japan and those in Hawaii (20.5% and 26.7%, respectively). However, the age-adjusted prevalence of the proliferative type of latent PC was higher in Japanese Hawaiians than in native Japanese (19.1% and 8.7%, respectively) [19].
Zlotta et al. prospectively compared the prevalence of latent PC in 100 Japanese men and 220 Russian men [8]. The prevalence was 35.0% and 37.3% in Japanese and Russian men, respectively, and did not differ significantly. However, Japanese men had a greater probability of having a PC Gleason score (GS) of 7 than Russian men after adjusting for age and prostate weight. These results suggest the increasing prevalence and grade of latent PC in Asian men over the past few decades.
5. Pathological Findings from Latent PC
Although latent PC does not cause clinical symptoms and is not generally detected during the lifetime, most studies showed that a significant proportion of latent PC had high-grade, capsular, or seminal vesicle invasion. In studies in the US and Europe, 5.43% of cancers were GS 7 or greater and 11–13% were pT3 or greater [8,27,29,30,31]. In contrast, in Asian studies, 35.7–51.4% of latent PC was GS 7 or higher, with proportions higher than those in Western reports, although the proportion of cancers with pT3 or greater was similar (11.5–12.7%) [7,8,42]. Consequently, these cases of latent PC included clinically significant cancer as defined by Epstein (the presence of T3 or greater and/or index tumor volume of 500 mm3 or greater and/or GS ≥ 7 [47]). A prospective comparative study by Zlotta et al. reported that 29.3% and 51.4% of latent PC cases were clinically significant in Russian and Japanese men, respectively [8]. A comparative study of contemporary latent PC and historical controls in Japan reported an index cancer volume of 500 mm3 in 9.6% of cancers in men in 1983–1987 and 25.5% in 2008–2013, a significant difference [7]. The increase in proportion of significant cancer in latent PC notwithstanding the spread of PSA screening might suggest an increase in high-grade cancer in Asian countries, especially in Japan. These results also suggest the need to reexamine the definition of clinically insignificant PC. Stamey et al. defined clinically significant PC as organ-confined tumors of <0.5 cm3, GS 3+3 with no grade 4 or 5 [48]. However, it can also be defined as a cancer that does not affect the patient during the natural course of his lifetime. The requirements of Stamey’s definition may be too stringent.
Investigating the tumor location of latent PC could improve our understanding of the origin of PC and how it grows [49]. Racial differences between Caucasian and Asian men have been suggested to affect not only the prevalence, but also the tumor location of PC. A comparative study of radical prostatectomy specimens reported that 35.5% and 0.6% of PCs originated in the transition zone (TZ) in Japanese men and US men, respectively [50]. Studies that categorized tumor location into anterior or posterior regions reported that anterior cancer was more prevalent in Asian men than in Caucasian men [50,51,52,53,54,55,56]. However, most studies evaluating tumor location in the prostate have analyzed only prostatectomy specimens. The tumor location of the prostatectomy specimens may overestimate the prevalence in the peripheral zone (PZ) or posterior cancer because of its higher detectability by digital rectal examination and transrectal prostate biopsy compared to in the TZ or anterior cancer. In this sense, the tumor location in latent PC may be less biased. There are limited reports regarding tumor location in latent PC. An autopsy study in Hungary including 139 men aged 18–95 years reported a latent PC prevalence of 38.8%; among the 64 tumor foci, 82.8% and 18.9% were present in the PZ and TZ, respectively [29]. Another study in the US including 164 men aged 54–73 years reported that latent PC was present in 29% of the cases, with 62% and 36% of PCs located in the posterior and anterior regions, respectively, and 77% and 16%—in the PZ and central zone in the prostate, respectively [31].
Reports on tumor locations of latent PC in Asian men are limited. A report of 149 autopsies of Iranian men over 50 years of age detected invasive adenocarcinoma in 14 (9.4%) cases, including nine cases (64%) in the posterior region, one case (7%) in the anterior region, and four cases (29%) in both lobes of the prostate [42]. A report of 182 Japanese men observed latent PC in 39.0% of cases, occurring in the TZ, PZ, or without dominance in 38.0%, 57.8%, and 4.2% of cases, respectively [43]. The tumors were located in the anterior and posterior regions in 49.3% and 40.8% of the cases, respectively. Approximately 40% of the tumors were located in the TZ and anterior region of the prostate, a rate higher than that reported in Western studies. The age distribution also differed between TZ and PZ cancers. In elderly men, cancer is more frequently diagnosed in the PZ than in the TZ [43]. This was consistent with the report by Takahashi et al. on autopsies in men over 90 years of age, which revealed that all latent PCs were localized in the PZ of the prostate [40]. An autopsy study in the US reported that most TZ cancers showed a different pathological pattern from that of PZ cancers, with lower GS and less aggressiveness [57]. However, a Japanese study reported that the pathological features did not differ between the TZ and PZ and between anterior and posterior cancers in terms of GS, tumor volume, or prevalence of clinically significant cancer; however, there is variation in the pT stage—PZ cancer has a significantly higher pT stage than TZ cancer. Several anatomical explanations have been proposed to explain this difference. For example, the TZ is separated from the surrounding area by fibromuscular tissue, whereas no such structure exists in the PZ. Moreover, the TZ contacts with the prostate capsule from the outside to the back of the PZ, and T3b cases of the TZ are few because of the anatomical position [43]. However, a prospective comparative study of Japanese and Russian autopsy cases reported similar tumor locations between cohorts, in which latent PC was located in the TZ in 25.9% and 20.7% and in the anterior region in 20.0% and 21.9% of the cases in Japanese and Russian men, respectively. Further investigation is required to determine whether there is a racial difference in tumor location and whether the location in Asian men has changed due to Western diet and lifestyle.
Few studies have investigated tumor location in the vertical direction. In their international multicenter study investigating 1327 autopsies from seven counties or regions, Breslow et al. reported latent PC in 350 cases. In the vertical direction, more tumors were present at the middle and apex levels than at the base. However, the evaluation method to describe the tumor distribution has not yet been standardized, and further studies are warranted.
Most latent PC cases represent the less aggressive forms of PC. Thus, comparing molecular markers or genomic aberrations between latent and clinical PCs is an ideal method to investigate their effects. Igawa et al. reported a significantly higher nm23-HI gene expression level in clinical PC than in normal prostatic tissues, latent PC, and clinical PC [58]. Watanabe et al. reported that Ras gene mutations in latent PC varied among ethnic groups and that the frequency in Japanese men was higher than that in US Black or Caucasian men [59]. Alipov et al. compared the expression of the ETS1 proto-oncogene in latent PC, benign prostatic hyperplasia, normal prostatic tissues, and clinical PC [60], reporting negative expression in benign tissues and higher levels in clinical PC than in latent PC. Maekawa et al. investigated the TMPRSS2 Met160Val polymorphism in Japanese men, including 518 men with sporadic PC, 433 healthy controls, and 154 men with latent PC [61]. The TMPRSS2 Met160Val polymorphism is a genetic risk factor for sporadic PC but not for latent PC in the Japanese population. However, molecular studies using latent PC are limited, possibly because of the limited quality and availability of latent PC specimens.
6. Limitations of Autopsy Studies
Latent PC has a unique cancer status compared to the malignancies of other origins. Although it has been investigated for a long time, several problems remain to be solved. Section 5 described variations in the methodologies used for sample preparation and diagnosis. Although step-sectioning of the whole prostate is a standard method, the duration from death to autopsy is difficult to control. A central review for diagnosis is mandatory because of inter- and intra-observer variability for the pathological diagnosis of PC [62]. A fundamental bias also exists in autopsy studies. As the subjects were men who died in the hospital, their backgrounds differed from those of healthy men. Inaba et al. reported that 106 of the 182 autopsy cases (58.2%) had been performed due to death of malignancy other than PC (unpublished data).
The available data on latent PC were provided by a limited number of countries and regions. Most studies were from North America, Western Europe, and Japan, whereas data from Africa and Latin America are limited. More importantly, the number of autopsies has been steadily declining over the past 30–40 years worldwide [63]. After the 2000s, the autopsy rate was only 7–9% in the US, compared to approximately 25–35% in the mid-1960s and 50% of all hospital deaths in the 1940s and 1950s [64,65]. In Japan, more than 40,000 autopsies were performed in 1985, but this number had gradually decreased to approximately 10,000 by 2018 [66].
One explanation for the limited number of studies evaluating molecular markers in latent PC is the low quality of specimens from autopsies. RNA and proteins were extracted during the time between death and autopsy. To overcome such limitations in autopsy studies, rapid autopsies have emerged [67]. In this new methodology, tissues are collected as soon as possible after the patient’s death. Ideally, the quality of a rapid autopsy tissue can be considered comparable to the quality of a fresh surgical biopsy tissue.
7. Learning from Latent PC and Future Directions
While latent PC studies have a long history, the available evidence remains limited. Latent PC studies have revealed a larger prevalence of insignificant PC than the incidence of clinical PC. PC prevalence increases with age and more than half of both Caucasian and Asian men over 80 years of age have indolent PC. The recent increase in latent PC in Asian men is consistent with an increase in clinical PC in Asian countries. These findings suggest that this increase in clinical PC in Asian countries is due not only to the spread of PSA screening, but also to the adoption of Westernized lifestyles.
In addition, the results of autopsy studies suggest the need to reconsider the definition of clinically insignificant PC, which is thought to be an ideal candidate for active surveillance. The present definition might be too strict, as latent PC included a significant proportion of cancer cases thought to be life-threatening, such as with GS ≥ 7 and pT3 or greater. Cancer volume and the percentage of high-grade cancer cases also increased with age. However, the individuals lived without the influence of PC throughout their lives. Molecular analyses are required in latent PC studies to distinguish between indolent and life-threatening PC. Methodologies such as rapid autopsies have opened the door for new studies of latent PC.
Author Contributions
Writing—original draft preparation, T.K.; writing—review and editing, S.S., H.T., and S.E. Authorship must be limited to those who have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this study.
Conflicts of Interest
T.K. is a paid consultant/advisor to Astellas, Bayer, Janssen, and Sanofi. S.E. is a paid consultant/advisor to Takeda, Astellas, AstraZeneca, Sanofi, Janssen, and Pfizer. The funders had no role in the study design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ito K. Prostate cancer in asian men. Nat. Rev. Urol. 2014;11:197–212. doi: 10.1038/nrurol.2014.42. [DOI] [PubMed] [Google Scholar]
- 3.Yoshita K., Arai Y., Nozue M., Komatsu K., Ohnishi H., Saitoh S., Miura K., Group N.D.R. Total energy intake and intake of three major nutrients by body mass index in japan: Nippon data80 and nippon data90. J. Epidemiol. 2010;20(Suppl. S3):S515–S523. doi: 10.2188/jea.JE20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki M., Mameri C.P., Hamada G.S., Tsugane S. Cancer mortality among japanese immigrants and their descendants in the state of sao paulo, brazil, 1999–2001. JPN J. Clin Oncol. 2004;34:673–680. doi: 10.1093/jjco/hyh123. [DOI] [PubMed] [Google Scholar]
- 5.Mintz E.R., Smith G.G. Autopsy findings in 100 cases of prostatic cancer. N. Engl. J. Med. 1934;211:479–487. doi: 10.1056/NEJM193409132111101. [DOI] [Google Scholar]
- 6.Bell K.J., Del Mar C., Wright G., Dickinson J., Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer. 2015;137:1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura T., Takahashi H., Okayasu M., Kido M., Inaba H., Kuruma H., Yamamoto T., Furusato B., Furusato M., Wada T., et al. Time trends in histological features of latent prostate cancer in japan. J. Urol. 2016;195:1415–1420. doi: 10.1016/j.juro.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 8.Zlotta A.R., Egawa S., Pushkar D., Govorov A., Kimura T., Kido M., Takahashi H., Kuk C., Kovylina M., Aldaoud N., et al. Prevalence of prostate cancer on autopsy: Cross-sectional study on unscreened caucasian and asian men. J. Natl. Cancer Inst. 2013;105:1050–1058. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]
- 9.Rebbeck T.R., Haas G.P. Temporal trends and racial disparities in global prostate cancer prevalence. Can. J. Urol. 2014;21:7496–7506. [PMC free article] [PubMed] [Google Scholar]
- 10.Moore R.A. The morphology of small prostatic carcinoma. J. Urol. 1935;33:224–234. doi: 10.1016/S0022-5347(17)72261-6. [DOI] [Google Scholar]
- 11.Andrews G.S. Latent carcinoma of the prostate. J. Clin. Pathol. 1949;2:197–208. doi: 10.1136/jcp.2.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards C.N., Steinthorsson E., Nicholson D. An autopsy study of latent prostatic cancer. Cancer. 1953;6:531–554. doi: 10.1002/1097-0142(195305)6:3<531::AID-CNCR2820060311>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Franks L.M. Latent carcinoma. Ann. R Coll. Surg. Engl. 1954;15:236–249. [PMC free article] [PubMed] [Google Scholar]
- 14.Viitanen I., Von Hellens A. Latent carcinoma of the prostate in finland; preliminary report. Acta Pathol. Microbiol. Scand. 1958;44:64–67. doi: 10.1111/j.1699-0463.1958.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 15.Halpert B., Schmalhorst W.R. Carcinoma of the prostate in patients 70 to 79 years old. Cancer. 1966;19:695–698. doi: 10.1002/1097-0142(196605)19:5<695::AID-CNCR2820190515>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Liavag I. The localization of prostatic carcinoma. An autopsy study. Scand. J. Urol. Nephrol. 1968;2:65–71. doi: 10.3109/00365596809136971. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg S., Berge T. Prostatic carcinoma. An autopsy study. Scand. J. Urol. Nephrol. 1970;4:93–97. doi: 10.3109/00365597009137581. [DOI] [PubMed] [Google Scholar]
- 18.Harbitz T.B. Testis weight and the histology of the prostate in elderly men. An analysis in an autopsy series. Acta Pathol. Microbiol. Scand. A. 1973;81:148–158. doi: 10.1111/j.1699-0463.1973.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 19.Akazaki K., Stemmerman G.N. Comparative study of latent carcinoma of the prostate among japanese in Japan and Hawaii. J. Natl. Cancer Inst. 1973;50:1137–1144. doi: 10.1093/jnci/50.5.1137. [DOI] [PubMed] [Google Scholar]
- 20.Breslow N., Chan C.W., Dhom G., Drury R.A., Franks L.M., Gellei B., Lee Y.S., Lundberg S., Sparke B., Sternby N.H., et al. Latent carcinoma of prostate at autopsy in seven areas. The international agency for research on cancer, lyons, france. Int. J. Cancer. 1977;20:680–688. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 21.Holund B. Latent prostatic cancer in a consecutive autopsy series. Scand. J. Urol. Nephrol. 1980;14:29–35. doi: 10.3109/00365598009181186. [DOI] [PubMed] [Google Scholar]
- 22.Guileyardo J.M., Johnson W.D., Welsh R.A., Akazaki K., Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. J. Natl. Cancer Inst. 1980;65:311–316. doi: 10.1093/jnci/65.2.311. [DOI] [PubMed] [Google Scholar]
- 23.Yatani R., Chigusa I., Akazaki K., Stemmermann G.N., Welsh R.A., Correa P. Geographic pathology of latent prostatic carcinoma. Int. J. Cancer. 1982;29:611–616. doi: 10.1002/ijc.2910290602. [DOI] [PubMed] [Google Scholar]
- 24.Stemmermann G.N., Nomura A.M., Chyou P.H., Yatani R. A prospective comparison of prostate cancer at autopsy and as a clinical event: The Hawaii Japanese experience. Cancer Epidemiol. Biomark. Prev. 1992;1:189–193. [PubMed] [Google Scholar]
- 25.Sakr W.A., Haas G.P., Cassin B.F., Pontes J.E., Crissman J.D. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J. Urol. 1993;150:379–385. doi: 10.1016/S0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 26.Brawn P.N., Jay D.W., Foster D.M., Kuhl D., Speights V.O., Johnson F.H., Riggs M., Lind M.L., Coffield K.S., Weaver B. Prostatic acid phosphatase levels (enzymatic method) from completely sectioned, clinically benign, whole prostates. Prostate. 1996;28:295–299. doi: 10.1002/(SICI)1097-0045(199605)28:5<295::AID-PROS4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Billis A. Latent carcinoma and atypical lesions of prostate an autopsy study. Urology. 1986;28:324–329. doi: 10.1016/0090-4295(86)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Chapado M., Olmedilla G., Cabeza M., Donat E., Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in caucasian mediterranean males: An autopsy study. Prostate. 2003;54:238–247. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- 29.Soos G., Tsakiris I., Szanto J., Turzo C., Haas P.G., Dezso B. The prevalence of prostate carcinoma and its precursor in hungary: An autopsy study. Eur. Urol. 2005;48:739–744. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Stamatiou K., Alevizos A., Perimeni D., Sofras F., Agapitos E. Frequency of impalpable prostate adenocarcinoma and precancerous conditions in greek male population: An autopsy study. Prostate Cancer Prostatic Dis. 2006;9:45–49. doi: 10.1038/sj.pcan.4500847. [DOI] [PubMed] [Google Scholar]
- 31.Haas G.P., Delongchamps N.B., Jones R.F., Chandan V., Serio A.M., Vickers A.J., Jumbelic M., Threatte G., Korets R., Lilja H., et al. Needle biopsies on autopsy prostates: Sensitivity of cancer detection based on true prevalence. J. Natl. Cancer Inst. 2007;99:1484–1489. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 32.Polat K., Tüzel E., Aktepe F., Akdoğan B., Güler C., Uzun I. Investigation of the incidence of latent prostate cancer and high-grade prostatic intraepithelial neoplasia in an autopsy series of Turkish males. Turk. J. Urol. 2009;35:96–100. [Google Scholar]
- 33.Powell I.J., Bock C.H., Ruterbusch J.J., Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white american men, and influences racial progression and mortality disparity. J. Urol. 2010;183:1792–1796. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahn J.L., Giovannucci E.L., Stampfer M.J. The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int. J. Cancer. 2015;137:2795–2802. doi: 10.1002/ijc.29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konety B.R., Bird V.Y., Deorah S., Dahmoush L. Comparison of the incidence of latent prostate cancer detected at autopsy before and after the prostate specific antigen era. J. Urol. 2005;174 doi: 10.1097/01.ju.0000177470.84735.55. [DOI] [PubMed] [Google Scholar]
- 36.Karube K. Study of latent carcinoma of the prostate in the japanese based on necropsy material. Tohoku J. Exp. Med. 1961;74:265–285. doi: 10.1620/tjem.74.265. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y.S., Shanmugaratnam K. Latent prostate carcinoma in Singapore Chinese. Singap. Med. J. 1972;13:1–6. [PubMed] [Google Scholar]
- 38.Bean M.A., Yatani R., Liu P.I., Fukazawa K., Ashley F.W., Fujita S. Prostatic carcinoma at autopsy in hiroshima and nagasaki japanese. Cancer. 1973;32:498–506. doi: 10.1002/1097-0142(197308)32:2<498::AID-CNCR2820320231>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 39.Yatani R., Shiraishi T., Nakakuki K., Kusano I., Takanari H., Hayashi T., Stemmermann G.N. Trends in frequency of latent prostate carcinoma in japan from 1965–1979 to 1982–1986. J. Natl. Cancer Inst. 1988;80:683–687. doi: 10.1093/jnci/80.9.683. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi S., Shirai T., Hasegawa R., Imaida K., Ito N. Latent prostatic carcinomas found at autopsy in men over 90 years old. JPN J. Clin. Oncol. 1992;22:117–121. doi: 10.1093/oxfordjournals.jjco.a039525. [DOI] [PubMed] [Google Scholar]
- 41.Gu F.-L., Xia T.-L., Kong X.-T. Preliminary study of the frequency ofbenign prostatic hyperplasia and prostatic cancer in china. Urology. 1994;44:688–691. doi: 10.1016/S0090-4295(94)80207-6. [DOI] [PubMed] [Google Scholar]
- 42.Zare-Mirzaie A., Balvayeh P., Imamhadi M.A., Lotfi M. The frequency of latent prostate carcinoma in autopsies of over 50 years old males, the Iranian experience. Med. J. Islam. Repub. Iran. 2012;26:73–77. [PMC free article] [PubMed] [Google Scholar]
- 43.Inaba H., Kimura T., Onuma H., Sato S., Kido M., Yamamoto T., Fukuda Y., Takahashi H., Egawa S. Tumor location and pathological features of latent and incidental prostate cancer in contemporary japanese men. J. Urol. 2020;204:267–272. doi: 10.1097/JU.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 44.Billis A., Souza C.A.F., Piovesan H. Histologic carcinoma of the prosate in autopsies frequency, origin, extension, grading and terminology. Braz. J. Urol. 2002;28:197–205. [Google Scholar]
- 45.Kimura T., Egawa S. Epidemiology of prostate cancer in asian countries. Int. J. Urol. 2018;25:524–531. doi: 10.1111/iju.13593. [DOI] [PubMed] [Google Scholar]
- 46.Cook L.S., Goldoft M., Schwartz S.M., Weiss N.S. Incidence of adenocarcinoma of the prostate in asian immigrants to the united states and their descendants. J. Urol. 1999;161:152–155. doi: 10.1016/S0022-5347(01)62086-X. [DOI] [PubMed] [Google Scholar]
- 47.Epstein J.I., Walsh P.C., Carmichael M., Brendler C.B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage t1c) prostate cancer. JAMA. 1994;271:368–374. doi: 10.1001/jama.1994.03510290050036. [DOI] [PubMed] [Google Scholar]
- 48.Stamey T.A., Freiha F.S., McNeal J.E., Redwine E.A., Whittemore A.S., Schmid H.P. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71:933–938. doi: 10.1002/1097-0142(19930201)71:3+<933::AID-CNCR2820711408>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y., Yan W. Implications from autopsy studies of latent prostate cancer. Nat. Rev. Urol. 2020;17:428–429. doi: 10.1038/s41585-020-0327-7. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi H., Epstein J.I., Wakui S., Yamamoto T., Furusato B., Zhang M. Differences in prostate cancer grade, stage, and location in radical prostatectomy specimens from united states and japan. Prostate. 2014;74:321–325. doi: 10.1002/pros.22754. [DOI] [PubMed] [Google Scholar]
- 51.Hashine K., Ueno Y., Shinomori K., Ninomiya I., Teramoto N., Yamashita N. Correlation between cancer location and oncological outcome after radical prostatectomy. Int. J. Urol. 2012;19:855–860. doi: 10.1111/j.1442-2042.2012.03041.x. [DOI] [PubMed] [Google Scholar]
- 52.Takashima R., Egawa S., Kuwao S., Baba S. Anterior distribution of stage t1c nonpalpable tumors in radical prostatectomy specimens. Urology. 2002;59:692–697. doi: 10.1016/S0090-4295(02)01525-X. [DOI] [PubMed] [Google Scholar]
- 53.Koppie T.M., Bianco F.J., Jr., Kuroiwa K., Reuter V.E., Guillonneau B., Eastham J.A., Scardino P.T. The clinical features of anterior prostate cancers. BJU Int. 2006;98:1167–1171. doi: 10.1111/j.1464-410X.2006.06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Ahmadie H.A., Tickoo S.K., Olgac S., Gopalan A., Scardino P.T., Reuter V.E., Fine S.W. Anterior-predominant prostatic tumors: Zone of origin and pathologic outcomes at radical prostatectomy. Am. J. Surg. Pathol. 2008;32:229–235. doi: 10.1097/PAS.0b013e31812f7b27. [DOI] [PubMed] [Google Scholar]
- 55.Hossack T., Patel M.I., Huo A., Brenner P., Yuen C., Spernat D., Mathews J., Haynes A.M., Sutherland R., del Prado W., et al. Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J. Urol. 2012;188:781–785. doi: 10.1016/j.juro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Mygatt J., Sesterhenn I., Rosner I., Chen Y., Cullen J., Morris-Gore T., Barton J., Dobi A., Srivastava S., McLeod D., et al. Anterior tumors of the prostate: Clinicopathological features and outcomes. Prostate Cancer Prostatic Dis. 2014;17:75–80. doi: 10.1038/pcan.2013.54. [DOI] [PubMed] [Google Scholar]
- 57.McNeal J.E. Cancer volume and site of origin of adenocarcinoma in the prostate: Relationship to local and distant spread. Hum. Pathol. 1992;23:258–266. doi: 10.1016/0046-8177(92)90106-D. [DOI] [PubMed] [Google Scholar]
- 58.Igawa M., Urakami S., Shiina H., Ishibe T., Usui T., Chodak G.W. Association of nm23 protein levels in human prostates with proliferating cell nuclear antigen expression at autopsy. Eur. Urol. 1996;30:383–387. doi: 10.1159/000474200. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe M., Shiraishi T., Yatani R., Nomura A.M., Stemmermann G.N. International comparison on ras gene mutations in latent prostate carcinoma. Int. J. Cancer. 1994;58:174–178. doi: 10.1002/ijc.2910580205. [DOI] [PubMed] [Google Scholar]
- 60.Alipov G., Nakayama T., Ito M., Kawai K., Naito S., Nakashima M., Niino D., Sekine I. Overexpression of ets-1 proto-oncogene in latent and clinical prostatic carcinomas. Histopathology. 2005;46:202–208. doi: 10.1111/j.1365-2559.2005.02059.x. [DOI] [PubMed] [Google Scholar]
- 61.Maekawa S., Suzuki M., Arai T., Suzuki M., Kato M., Morikawa T., Kasuya Y., Kume H., Kitamura T., Homma Y. Tmprss2 met160val polymorphism: Significant association with sporadic prostate cancer, but not with latent prostate cancer in Japanese men. Int. J. Urol. 2014;21:1234–1238. doi: 10.1111/iju.12578. [DOI] [PubMed] [Google Scholar]
- 62.Allsbrook W.C., Jr., Mangold K.A., Johnson M.H., Lane R.B., Lane C.G., Amin M.B., Bostwick D.G., Humphrey P.A., Jones E.C., Reuter V.E., et al. Interobserver reproducibility of gleason grading of prostatic carcinoma: Urologic pathologists. Hum. Pathol. 2001;32:74–80. doi: 10.1053/hupa.2001.21134. [DOI] [PubMed] [Google Scholar]
- 63.Dehner L.P. The medical autopsy: Past, present, and dubious future. Mo. Med. 2010;107:94–100. [PMC free article] [PubMed] [Google Scholar]
- 64.Nemetz P.N., Tanglos E., Sands L.P., Fisher W.P., Jr., Newman W.P., 3rd, Burton E.C. Attitudes toward the autopsy—An 8-state survey. MedGenMed. 2006;8:80. [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao J., Krueger G.R., Buja L.M., Covinsky M. The impact of declining clinical autopsy: Need for revised healthcare policy. Am. J. Med. Sci. 2009;337:41–46. doi: 10.1097/MAJ.0b013e318184ce2b. [DOI] [PubMed] [Google Scholar]
- 66.Japanese Pathological Society Annual of the Pathological Autopsy Cases in Japan. [(accessed on 19 January 2021)]; Available online: http://pathology.or.jp/kankoubutu/autopsy-index.html.
- 67.Duregon E., Schneider J., DeMarzo A.M., Hooper J.E. Rapid research autopsy is a stealthy but growing contributor to cancer research. Cancer. 2019;125:2915–2919. doi: 10.1002/cncr.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this study.