Abstract
The α7 nicotinic acetylcholine receptor (α7 nAChR) is a ligand-gated ion channel that is involved in cognition disorders, schizophrenia, pain, and inflammation. Allosteric modulation of this receptor might be advantageous to reduce the toxicity in comparison with full agonists. Our previous results obtained with some hydroxy-chalcones, which were identified as positive allosteric modulators (PAMs) of α7 nAChR, prompted us to evaluate the potential of some structurally related naturally occurring flavonoids and curcuminoids and some synthetic curcumin analogues, with the aim of identifying new allosteric modulators of the α7 nAChR. Biological evaluation showed that phloretin, demethoxycurcumin, and bis-demethoxicurcuming behave as PAMs of α7 nAChR. In addition, some new curcumin derivatives were able to enhance the signal evoked by ACh; the activity values found for the tetrahydrocurcuminoid analog 23 were especially promising.
Keywords: curcuminoids, tetrahydrocurcuminoids, α7 nicotinic receptors, positive allosteric modulation
1. Introduction
The α7 acetylcholine nicotinic receptors (α7 nAChRs) are excitatory neurotransmitter receptors of the ligand-gated ion channels superfamily. They are involved in neurological/cognition disorders, as well as in pain and inflammation processes [1,2,3], being potential therapeutic targets for the treatment of several diseases such as Alzheimer or schizophrenia [4,5,6,7]. Therefore, the development of new agents that target these receptors has great therapeutic significance. Research efforts until now lead to the identification of some clinical candidates; however, more research will be needed in order to identify new more potent and effective ligands that could end in clinical candidates.
In this context, positive allosteric modulators (PAMs) might be advantageous, since they facilitate receptor responses without directly interacting with the agonist binding site, which can avoid many of the adverse effects. PAMs are compounds that modulate the α7 nAChRs activity, binding to sites different to that of the endogenous ligand. This could represent an advantage regarding selectivity for α7 nAChRs versus other nicotinic receptors and also versus 5-HT3 serotonine receptors that bear a high structural homology with the α7 nAChRs, avoiding cross-reactivity and consequently undesired effects [8,9]. According to their capacity to delay the desensitization of the receptors, two kinds of PAMS can be distinguished, Type I and II. Type I PAMs do not practically affect the agonist-induced desensitization, while Type II PAMs affect receptors’ kinetics causing a delay in desensitization and, as a consequence, a prolonged channel opening [10]. More recent studies lead to identify other types of allosteric modulators, like the silent allosteric modulators (SAMs) that block allosteric potentiation without interfering the responses of orthosteric agonist. There are also compounds which can activate the receptor through sites different from the orthosteric sites, not affecting the PAM action and without needing the presence of the orthosteric agonist, the so called ago-PAMs. Lastly, the negative allosteric modulators (NAMs), are able to allosterically block the open channel [11]. The interest for α7 nAChRs PAMs is still alive and is evidenced by the approval of a number of activators to be advanced into clinical trials [12,13], for instance, AVL-3288, currently in phase I for the treatment of schizophrenia [14,15], or Encenicline (EVP-6124), in phase III for smoke cessation [16,17].
In the search for new chemical entities able to modulate the α7 nAChRs, a few years ago we evaluated a heterogeneous collection of commercially available small-molecule natural products. As a result of this screening, a natural flavonoid with a chalcone structure, isoliquiritigenine (4,2′,4′-trihydroxychalcone), was identified as a selective PAM of the α7 nAChRs. This finding led to the evaluation of a collection of chalcones and related compounds, leading to the discovery of two new families of potent PAMs of the α7 nicotinic channels—polyhydroxychalcones [18] and polyhydroxydiphenyl propanones. This permitted the identification of a new chemotype of α7 nAChRs PAMs and compounds that exhibit promising analgesic and neuroprotective activities [19,20,21].
We report here the extension of the evaluation to a small collection of polyhydroxy flavonoids of natural origin together with known curcuminoids and new synthetic curcumin derivatives in an effort to explore the potential of these scaffolds in our ongoing interest for identifying new PAMs of the α7 nAChR with improved potency and efficacy. The structural similarity of flavones, chalcones, and curcumins invite to think that they could behave as α7 nAChRs PAMs interacting with the receptors in the same way as the previously described chalcones. Preliminary studies of selected compounds in a primary screening led to the identification of some curcuminoids able to significantly enhance the acetylcholine-evoked currents.
2. Results and Discussion
2.1. Evaluation of Polyhydroxy Natural Products (NPs)
Considering the biological activity reported for several natural products (NPs) in reference to the modulation of ion channels, and the results previously found for our chalcone collection, we decided to investigate the ability of a small collection of commercially available NPs to activate α7 nicotinic channels. The chemical structure of the selected PNs was closely related to that of chalcones, mainly flavonoids and curcuminoids (Table 1). In our first screening of a structurally diverse heterogeneous collection of PNs, we already identified a flavonoid, isoliquiritigenin, as a PAM of the α7 [18]. Isoliquiritigenin, is a polyhydroxy substituted chalcone extracted from the licorice plant that was widely used in Chinese medicine and is described to have a wide spectrum of therapeutic properties, among other neuroprotective effects [22,23]. Flavonoids have been extensively used in traditional herbal medicine [24], having important biological effects associated to a wide spectrum of pharmacological activity. They play preventive and therapeutic roles in several pathological processes like arthritis [25], neurodegenerative [26,27,28] and cardiovascular processes [29], cancer [30], or pain [31,32]. Several studies revealed that the biological actions of some flavonoids are in part mediated by their ability to modulate the activity of some ion channels, for instance, the cholinergic nicotinic channels.
Table 1.
Structure of commercial naturally occurring compounds 1 to 14 and their potentiation percentage values of α7 nAChR currents.
| Compound Number | Molecular Structure | % ± s.e. Current vs. ACh [200 µM] a |
|---|---|---|
|
1
Apigenin |
|
108 ± 4 |
|
2
Quercetin |
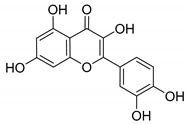
|
130 ± 10 |
|
3
Luteolin |
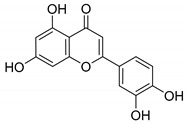
|
125 ± 5.5 |
|
4
Genistein |
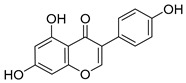
|
154 ± 10 |
|
5
Gossypetin |
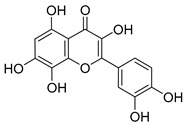
|
115 ± 2 |
|
6
Kaempferol |
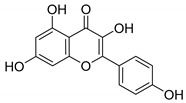
|
155 ± 5 |
|
7
Myricetin |
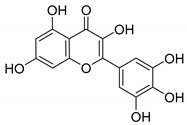
|
97 ± 6 |
|
8
Eriodictyol |
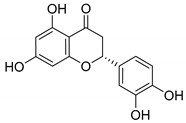
|
108 ± 2.5 |
|
9
Naringenin |
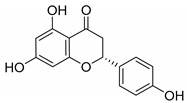
|
105 ± 3 |
|
10
Taxifolin |
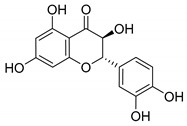
|
107 ± 5.5 |
|
11
Phloretin |
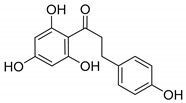
|
231 ± 0.5 |
|
12
Curcumin |
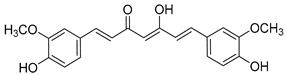
|
148 ± 43 |
|
13
Demethoxycurcumin (DMC) |
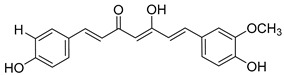
|
408 ± 18 |
|
14
Bisdemethoxycurcumin (BDMC) |
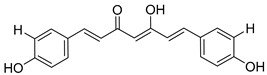
|
469 ± 42 |
a Compounds were tested at a unique dose of 10 µM, which was co-applied with 200 μM ACh. Responses were recorded at −80 mV and normalized with respect to that shown by only ACh (200 μM). Data are taken from 6–10 oocytes from at least 2 donors.
The findings reported above prompted us to comparatively screen a small, more directed collection of polyhydroxy-substituted naturally occurring and commercially available compounds with a chemical structure closely related to the chalcones previously identified by our group as PAMs of α7 nAChRs (Table 1). The selected compounds included some flavonoids, compounds 1 to 10, a diphenyl-2-propanone, phloretin 11, curcumin 12, and the curcuminoids 13 and 14. The results of the evaluation in a cellular assay, in xenopus oocytes selectively expressing α7 nAChRs, to determine its capacity to enhance the ion currents evoked by acetylcholine is shown in Table 1.
Among the results found, it is worth noting the values exhibited by some flavonoids, like quercetin (2), genistein (4), kaempferol (6), and especially phloretin (11). This last compound has a polyhydroxy-substituted diphenyl-2-propanone scaffold, similar to one of our already explored series that led to the identification of new potent PAM derivatives, as mentioned above [19]. Comparatively, some of our diphenyl-2-propanones are better PAMs of α7 nAChRs than phloretin itself. On the other hand, curcumin (12), especially the curcuminoids 13 and 14, significantly enhance the ACh-induced ion currents. The last two compounds, 13 and 14, showed strong potentiation, being able to increase the currents in more than 4-fold (408 and 469%, respectively).
Previous work described concerning some of the compounds that showed good results in our assay, already revealed their capacity to modulate ion channels and in particular α7 nAChRs. Therefore, in 2011, Dey R. et al. performed a virtual screening study of about 5000 small molecules, at three potential binding sites looking for allosteric modulators of the α7 receptors, which resulted in the identification of genistein (4) as one of the 100 compounds that could behave as PAM of the α7 receptors [33]. Moreover, the ability of genistein to potentiate the α7 nAChRs-mediated responses was already reported in 2005 by Robin A. J. and coworkers [34]. Later work published in 2007 pointed to its behavior as PAM [35], which was in accordance with the findings of the virtual screening carried out by Dey R. and Chen L. in 2011 [33]. Genistein promotes the overexpression of α7 nAChRs in adipose tissue of isolated mature adipocytes from obese subjects, where the levels of these protein are down regulated together with an inflammatory profile providing evidence of its role in the modulation of these receptors [36,37]. In agreement with that, work recently reported by Nielsen et al. evidenced that genistein and quercetin (2) were able to enhance the α7 nAChRs currents evoked by acetylcholine (ACh) in oocytes expressing α7 nAChRs, showing a PAM profile [38].
On the other hand, curcumin (12), a yellow pigment isolated from the rhizome of curcuma longa (turmeric), extensively used in traditional medicine for thousands of years, has been widely studied because of its uncountable therapeutic properties [39], from cancer [40,41] and arthritis [42] to neurodegenerative diseases [43] like Parkinson [44] and Alzheimer [45], as well as pain [46]. Its biological activity is mainly attributed to its particular chemical structure, having the advantage of low toxicity. Moreover, its chemical structure is very closely related to that of chalcone, which moved us to consider curcumin for our purposes, together with curcuminoids, demethoxycurcumin (13), and bisdemethoxycurcumin (14). Additionally, the interaction of curcumin with ion channels was also studied, trying to shed light into the underlying molecular mechanisms, and was broadly reviewed [47,48]. In fact, curcumin itself has recently been reported as an α7 nAChRs PAM, reversing nociception in mouse models [49,50]. Moreover, a recent work that describes the curcumin ability to activate vagal afferent neurons increasing neuron excitability suggests the mediation of the α7 nAChRs [51].
Compounds 12, 13, and 14 were characterized in more detail (Figure 1). Figure 1a–c features the potentiating effect that was observed in ACh-evoked ionic currents. In all cases, the currents decayed relatively fast; hence, the changes in kinetics were similar to those found for Type I PAMs. Recently, it has been reported that curcumin acts as a Type II PAM of α7 nACRs. Despite that, in the presence of this compound, α7 currents were observed to decay over time [50]. We and others (see, for instance, Grønlien et al., 2007 [35]) consider that Type II PAMs act by increasing maximum responses but also avoiding the typical desensitization of α7 nAChRs, which is not the case of curcumin. The three compounds potentiated α7 currents in a concentration-dependent manner (Figure 1d). Compound 12 seemed to be the most effective (1100% enhancement of ACh-induced currents, although this result should be considered only approximated), whereas compound 13 seemed to be the most potent (EC50 4.9 µM). Our results regarding the strong potentiating effect of curcumin (compound 12) confirm previous ones by El Nebrisi et al., although they differ quantitatively [50]. This could be due to the different conditions used in the experiments. For instance, these authors used a 20-fold lower concentration of ACh in their concentration–response experiments. Moreover, pre-treatment time was different, 10 min vs. 2 min in our case. Dose–response curves for ACh in the absence and in the presence of compound 13 were obtained (Figure 1e). Compound 13 induced a slight leftward shift (3-fold) and, most importantly, a 2-fold increase in apparent efficacy.
Figure 1.
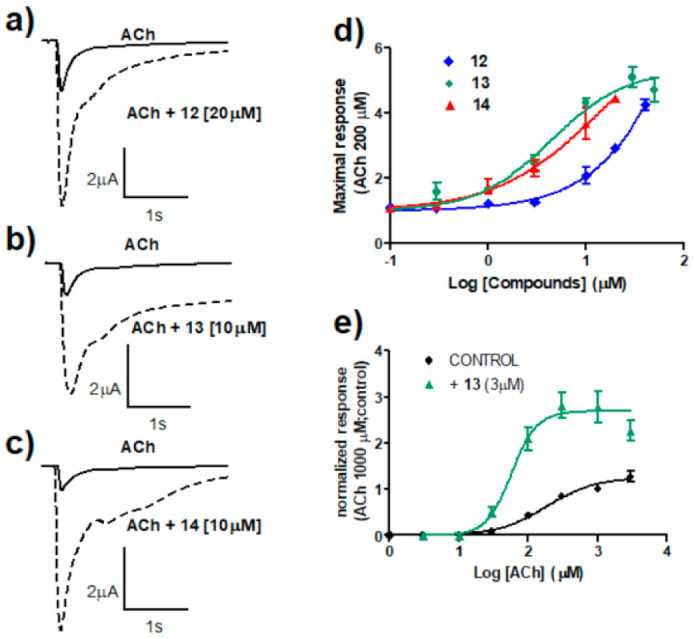
Characterization of curcumin (12) and two curcuminoids (compounds 13 and 14). (a–c) Ionic currents recorded in representative oocytes expressing human α7 nAChRs. Currents were evoked by 600 ms applications of ACh 200 μM in the absence (continuous line) and in the presence (dotted line) of 20 μM of 12 (panel (a)) or 10 μM of 13 (panel (b)) and 14 (panel (c)). All currents were recorded at a holding potential of –80 mV. (d) Concentration–response relationships for the potentiating effect of 12, 13, and 14 co-applied with ACh 200 μM. Continuous lines represent the fit to the Hill equation. Limited solubility of these compounds, especially 12 and 14, hindered the use of higher concentrations in order to adequately complete the curves, so that the following values should be considered with caution. Thus, maximal potentiating effects were estimated as 11 ± 3-, 5.5 ± 0.3-, and 5.5 ± 1.0-fold for compounds 12, 13, and 14, respectively. The EC50 values were 85 ± 35, 4.9 ± 1.0, and 6.9 ± 3.1 μM for 12, 13, and 14, respectively. (e) Concentration–response curves of peak currents elicited by ACh in control conditions or in the presence of 3 μM 13. Data have been normalized to the peak current obtained in control conditions with 1 mM ACh. Lines are fit to the Hill equation with parameters (Imax, EC50, nH): control (1.25, 176 μM, 1.25), 13 (2.70, 57 μM, 2.36). Data were taken from 3–7 oocytes from at least 2 donors.
The strong potentiation of ACh-induced currents found for demethylated curcuminoids, encouraged us to synthesize some new curcumin analogues, taking previous results found for the chalcone and diphenylpropanone series as a model [18,19].
2.2. Design and Synthesis of New Curcumin Derivatives
Despite the outstanding and multiple therapeutic properties of curcumin, there is a major limitation that restricts its therapeutic use, its poor pharmacokinetic properties, low aqueous solubility, low metabolic stability, and low oral bioavailability [52]. This promoted important research efforts to improve its therapeutic profile while keeping the beneficial biological properties, like modification of its chemical structure leading to the preparation of different curcuminoids. Among them, dimethoxycurcumin (DiMC), a synthetic analogue with higher metabolic stability [53], and tetrahydrocurcumin (THC), both exhibiting pleiotropic biological activities. Tetrahydrocurcumin, is one of the major metabolites of curcumin, being equally or even more effective than curcumin in a number of targets [54,55,56]. For the selection of the new curcumin derivatives to be synthesized, we designed tetramethoxy curcuminoids, analogues of DiMC, following the pattern substitution that led to the best results on the previously described chalcone series [18]. Besides, providing the good results found for the propen-2-one series [19], the saturated analogs of chalcones, preparation of the corresponding tetrahydrocurcumins, by reduction of the linker double bonds was also contemplated. Finally, assays to prepare the corresponding fully demethylated analogues were performed.
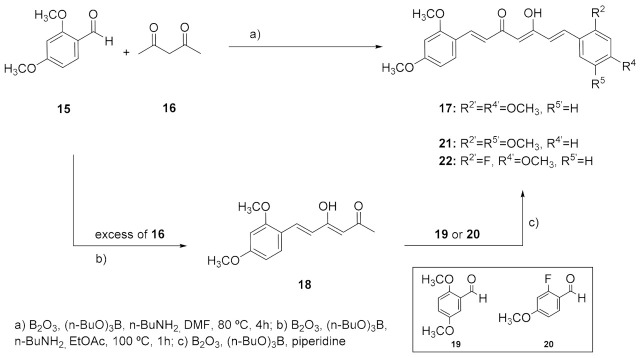
The preparation of the differently substituted curcumin analogues was tackled following the synthetic strategy originally described by Pabon, starting from the appropriate benzaldehydes and 2,4-pentanedione-boric anhydride in EtOAc in the presence of tributyl borate and n-butylamine [57]. The strategy consisted in protecting the highly acidic methylene protons of the 2,4-pentanedione system from the Knoevenagel condensation by forming a boron complex, so that the reaction can only take place at the terminal methyl groups of the diketone [58]. Compound 17, the tetramethoxy analogue symmetrically substituted in positions 2 and 4 of the phenyl rings, was firstly prepared. However, given the low yield obtained in the synthesis of 17 (17%), an alternative procedure described by Ferrari et al. was tried, that makes use of DMF instead of EtOAc as a solvent (Scheme 1) [59]. This method allowed us to shorten the reaction times as well as to reduce the volume of the solvent. Moreover, the easier reaction workup simplified the isolation and separation of 17 from byproducts. On the whole, this one-pot reaction resulted in a higher yield of 52% after crude recrystallization.
Scheme 1.
Synthesis of symmetrically substituted (17) and asymmetrically substituted (21, 22) curcumin derivatives.
Having in mind the good results obtained with the chalcone analogues, and searching for a possible parallelism, the synthesis of asymmetrically substituted curcumins 21 and 22, was explored. Compound 21 responds to the pattern substitution that led to the best results in the chalcone series, 2,4,2′,5′ -tetrasubstitution. On the other hand, considering the low stability and low bioavailability of curcumin systems, the incorporation of a fluorine atom in the aromatic ring (22) was also considered. The C-F bond is particularly strong and thus resistant to metabolic cleavage, given that fluorine is highly electron-withdrawing and reduces the potential for oxidative metabolism. Furthermore, fluorine substitution normally increases bioavailability [60]. The synthesis of these asymmetric analogues was carried out by preparing, in first instance, compound 18, which required the use of an excess of pentanedione to avoid the aldol condensation taking place at both terminal ends [61]. Subsequent condensation of isolated 18 with the appropriate aldehyde, 19 or 20, led to the asymmetrically substituted curcuminoids 21 and 22, respectively (Scheme 1). Unlike what was observed in the preparation of 17, in the case of the monophenyl intermediate 18, the use of EtOAc as solvent (27%) led to a slightly better result compared to DMF (15%). Coupling 18 with aldehyde 19 led to 21 in moderate yield (32%). In this case, the use of n-BuNH2 as a catalyst led to complex reaction mixtures, but the reaction proceeded using piperidine instead, which finally gave the desired compound 21 [62]. Fluorine derivative 22 was obtained by coupling 18 with aldehyde 20 using also piperidine as a catalyst. Unfortunately, several attempts to improve the yield and reduce the reaction time by using microwave irradiation and calcium oxide as a catalyst failed to provide the desired products and only complex mixtures were obtained [63].
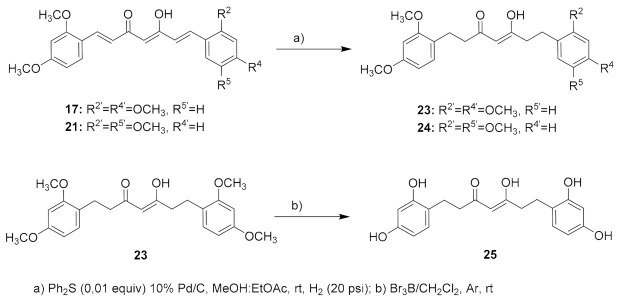
Additionally, searching for a parallelism with the chalcone series, the corresponding tetrahydrocurcumin analogues of 17 and 21 were prepared by reduction of the linker-chain double bonds, leading to 23 and 24. The reduction conditions were similar to those used for the reduction of the chalcones linker, by Pd/C hydrogenation in the presence of diphenyl sulfide, a catalyst poison that permits the selective hydrogenation of the double bonds without affecting the carbonyl groups (Scheme 2) [64].
Scheme 2.
Synthesis of tetrahydrocurcumins 23-25.
Finally, preparation of deprotected hydroxyl substituted derivatives of curcuminoid 17 and tetrahydrocurcumins 23 and 24 was attempted by reaction with Br3B in CH2Cl2 under inert atmosphere, as described by McOmie et al. [65]. However, the synthesis showed more difficulties than expected, and only the preparation of analogue 25 was achieved (Scheme 2). In order to determine the most favorable reaction conditions to arrive at the desired totally deprotected analog of 17, several assays for demethylation (Scheme 2) were performed using different amounts of reagent, 2, 1.5, and 1 equiv, for each reacting group. The best results were found using 1.5 equiv of Br3B as shown by the HPLC-MS data of the crude product, although the corresponding tetrahydroxy compound was obtained at quite a low purity (75%, HPLC data). All attempts to purify this material were unsuccessful resulting in decomposition of the product. The reaction worked better for the corresponding tetrahydrocurcumin analogue 23 to give the expected tetrahydroxy compound 25 in a moderate yield (24%), while efforts to isolate the demethylation product derived from the asymmetric curcuminoid 24 failed. A possible explanation could be the rapid oxidation, experimented by the para-dihydroxy substituted ring, which would lead to the corresponding benzoquinone product the presence of which was detected in the MS spectra of the reaction untreatable raw material.
Concerning the structure of the isolated compounds, the formation of the E,E isomer as a unique product was observed in all cases, as shown by the NMR spectra. On the other hand, it is worth noting that curcumins can exist in a tautomeric equilibrium between diketo and keto-enol forms, where the intramolecular hydrogen bond usually predominates in organic solvents, whereas the diketo form was mainly found in aqueous media, being stabilized by an increase in polarity [66] (Figure 1). In view of the NMR spectra, it can be said that the compounds were isolated in the enol form, with the only exception of tetrahydroxy tetrahydrocurcuminoid 25. In the HPLC chromatogram of this compound only one peak was observed, which indicates a high degree of purity, and that peak corresponds to the expected mass. However, in the NMR spectra, the presence of the two tautomeric forms was in fact detected. This is confirmed by the identification of two signals for C4, corresponding to the enol and keto forms, in the 13C spectrum and to linker H4 in the 1H-NMR spectrum. Additionally, while in the 1H-NMR the signals of the aliphatic protons were overlapped with that of the solvent, the 13C spectra denotes the presence of a duplicated number of signals in the aliphatic region, which were tentatively assigned on the basis of the bidimensional HSQC-NMR spectra.
To discard any possible artefacts due to decomposition of the compounds in solution, the chemical stability of the best compounds 17, 23, and 25 was checked in aqueous mixtures of H2O/acetonitrile 20/80 for a period of two months. The samples were periodically checked in the same HPLC conditions (gradient, column, and equipment), at different time intervals for a period of two months, observing no alteration on the retention times and purity along the tested period.
2.3. Biological Evaluation of New Curcumin Derivatives
The obtained compounds were screened to determine their capacity to modulate the α7 nAChR activity (Table 2). Each compound (10 μg/mL) was co-applied with ACh (200 μM) to oocytes expressing homomeric α7 nAChRs previously incubated with the same concentration of compound. Currents were measured and compared with those induced by ACh alone.
Table 2.
Effects of compounds 17 and 21–25 on α7 nAChR currents.
| Compound Number | Molecular Structure | % ± s.e. Current Vs. ACh [200 µM] a |
|---|---|---|
| 17 |
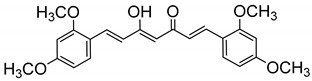
|
143 ± 3.5 |
| 21 |
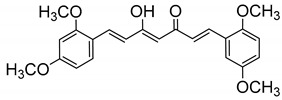
|
107 ± 4 |
| 22 |
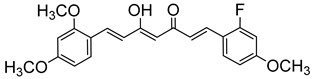
|
153 ± 17 |
| 23 |
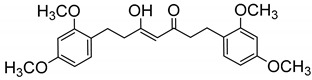
|
498 ± 7 |
| 24 |
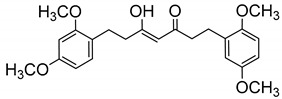
|
53.5 ± 3.5 |
| 25 |
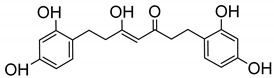
|
251 ± 24 |
a Compounds were tested at a unique dose of 10 µM, which was co-applied with 200 μM ACh. Responses were recorded at −80 mV and normalized with respect to that shown by only ACh (200 μM). Data are taken from 6–10 oocytes from at least 2 donors.
Looking at the data altogether, the most significant result was found for tetrahydrocurcuminoid 23, with 2,4-dimethoxy symmetrically substituted phenyls. No significant PAM properties were found for the 2,4,2′,5′-asymmetric compounds 21 and 24, the latter even decreasing the ACh-induced currents, while the fluorine-derivative 22 increases them only slightly. On the other hand, the reduction of the unsaturated linker is convenient, because compound 23, the tetrahydrocurcuminoid analogue of 17, enhanced the current at a higher percentage than its curcuminoid precursor. The only isolated tetrahydroxyl analogue, 25, showed a tendency for increasing the current but at a lower percentage compared to its MeO-substituted precursor 23. Regarding the latter, it is interesting that just the displacement of a methoxy substituent from position 3 to 4 resulted in the conversion of a potentiator into an antagonist (compound 24).
In parallel with the chalcone/diphenyl propenone series, it can be ventured, that also in this case compounds with the saturated linker chain were able to enhance the α7 nAChR currents evoked by ACh. However, considering the values found for 23 and 25, on this occasion, the methoxy substitution was preferred over the hydroxyl analogue in contrast with the results found for chalcones and diphenylpropanones, for which methoxy-substituted derivatives inhibited α7 nACh channels, while hydroxyl compounds behaved as α7 nAChR PAMs, increasing the activity promoted by ACh [18,19]. Regarding the results obtained for curcumin 12 and curcuminoids 13 and 14, the capacity to potentiate the currents increases with the decrease in the number of MeO- groups, the percentage values being higher in the following order 14 > 13 >> 12 (Table 1). However, compared with the new compounds prepared (17, 20-25), compound 23, a 2,4,2′,4′-tetramethoxy tetrahydrocurcumin analogue, is the compound that showed the best value, comparable to that found for BDMC, 14, the 4,4′-dihydroxy curcuminoid (Table 2).
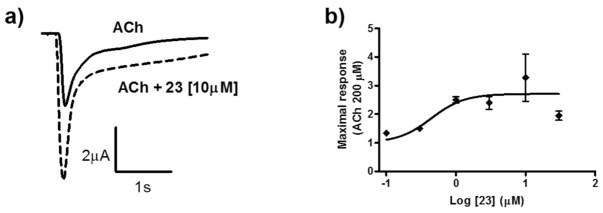
Compound 23 showed the highest increase in ACh-induced currents (Table 2) and, therefore, was further characterized (Figure 2). As it was previously observed for curcumin and derivatives (Figure 1a), currents potentiated by compound 23 decayed relatively fast (Figure 2a), as in control. Maximal activation of about 3-fold over the control was already observed at a relatively low concentration of 1 μM (see Figure 2b). Compound 23 showed a one and two orders of magnitude higher PAM potency than curcuminoids 13/14 and curcumin 12, respectively.
Figure 2.
Characterization of compound 23. (a) Ionic currents recorded in representative oocytes expressing human α7 nAChRs. Currents were evoked by 600 ms applications of ACh 200 μM in the absence (continuous line) and in the presence (dotted line) of 10 μM of 23. All currents were recorded at a holding potential of −80 mV. (b) Concentration–response relationship for the potentiating effect of 23 co-applied with ACh 200 μM. Continuous line represents the fit to the Hill equation. Thus, maximal potentiating effect was estimated as 2.7 ± 0.3-fold, and the EC50 value was 0.44 ± 0.28 μM. Data are taken from 3–7 oocytes from at least 2 donors.
3. Materials and Methods
3.1. Chemistry
General Methods
All reagents were of commercial quality. Solvents were dried and purified by standard methods. Analytical thin-layer chromatography (TLC) was performed on aluminum sheets coated with a 0.2 mm layer of silica gel 60 F254. Silica gel 60 (230–400 mesh) was used for flash chromatography. Some compounds were purified by flash chromatography on an ISOLERA ONE (Biotage) apparatus using normal phase: SNAP 10 g or 25 g KP-SIL (Biotage® KP-Sil Flash) cartridges and mixtures of ethyl acetate (solvent A) and n-hexane (solvent B) were used as mobile phase. The gradient will be indicated in each case. HPLC-MS was conducted in a Waters 2695 separation module using a Sunfire C18 (2.1 × 50 mm, 3.5 μm) reversed-phase column, gradient of 0.1% formic acid in CH3CN (solvent A) and 0.1% formic acid in H2O (solvent B) as mobile phase, coupled to a Waters 2996 Photodiode Array detector and a Waters micromass ZQ (ESI+). 1H and 13C-NMR spectra were registered in a Varian 300, 400, or 500 MHz spectrometer, using TMS as the internal standard.
Synthesis of Compounds 17, 18, 21 and 22
(1E,4Z,6E)-1,7-Bis(2,4-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one (17). A suspension of B2O3 (4.87 mmol) and 2,4-pentanedione (4.87 mmol) in DMF (6 mL) was stirred for 0.5 h at 80 °C, and then tributylborate (19.48 mmol) was added. After 0.5 h, 2,4-dimethoxybenzaldehyde (8.76 mmol) was added, followed by slow addition of n-butylamine (1.95 mmol in 2 mL of DMF). After stirring at 80 °C for 4 h, the solution was acidified with 0.5N HCl (16 mL) and cooled down to room temperature. The resulting solid was suspended in ice water, filtered, and dried under vacuum. The crude residue was recrystallized from MeOH. Brown yellow prisms (52%). m.p.: 140–142 °C (MeOH) (Lit.[61]: 135–137 °C). HPLC-MS: tR = 9.50 (10 min gradient of 40 to 95% of A in B). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 3.85 (s, 6H, OCH3), 3.88 (s, 6H, OCH3), 5.80 (s, 1H, H4), 6.46 (d, J = 2.4 Hz, 2H, 3-H, 3′-H), 6.52 (dd, J = 8.5 and 2.4 Hz, 2H, 5-H, 5′-H), 6.63 (d, J = 16,0 Hz, 2H, H2, H6), 7.49 (d, J = 8.5 Hz, 2H, 6-H, 6′-H), 7.89 (d, J = 16.0 Hz, 2H, H1, H7), 16.19 (br s, 1H, OH). 13C-NMR: (125 MHz, CDCl3) δ (ppm): 55.63 and 55.65 (4OCH3), 98.6 (C3, 3′), 101.3 (C4), 105.5 (C5, 5′), 117.5 (C5), 122.6 (C2, C6), 130.2 (C6, 6′), 135.6 (C1, C7), 160.0, 162.7, 182.25 (C3-OH), 184.0 (CO). MS (ESI+): m/z 397 [M + H]+. HRMS (ESI+): m/z [M ]+ calc for C23H24O6 [M ]+ 396.1573, found 396.1581.
(3Z,5E)-6-(2,4-Dimethoxyphenyl)-4-hydroxyhexa-3,5-dien-2-one (18). To a solution of 2,4-pentanedione (9 mmol) in EtOAc (5 mL) B2O3 (2.1 mmol) was added. The solution was stirred at 70 °C for 0.5 h, and then the corresponding aldehyde (3 mmol) and tributyl borate (3 mmol) were added. The mixture was stirred for 30 min, and then a solution of n-butylamine (3 mmol) in EtOAc (5 mL) was added at 85 °C, dropwise over 15 min. The stirring continued for 1 h at 100 °C. The mixture was then hydrolyzed by adding 1N HCl (12 mL) at 50 °C and then stirred at the same temperature for 0.5 h. After that, the organic layer was separated, and the aqueous layer was extracted with EtOAc. The combined organic extracts were washed with water until neutral pH, and finally with brine and dried over anhydrous MgSO4. After removal of the solvent under reduced pressure, the crude product was purified by flash HPLC and crystallized from MeOH giving 18 as yellow needles (27%). m.p.: 108–111 °C (MeOH) (Lit.[61] 92–93 °C). Purified by flash chromatography (gradient: 0 to 30 of EtOAc in hexane). HPLC-MS: tR = 6.62 (10 min gradient of 30 to 95% of A in B; column Sunfire 4.6 × 15 mm). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.14 (s, 3H, H1), 3.84 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 5.62 (s, 1H, H3), 6.45 (d, J = 2.5 Hz, 1H, 3-H), 6.49 (d, J = 16.0 Hz, 1H, H5), 6.50 (dd, J = 8.5 and 2.5 Hz, 1H, 5-H), 7.45 (d, J = 8.5 Hz, 1H, 6-H), 7.84 (d, J = 16.0 Hz, 1H, H6), 15.57 (br s, 1H, OH). MS (ESI+): m/z 249 [M + H]+.
(1E, 4Z, 6E)-1-(2,4-Dimethoxyphenyl)-7-(2,5-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one (21). The 2,5-dimethoxy benzaldehyde (21) (0.171 g, 1.03 mmol) and B2O3 (90 mg, 1.29 mmol) was dissolved in EtOAc (2 mL) at 80 °C. To this mixture an EtOAc solution (3 mL) of 18 (159 mg, 0.64 mmol) and (n-BuO)3B (0.35 mL, 1.40 mmol) was added. After stirring for 0.5 h, the reaction mixture was treated with piperidine (26 μL, 0.26 mmol) at 80 °C for 0.5 h. Then 1N HCl (10 mL) was added at room temperature, and the reaction mixture was stirred overnight. The aqueous phase was extracted with EtOAc, washed with water, and then dried over MgSO4. Normal-phase flash chromatography (gradient: 0–30% of EtOAc in hexane) and further crystallization from MeOH led to the desired product 23. Yellow prisms (32%). m.p.: 115–118 °C (MeOH). HPLC-MS: tR = 5.62 (10 min gradient of 60 to 95% of A in B). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 3.81, 3.85, 3.86 and 3.89 (s, 3H, OCH3), 5.85 (s, 1H, H4), 6.45 (d, J = 2.5 Hz, 1H, 3-H), 6.52 (dd, J = 8.5 and 2.5 Hz, 1H, 5-H), 6.64 (d, J = 16.0 Hz, 1H, H2), 6.67 (d, J = 16.0 Hz, 1H, H6), 6.85 (d, J = 9.0 Hz, 1H, 6′-H), 6.90 (dd, J = 9.0 and 2.9 Hz, 1H, 5′-H), 7.08 (d, J = 2.9 Hz, 1H, 3′-H), 7.49 (d, J = 8.5 Hz, 1H, 6-H), 7.92 (d, J = 16.0 Hz, 1H, H1), 7.93 (d, J = 16.0 Hz, 1H, H7), 16.08 (br s, 1H, OH). 13C-NMR: (125 MHz, CDCl3) δ (ppm): 55.6 (2OCH3), 55.9 (OCH3), 56.2 (OCH3), 98.5 (C3), 101.5 (C4), 105.6 (C5), 112.6 (C4′), 113.1 (C6′), 116.8 (C3′), 117.3 (C1), 122.5 (C2), 124.9 (C1′), 125.1 (C6), 130.3 (C6), 135.0 (C1), 136.2 (C7), 153.0, 153.7, 160.1 and 162.8 (C2′, C5′, C2 and C4), 182.5 (C3-OH), 185.2 (CO). MS (ESI+): m/z 397 [M + H] +. HRMS (ESI+): calc for C23H24O6 [M ]+ 396.1573, found 396.1580.
(1E, 4Z, 6E) -1-(2,4-Dimethoxyphenyl)-7-(2-fluoro-4-methoxyphenyl)-5-hydroxyhepta-1,4,6 -trien-3-one (22). Orange prisms (27%). From 20 and aldehyde 22, following the same method described for the preparation of compound 23. m.p.: 128–131 °C (MeOH). HPLC-MS: tR = 3.28 (10 min gradient of 70 to 95% of A in B). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 3.83 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 5.81 (s, 1H, H4), 6.45 (d, J = 2.4 Hz, 1H, 3-H), 6.52 (dd, J = 8.5 and 2.4 Hz, 1H, 5-H), 6.60 (d, J = 16.0 Hz, 2H, H2), 6.63 (d, J = 16.0 Hz, 2H, H6), 6.65 (dd, J = 12.8 and 2.5 Hz, 1H, 3′-H)], 6.72 (dd, J = 8.7 and 2.5 Hz, 1H, 5′-H), 7.44 (dd, J = 9.0, 8.9 Hz, 1H, 6′-H), 7.50 (d, J = 8.9 Hz, 1H, 6-H], 7.68 (d, J = 16.0 Hz, 1H, H1), 7.91 (d, J = 16.0 Hz, 1H, H7). 13C-NMR: (125 MHz, CDCl3) δ (ppm): 55.6 (2OCH3), 55.8 (OCH3), 98.5 (C3), 101.5 (C4), 102.0 (d, J = 25 Hz, C3′), 105.6 (C5), 110.9 (d, J = 3 Hz, C5′), 116.0 (d, J = 12 Hz, C1′), 117.3 (C1), 122.4 (C2), 124.3 (d, J = 7 Hz, C6), 130.1 (d, J = 5 Hz, C6′), 130.4 (C6), 132.6 and 136.3 (C1, C7), 160.1, 162.9 (C2, C4), 162.3 (d, J = 11 Hz, C4′), 162.6 (d, J = 253 Hz, C2′), 182.3 (C3-OH), 185.1 (CO). MS (ESI+): m/Iz: 385 [M + H]+. HRMS (ESI+): calc for C22H21FO5 [M ]+ 384.1373, found 384.1367.
General Procedure for the Synthesis of Tetrahydrocurcuminoids 23 and 24
The corresponding curcumin analogue (17 or 21, 0.25 mmol) was dissolved in 70 mL of a mixture of MeOH:THF (1:1) and Ph2S (0.0025 mmoL), and 10% Pd/C (0.025 mmoL) was added. The mixture was hydrogenated at 20 psi and room temperature overnight. The suspension was filtered and the solvent removed under vacuum. The crude product was purified by flash chromatography (gradient: 0 to 17% of EtOAc in hexane).
(Z)-1,7-Bis(2,4-dimethoxyphenyl)-5-hydroxyhept-4-en-3-one (23). From 17. Pale oil. (85% yield). HPLC-MS: tR = 5.25, tautomeric ratio: 72.1 (10 min gradient of 60 to 95% of A in B). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.49–2.54 (m, 3H), 2.77–2.86 (m, 5H), 3.79 (s, 12H, OCH3), 5.45 (s, 1H, 4-H), 6.38–6.44 (m, 4H, 5, 5′, 3, 3′-H), 7.01 (d, J = 8,0 Hz, 2H, 6, 6′-H), 15.50 (br s, 1H, OH).13C-NMR: (75 MHz, CDCl3) δ (ppm): 24.3, 26.2 (C1, C7), 38.8, 44.0 (C2, C6)), 55.3, 55.5 (OCH3), 98.6 (C3, C3′), 99.4 (C4), 103.9 (C5, C5′), 121.6 (C1, C1′), 130.2(C6, C6′), 158.4, 159.6 (CCH3), 194.0 (C3-OH), 204.2 (CO)). MS (ESI+): m/Iz 401 [M + H]+. HRMS (ESI+): calc for C23H28O6 [M]+ 400.1886, found 400.1870.
(Z)-1-(2,4-Dimethoxyphenyl)-7-(2,5-dimethoxyphenyl)-5-hydroxyhept-4-en-3-one (24). From 21. Pale oil. (70% yield). HPLC-MS: tR = 4.98 (10 min gradient of 60 to 95% of A in B). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 2.49–2.58 (m, 3H), 2.74–2.90 (m, 5H), 3.75 and 3.78 (s, 3H, OCH3), 3.79 (s, 6H, OCH3), 5.46 (s, 1H, H4), 6.38–6.44 (m, 2H, 3-H, 5′-H), 6.70–6.78 (m, 3H, 3′, 4′, 6′-H), 7.01 (d, J = 8.1, 1H, 6-H), 15.49 (s, 1H, OH). 13C-NMR: (75 MHz, CDCl3) δ (ppm): 24.6 , 26.5 (C1, C7), 38.6, 44.1 (C2, C6,), 55.3, 55.5, 55.8 and 55.9 (OCH3), 98.6 (C3), 99.4 (C4), 103.9 (C5), 111.3 (C4′), 111.6 (C3′) 116.4 (C6′), 121.6 (C1), 130.2 (C6′), 130.4 (C1′), 151.8, 153.6, 158.4, 159.6 (COCH3), 193.8 (C3-OH), 204.0 (CO). MS (ESI+): m/Iz 401 [M + H]+. HRMS (ESI+): calc for C23H28O6 [M]+ 400.1886, found 400.1881.
Synthesis of Compound 25
(Z)-1,7-Bis(2,4-dihydroxyphenyl)-5-hydroxyhept-4-en-3-one (25). To a solution of 23 in dry CH2Cl2 (0.66 mmol/15 mL), a 1M solution of Br3B in CH2Cl2 (9 equiv, 1.5 equiv for every O and/or N present in the molecule) was slowly added under Ar atmosphere. The reaction mixture was stirred at room temperature, and the progress of the reaction was monitored by thin-layer chromatography (TLC). After completion, the reaction was quenched by adding water (10 mL) and the precipitated solid filtered and washed with water. The product was extracted with EtOAc from the aqueous reaction mixture. The organic layer was washed with water until neutral pH, and then with brine and dried over anhydrous MgSO4. After removal of the solvent in vacuum, the crude product was purified by flash column chromatography eluting with CH2Cl2:MeOH, 20:1 solution. Yellow oil. 24% yield. HPLC-MS: tR = 7.46 (10 min gradient, 30–95% of A in B). 1H-NMR: (400 MHz, CD3)2SO) δ (ppm): Keto-enol tautomeric equilibrium, 1:1, 2.45–2.68 (m, 8H), 3.66 (s, 2H, 4-CH2, keto form), 5.68 (s, 1H, 4-CH, enol form), 6.09–6.13 (m, 2H, 6, 6′-H), 6.23–6.26 (m, 2H, 3, 3′-H), 6.7–6.8 (m, 2H, 5, 5′-H), 8.95 (s, 1H, OH), 8.97 (s, 1H, OH), 9.13 (s, 1H, OH), 9.18 (s, 1H, OH), 15.59 (s, 1H, 4-OH). 13C-NMR: (100 MHz, CD3)2SO)) δ (ppm): 23.28, 23.65, 24.99, 25.34 (CH2, enol tautomer), 34.45, 38.10, 43.33 and 43.45 (CH2, keto tautomer), 56.19 (C4, keto tautomer), 99.21 (C4, enol tautomer), 102.35, 102.44 (C3, C3′), 105.82, 105.89 (C5, C5′), 117.22, 117.49 (C1, C1′), 129.87, 129.95 (C6, C6′), 155.71, 155.76, 155.84, 156.41, 156.45, 156.52, 156.56 (C-OH), 205.12 (C3-OH), 208.37 (CO). MS (ESI+): m/Iz m/Iz: 345 [M + H]+. HRMS (ESI-): calc for C19H20O6 [M]+ 344.1260, found 344.1260.
3.2. Biological Evaluation
Oocyte Expression and Electrophysiological Studies
All human nAChR cDNAs were cloned in derivatives of the pSP64T vector containing part of the pBluescript polylinker. Capped mRNA was synthesized in vitro using SP6 RNA polymerase, the mMESSAGEmMACHINE kit from Ambion (Thermo Fisher Scientific, Madrid, Spain), and the pSP64T derivatives mentioned above. Defoliculated Xenopus laevis oocytes were injected with 5 ng of each subunit cRNA in 50 nL of sterile water. All experiments were performed within 2–3 days after cRNA injection. Two-electrode voltage-clamp recordings were carried out in oocytes located in a chamber and perfused with a modified frog Ringer containing (in mM) NaCl 82.5, KCl 2.5, BaCl2 2.5, MgCl2 1, and HEPES 5 (pH 7.4). Barium, instead of calcium, was used as the extracellular divalent cation to avoid the presence of calcium-activated chloride currents. Unless otherwise specified, compounds were pre-applied in the bath for 2 min and then co-applied with ACh through a pipette held very close to the oocyte for fast application (18–22 mL/min), as previously described [67]. All experiments were performed at 22 °C. The holding potential was usually −80 mV. Current records were measured with Clampfit 10.0 (MDS Analytical Technologies, Sunnyvale, CA, USA). Normalized peak currents were obtained by dividing the maximum value of the current obtained in the presence of compound by the maximum value of the current obtained in control conditions (200 μM ACh, a value close to its EC50). Dose–response curves for the peak current obtained with ACh were fitted to the Hill equation: normalized current = Imax/(1 + (EC50/[ACh])nH). Data are expressed as mean ± SEM. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4. Conclusions
With the aim of identifying new positive allosteric modulators of α7 nAChRs, we describe here the biological evaluation of a small collection of naturally occurring compounds, together with the synthesis and evaluation of new curcuminoids and tetrahydrocurcuminoids concerning their capacity as modulators of the α7 nAChRs with the aim of identifying new positive allosteric modulators.
The selected natural products include several polyhydroxyflavonoids, a polyhydroxy diphenylpropanone, phloretin, curcumin, and two curcuminoids, DMC and BDMC. The results obtained revealed the ability of phloretin, curcumin, and curcuminoids to enhance the ACh-induced currents in α7 nAChRs. Concerning phloretin (11), a polyhydroxydiphenylpropanone, its tendency to increase the current is in agreement with our previously described findings for this chemical series [19]. On the other hand, regarding curcumin, our results confirm its capacity as PAM of the α7 receptors, which together with the even better values found for curcuminoids DMC and BDMC prompted us to the preparation of new curcumin analogues. Three of the new compounds prepared, the three tetrahydrocurcuminoids, responding to a bis(2,4-diphenyl) symmetric substitution pattern (17, 23 and 25), were able to activate the α7 nicotinic receptors in an allosteric manner. The best result was found for the tetrahydrocurcumin analogue 23, a tetramethoxy substituted compound that increased the current at superior levels than its tetrahydroxy analogue 25. This is in contrast with the behavior previously observed for our chalcone and diphenylpropanone series, for which only the polyhydroxy derivatives behave as PAMs of the α7 nAChRs.
These results confirm the interest of curcumin and curcuminoids in terms of activation of α7 nAChRs and additionally revealed the potential of the tetrahydrocurcumin as a new scaffold to be able to positively modulate these receptors. The results are very promising and open the way to further research in order to fully establish these new scaffolds as a new series of α7 nAChRs PAMs.
Acknowledgments
We thank Jessy Medina for technical assistance with stability studies.
Author Contributions
Conceptualization, M.C., R.G.-M. and M.J.P.d.V.; Funding acquisition, M.C. and R.G.-M.; Investigation, M.X., J.M., S.S. and F.S.; Methodology, M.X., J.M., S.S. and F.S.; Project administration, R.G.-M. and M.J.P.d.V.; Resources, M.C.; Supervision, M.J.P.d.V.; Visualization, M.C. and M.J.P.d.V.; Writing—original draft, M.J.P.d.V.; Writing—review & editing, M.C., F.S., R.G.-M. and M.J.P.d.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the Spanish Ministerio de Ciencia, Innovación y Universidades (MICIU-FEDER) grant number RTI2018-097189-B-C22 and CSIC grant number 2019E030 to RGM. BFU2008-02160 to MC and SAF2011-22802 to SS.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Universidad Hernández (protocol code INA-MC-001-07 approved 01-29-2008).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bouzat C., Lasala M., Nielsen B.E., Corradi J., del C Esandi M. Molecular function of α7 nicotinic receptors as drug targets. J. Physiol. 2018;596:1847–1861. doi: 10.1113/JP275101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagdas D., Gurun M.S., Flood P., Papke R.L., Damaj M.I. New Insights on Neuronal Nicotinic Acetylcholine Receptors as Targets for Pain and Inflammation: A Focus on α7 nAChRs. Curr. Neuropharmacol. 2018;16:415–425. doi: 10.2174/1570159X15666170818102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titus D.J., Johnstone T., Johnson N.H., London S.H., Chapalamadugu M., Hogenkamp D., Gee K.W., Atkins C.M. Positive allosteric modulation of the α7 nicotinic acetylcholine receptor as a treatment for cognitive deficits after traumatic brain injury. PLoS ONE. 2019;14:e0223180. doi: 10.1371/journal.pone.0223180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma K.G., Qian Y.H. Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides. 2019;73:96–106. doi: 10.1016/j.npep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Sinha N., Karche N.P., Verma M.K., Walunj S.S., Nigade P.B., Jana G., Kurhade S.P., Hajare A.K., Tilekar A.R., Jadhav G.R., et al. Discovery of Novel, Potent, Brain-Permeable, and Orally Efficacious Positive Allosteric Modulator of α7 Nicotinic Acetylcholine Receptor [4-(5-(4-Chlorophenyl)-4-methyl-2-propionylthiophen-3-yl)benzenesulfonamide]: Structure-Activity Relationship and Preclinical Characterization. J. Med. Chem. 2020;63:944–960. doi: 10.1021/acs.jmedchem.9b01569. [DOI] [PubMed] [Google Scholar]
- 6.Antonio-Tolentino K., Hopkins C.R. Selective α7 nicotinic receptor agonists and positive allosteric modulators for the treatment of schizophrenia—A review. Expert Opin. Investig. Drugs. 2020;29:603–610. doi: 10.1080/13543784.2020.1764938. [DOI] [PubMed] [Google Scholar]
- 7.Terry A.V., Callahan P.M. α7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology. 2020;170:108053. doi: 10.1016/j.neuropharm.2020.108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand D., Terry A.V. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2018;151:214–225. doi: 10.1016/j.bcp.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Mitra S., Khatri S.N., Maulik M., Bult-Ito A., Schulte M. Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders. Int. J. Mol. Sci. 2020;21:4918. doi: 10.3390/ijms21144918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams D.K., Wang J., Papke R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatzidaki A., Millar N.S. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015;97:408–417. doi: 10.1016/j.bcp.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Yang T., Xiao T., Sun Q., Wang K. The current agonists and positive allosteric modulators of α 7 nAChR for CNS indications in clinical trials. Acta Pharm. Sin. B. 2017;7:611–622. doi: 10.1016/j.apsb.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tregellas J.R., Wylie K.P. Alpha7 Nicotinic Receptors as Therapeutic Targets in Schizophrenia. Nicotine Tob. Res. 2019;21:349–356. doi: 10.1093/ntr/nty034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantrowitz J.T., Javitt D.C., Freedman R., Sehatpour P., Kegeles L.S., Carlson M., Sobeih T., Wall M.M., Choo T.-H., Vail B., et al. Double blind, two dose, randomized, placebo-controlled, cross-over clinical trial of the positive allosteric modulator at the alpha7 nicotinic cholinergic receptor AVL-3288 in schizophrenia patients. Neuropsychopharmacology. 2020;45:1339–1345. doi: 10.1038/s41386-020-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Trial of AVL-3288 in Schizophrenia Patients—Full Text View—ClinicalTrials.gov. [(accessed on 1 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02978599.
- 16.Deardorff W.J., Shobassy A., Grossberg G.T. Safety and clinical effects of EVP-6124 in subjects with Alzheimer’s disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev. Neurother. 2014;15:7–17. doi: 10.1586/14737175.2015.995639. [DOI] [PubMed] [Google Scholar]
- 17.A Safety and Cognitive Function Study of EVP-6124 versus Placebo in Subjects with Nicotine Dependence—Full Text View—ClinicalTrials.gov. [(accessed on 21 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01480232.
- 18.Balsera B., Mulet J., Fernández-Carvajal A., de la Torre-Martínez R., Ferrer-Montiel A., Hernández-Jiménez J.G., Estévez-Herrera J., Borges R., Freitas A.E., López M.G., et al. Chalcones as positive allosteric modulators of α7 nicotinic acetylcholine receptors: A new target for a privileged structure. Eur. J. Med. Chem. 2014;86:724–739. doi: 10.1016/j.ejmech.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Criado M., Balsera B., Mulet J., Sala S., Sala F., de la Torre-Martínez R., Fernández-Carvajal A., Ferrer-Montiel A., Moreno-Fernández S., Miguel M., et al. 1,3-diphenylpropan-1-ones as allosteric modulators of α7 nACh receptors with analgesic and antioxidant properties. Future Med. Chem. 2016;8:731–749. doi: 10.4155/fmc-2015-0001. [DOI] [PubMed] [Google Scholar]
- 20.Balsera B., Mulet J., Sala S., Sala F., de la Torre-Martínez R., González-Rodríguez S., Plata A., Naesens L., Fernández-Carvajal A., Ferrer-Montiel A., et al. Amino acid and peptide prodrugs of diphenylpropanones positive allosteric modulators of α7 nicotinic receptors with analgesic activity. Eur. J. Med. Chem. 2018;143:157–165. doi: 10.1016/j.ejmech.2017.10.083. [DOI] [PubMed] [Google Scholar]
- 21.Pérez de Vega M.J., Fernandez-Mendivil C., de la Torre Martínez R., González-Rodríguez S., Mullet J., Sala F., Sala S., Criado M., Moreno-Fernández S., Miguel M., et al. 1-(2′,5′-Dihydroxyphenyl)-3-(2-fluoro-4-hydroxyphenyl)-1-propanone (RGM079): A Positive Allosteric Modulator of α7 Nicotinic Receptors with Analgesic and Neuroprotective Activity. ACS Chem. Neurosci. 2019;10:3900–3909. doi: 10.1021/acschemneuro.9b00364. [DOI] [PubMed] [Google Scholar]
- 22.Zhan C., Yang J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol. Res. 2006;53:303–309. doi: 10.1016/j.phrs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Min J.L., Chae H.Y., Jeon J.P., Hwang M. Protective effects of isoliquiritigenin against methamphetamine-induced neurotoxicity in mice. J. Pharmacol. Sci. 2009;111:216–220. doi: 10.1254/jphs.09153SC. [DOI] [PubMed] [Google Scholar]
- 24.Saudagar R.B., Saokar S. Anti-inflammatory Natural Compounds from Herbal and Marine Origin. J. Drug Deliv. Ther. 2019;9:669–672. doi: 10.22270/jddt.v9i3.2906. [DOI] [Google Scholar]
- 25.Sung S., Kwon D., Um E., Kim B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules. 2019;24:1589. doi: 10.3390/molecules24081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin M.S., Kabir M.T., Niaz K., Jeandet P., Clément C., Mathew B., Rauf A., Rengasamy K.R.R., Sobarzo-Sánchez E., Ashraf G.M., et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules. 2020;25:1267. doi: 10.3390/molecules25061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maan G., Sikdar B., Kumar A., Shukla R., Mishra A. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 2020;20:1169–1194. doi: 10.2174/1568026620666200416085330. [DOI] [PubMed] [Google Scholar]
- 28.Dajas F., Juan Andres A.-C., Florencia A., Carolina E., Felicia R.-M. Neuroprotective Actions of Flavones and Flavonols: Mechanisms and Relationship to Flavonoid Structural Features. Cent. Nerv. Syst. Agents Med. Chem. 2013;13:30–35. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- 29.Fusi F., Spiga O., Trezza A., Sgaragli G., Saponara S. The surge of flavonoids as novel, fine regulators of cardiovascular Cav channels. Eur. J. Pharmacol. 2017;796:158–174. doi: 10.1016/j.ejphar.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Montané X., Kowalczyk O., Reig-Vano B., Bajek A., Roszkowski K., Tomczyk R., Pawliszak W., Giamberini M., Mocek-Płóciniak A., Tylkowski B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules. 2020;25:3342. doi: 10.3390/molecules25153342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraz C.R., Carvalho T.T., Manchope M.F., Artero N.A., Rasquel-Oliveira F.S., Fattori V., Casagrande R., Verri W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules. 2020;25:762. doi: 10.3390/molecules25030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu P., Basu A. In Vitro and In Vivo Effects of Flavonoids on Peripheral Neuropathic Pain. Molecules. 2020;25:1171. doi: 10.3390/molecules25051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey R., Chen L. In search of allosteric modulators of α7-nAChR by solvent density guided virtual screening. J. Biomol. Struct. Dyn. 2011;28:695–715. doi: 10.1080/07391102.2011.10508600. [DOI] [PubMed] [Google Scholar]
- 34.Cho C.-H. Rapid Upregulation of 7 Nicotinic Acetylcholine Receptors by Tyrosine Dephosphorylation. J. Neurosci. 2005;25:3712–3723. doi: 10.1523/JNEUROSCI.5389-03.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gronlien J.H., Hakerud M., Ween H., Thorin-Hagene K., Briggs C.A., Gopalakrishnan M., Malysz J. Distinct Profiles of 7 nAChR Positive Allosteric Modulation Revealed by Structurally Diverse Chemotypes. Mol. Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 36.Grønlien J.H., Ween H., Thorin-Hagene K., Cassar S., Li J., Briggs C.A., Gopalakrishnan M., Malysz J. Importance of M2-M3 loop in governing properties of genistein at the α7 nicotinic acetylcholine receptor inferred from α7/5-HT3A chimera. Eur. J. Pharmacol. 2010;647:37–47. doi: 10.1016/j.ejphar.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Cancello R., Zulian A., Maestrini S., Mencarelli M., Della Barba A., Invitti C., Liuzzi A., Di Blasio A.M. The nicotinic acetylcholine receptor a7 in subcutaneous mature adipocytes: Downregulation in human obesity and modulation by diet-induced weight loss. Int. J. Obes. 2012;36:1552–1557. doi: 10.1038/ijo.2011.275. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen B.E., Bermudez I., Bouzat C. Flavonoids as positive allosteric modulators of α7 nicotinic receptors. Neuropharmacology. 2019;160:107794. doi: 10.1016/j.neuropharm.2019.107794. [DOI] [PubMed] [Google Scholar]
- 39.Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Ramirez-Tortosa M. Curcumin and Health. Molecules. 2016;21:264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashrafizadeh M., Zarrabi A., Hashemi F., Moghadam E.R., Hashemi F., Entezari M., Hushmandi K., Mohammadinejad R., Najafi M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020;256:117984. doi: 10.1016/j.lfs.2020.117984. [DOI] [PubMed] [Google Scholar]
- 41.Fidelis G.K., Louis H., Fidelis Tizhe T., Solomon O. Curcumin and Curcumin-based derivatives as anti-cancer agents: Recent Nano-Synthetic Methodologies and Anti-cancer Therapeutic Mechanisms. J. Med. Chem. Sci. 2019;2:59–63. doi: 10.26655/JMCHEMSCI.2019.3.5. [DOI] [Google Scholar]
- 42.Mohammadian Haftcheshmeh S., Momtazi-Borojeni A.A. Immunomodulatory therapeutic effects of curcumin in rheumatoid arthritis. Autoimmun. Rev. 2020;19:102593. doi: 10.1016/j.autrev.2020.102593. [DOI] [PubMed] [Google Scholar]
- 43.Bhat A., Mahalakshmi A.M., Ray B., Tuladhar S., Hediyal T.A., Manthiannem E., Padamati J., Chandra R., Chidambaram S.B., Sakharkar M.K. Benefits of curcumin in brain disorders. BioFactors. 2019;45:666–689. doi: 10.1002/biof.1533. [DOI] [PubMed] [Google Scholar]
- 44.Rabiei Z., Solati K., Amini-Khoei H. Phytotherapy in treatment of Parkinson’s disease: A review. Pharm. Biol. 2019;57:355–362. doi: 10.1080/13880209.2019.1618344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calfio C., Gonzalez A., Singh S.K., Rojo L.E., MacCioni R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020;77:33–51. doi: 10.3233/JAD-200443. [DOI] [PubMed] [Google Scholar]
- 46.Sun J., Chen F., Braun C., Zhou Y.-Q., Rittner H., Tian Y.-K., Cai X.-Y., Ye D.-W. Role of curcumin in the management of pathological pain. Phytomedicine. 2018;48:129–140. doi: 10.1016/j.phymed.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 47.Tabeshpour J., Banaeeyeh S., Eisvand F., Sathyapalan T., Hashemzaei M., Sahebkar A. Effects of Curcumin on Ion Channels and Pumps: A Review. IUBMB Life. 2019;71:812–820. doi: 10.1002/iub.2054. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X., Chen Q., Wang Y., Peng W., Cai H. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014;5:94. doi: 10.3389/fphys.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Nebrisi E., Al Kury L.T., Yang K.H.S., Jayaprakash P., Howarth F.C., Kabbani N., Oz M. Curcumin potentiates the function of human α 7 -nicotinic acetylcholine receptors expressed in SH-EP1 cells. Neurochem. Int. 2018;114:80–84. doi: 10.1016/j.neuint.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 50.El Nebrisi E.G., Bagdas D., Toma W., Al Samri H., Brodzik A., Alkhlaif Y., Yang K.-H.S., Howarth F.C., Damaj I.M., Oz M. Curcumin Acts as a Positive Allosteric Modulator of α7 -Nicotinic Acetylcholine Receptors and Reverses Nociception in Mouse Models of Inflammatory Pain. J. Pharmacol. Exp. Ther. 2018;365:190–200. doi: 10.1124/jpet.117.245068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiao S., Dou Y., Hu H., Dai Y. Curcumin Activates Vagal Afferent Neurons Through the Modulation of Ion Channels via α7 nAChR. Nat. Prod. Commun. 2019;14:1–6. doi: 10.1177/1934578X19873738. [DOI] [Google Scholar]
- 52.Slika L., Patra D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2020;17:61–75. doi: 10.1080/17425247.2020.1702644. [DOI] [PubMed] [Google Scholar]
- 53.Teymouri M., Barati N., Pirro M., Sahebkar A. Biological and pharmacological evaluation of dimethoxycurcumin: A metabolically stable curcumin analogue with a promising therapeutic potential. J. Cell. Physiol. 2018;233:124–140. doi: 10.1002/jcp.25749. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal B., Deb L., Prasad S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules. 2014;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J.-C., Tsai M.-L., Lai C.-S., Wang Y.-J., Ho C.-T., Pan M.-H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014;5:12–17. doi: 10.1039/C3FO60370A. [DOI] [PubMed] [Google Scholar]
- 56.Lai C.-S., Ho C.-T., Pan M.-H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules. 2020;10:831. doi: 10.3390/biom10060831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pabon H.J.J. A synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays Bas. 1964;83:379–386. doi: 10.1002/recl.19640830407. [DOI] [Google Scholar]
- 58.Mazumder A., Neamati N., Sunder S., Schulz J., Pertz H., Eich E., Pommier Y. Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J. Med. Chem. 1997;40:3057–3063. doi: 10.1021/jm970190x. [DOI] [PubMed] [Google Scholar]
- 59.Ferrari E., Pignedoli F., Imbriano C., Marverti G., Basile V., Venturi E., Saladini M. Newly synthesized curcumin derivatives: Crosstalk between chemico-physical properties and biological activity. J. Med. Chem. 2011;54:8066–8077. doi: 10.1021/jm200872q. [DOI] [PubMed] [Google Scholar]
- 60.Gillis E.P., Eastman K.J., Hill M.D., Donnelly D.J., Meanwell N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 61.Lin L., Shi Q., Nyarko A.K., Bastow K.F., Wu C.-C., Su C.-Y., Shih C.C.-Y., Lee K.-H. Antitumor Agents. 250. † Design and Synthesis of New Curcumin Analogues as Potential Anti-Prostate Cancer Agents. J. Med. Chem. 2006;49:3963–3972. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryu E.K., Choe Y.S., Lee K.-H., Choi Y., Kim B.-T. Curcumin and Dehydrozingerone Derivatives: Synthesis, Radiolabeling, and Evaluation for β-Amyloid Plaque Imaging. J. Med. Chem. 2006;49:6111–6119. doi: 10.1021/jm0607193. [DOI] [PubMed] [Google Scholar]
- 63.Elavarasan S., Bhakiaraj D., Chellakili B., Elavarasan T., Gopalakrishnan M. One pot synthesis, structural and spectral analysis of some symmetrical curcumin analogues catalyzed by calcium oxide under microwave irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;97:717–721. doi: 10.1016/j.saa.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Mori A., Miyakawa Y., Ohashi E., Haga T., Maegawa T., Sajiki H. Pd/C-catalyzed chemoselective hydrogenation in the presence of diphenylsulfide. Org. Lett. 2006;8:3279–3281. doi: 10.1021/ol061147j. [DOI] [PubMed] [Google Scholar]
- 65.McOmie J.F.W., Watts M.L., West D.E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron. 1968;24:2289–2292. doi: 10.1016/0040-4020(68)88130-X. [DOI] [Google Scholar]
- 66.Caselli M., Ferrari E., Imbriano C., Pignedoli F., Saladini M., Ponterini G. Probing solute–solvent hydrogen bonding with fluorescent water-soluble curcuminoids. J. Photochem. Photobiol. A Chem. 2010;210:115–124. doi: 10.1016/j.jphotochem.2010.01.008. [DOI] [Google Scholar]
- 67.Sala F., Mulet J., Valor L.M., Criado M., Sala S. Effects of benzothiazepines on human neuronal nicotinic receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2002;136:183–192. doi: 10.1038/sj.bjp.0704699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.