Abstract
The related nematodes Pristionchus pacificus and Caenorhabditis elegans both eat bacteria for nutrition and are therefore competitors when they exploit the same bacterial resource. In addition to competing with each other, P. pacificus is a predator of C. elegans larval prey. These two relationships together form intraguild predation, which is the killing and sometimes eating of potential competitors. In killing C. elegans, the intraguild predator P. pacificus may achieve dual benefits of immediate nutrition and reduced competition for bacteria. Recent studies of P. pacificus have characterized many aspects of its predatory biting behaviour as well as underlying molecular and genetic mechanisms. However, little has been explored regarding the potentially competitive aspect of P. pacificus biting C. elegans. Moreover, aggression may also be implicated if P. pacificus intentionally bites C. elegans with the goal of reducing competition for bacteria. The aim of this review is to broadly outline how aggression, predation, and intraguild predation relate to each other, as well as how these concepts may be applied to future studies of P. pacificus in its interactions with C. elegans.
Keywords: intraguild predation, aggression, competition, nematode
Introduction
The nematode Pristionchus pacificus was first introduced by Sommer et al. (1996) to serve as a counterpoint species to Caenorhabditis elegans in comparative studies (Sommer, 2015). Since then, numerous studies have characterized the similarities, differences, and interactions between P. pacificus and C. elegans. P. pacificus and C. elegans are separated by an order of 100 million years of evolution (Dieterich et al., 2008), and share a remarkable level of similarity. On a gross morphological level, P. pacificus and C. elegans are both vermiform in shape and roughly the same size, approximately 1 mm long as young adults (Fig. 1). P. pacificus, like most nematodes, are also conveniently eutelic and have a fixed number of developmentally determined somatic cells (Hong & Sommer, 2006b; Sommer, 2015). While number, neuroanatomical positions, and processes of homologous neurons are highly conserved between the two nematodes, subtle changes in neuroanatomical features of amphid neurons (Hong et al., 2019; Srinivasan, 2008; Sommer, 2015) and massive wiring of the pharyngeal motor system have been reported (Bumbarger et al., 2013). Despite having similar life cycle length, early P. pacificus development differs from that of C. elegans in that P. pacificus eggs hatch at the J2 stage, one full larval stage later than the corresponding C. elegans L1 stage (von Lieven, 2005). Although dauer formation in both nematode species share conserved endocrine signalling (Ogawa et al., 2009), exit from dauer in P. pacificus strongly biases development of a non-predatory mouthform (Bento & Sommer, 2010).
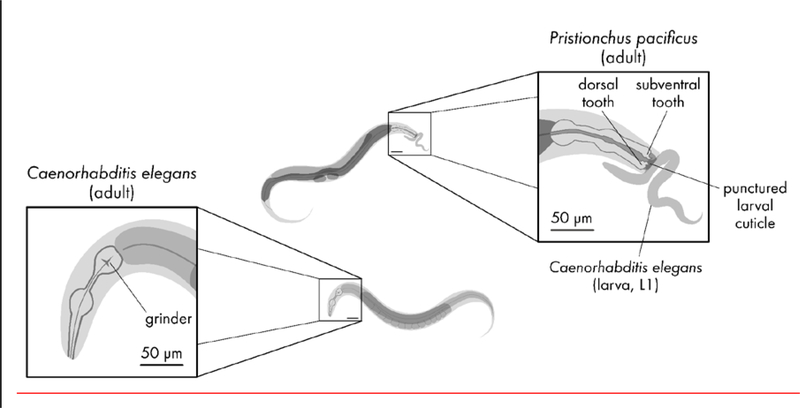
Figure 1.
P. pacificus and C. elegans are similar in size and body form at the young adult stage. C. elegans possesses a grinder that it uses to lyse bacteria for consumption. Instead of a grinder, P. pacificus instead has one or two teeth that it uses to puncture the cuticle of larval C. elegans prey. The non-predatory stenostomatous dimorph of P. pacificus has only dorsal tooth, while the predation-enabled eurystomatous dimorph possesses a larger claw-like dorsal tooth and an additional subventral tooth.
The most striking difference between P. pacificus and C. elegans relates to how they feed. While both species eat bacteria, P. pacificus, but not C. elegans, can also kill and consume non-self nematode larvae with the use of teeth-like denticles (Fig. 1). P. pacificus, as do most Diplogastrids, possesses a dorsal tooth and lacks the pharyngeal grinder (Fig. 1) that C. elegans uses to grind bacteria (von Lieven & Sudhaus, 2000). This dramatic restructuring of the buccal cavity is accompanied by drastic rewiring of the P. pacificus pharyngeal motor system relative to that of C. elegans (Bumbarger et al., 2013). P. pacificus exhibits a developmental dimorphism in which a proportion of individuals known as stenostomatous develop only a dorsal tooth, while eurystomatous individuals develop a larger dorsal tooth and an additional ventral tooth (Fig. 1). The relative proportions of eurystomatous and stenostomatous individuals in a population are affected by starvation, crowding, and the sulfatase EUD-1, all of which promote the eurystomatous mouth form (Bento & Sommer, 2010; Namdeo et al., 2018; Ragsdale et al., 2013). The eurystomatous mouth form is adaptive for predating on nematode larvae, while the stenostomatous mouth form is ineffective for killing prey and is restricted to bacteriovory and prey scavenging (Serobyan, 2014; Wilecki et al., 2015). The remainder of this review will only discuss eurystomatous individuals, since they are able to inflict harm on other nematodes and therefore have the potential to be aggressive.
Some indirect evidence exists to suggest that P. pacificus and C. elegans may compete with each other. Both nematodes have overlapping bacterial diets in the wild (Akduman et al., 2018; Samuel et al., 2016) and have been found to co-occur in nature on bacteria-rich rotting plant material (Félix et al., 2018). In exploiting the same bacterial resources, P. pacificus and C. elegans likely compete with each other in an indirect manner. Direct competition may also occur if P. pacificus interferes with C. elegans access to bacteria. P. pacificus may achieve this by using its teeth, which are the only implements of direct physical harm that P. pacificus possesses. However, P. pacificus teeth have traditionally been attributed to predatory function, so further research must be done before a competitive function can be ascribed to biting.
The killing and sometimes feeding on an interspecific potential competitor is called intraguild predation (Polis, 1989). When P. pacificus kills and feeds on C. elegans, it can simultaneously achieve both a prey meal and decreased competition for bacterial resources. However, it is unclear whether this competitive benefit is intentional or just a side effect of predation, and the motivation of an intraguild predator is notoriously difficult to dissect. An animal’s goal is obscured when a single behaviour produces multiple simultaneous benefits. Motivation further eludes simple inference when the eliciting stimuli and behavioural expression of killing appear similar regardless of whether killing is motivated by hunger, competition for a shared resource, or a combination of both. If P. pacificus is motivated by the goal of killing and eating prey, then killing of C. elegans is predation. On the other hand, if P. pacificus is motivated by the goal of reducing competition for bacteria, then killing of C. elegans is interspecific aggression. Although both involve intentional harm of others, aggression has been traditionally distinguished from predation in their respective competitive and nutritional goals for harm (Archer, 1988; Nelson, 2005).
While many studies have explored the ecological ramifications of intraguild predation on a community level, little is known about the motivation that drives attack behaviour on the individual intraguild predator level. Intraguild predation is widespread throughout the animal kingdom and is a key trophic module in many food webs (Arim & Marquet, 2004). After introducing intraguild predation as a concept (Polis & Holt, 1992), Holt & Polis (1997) articulated a theoretical framework of intraguild predation that predicted immense impacts on biodiversity and community structure. Since then, most studies of intraguild predation have focused on validating or invalidating those predictions by measuring population patterns and dynamics. Field studies are well-suited for these macroecological investigations of intraguild predation: with access to the full complexity of an open ecosystem, field studies of intraguild predation have unsurpassable ecological validity. However, open ecosystems preclude fine control and manipulation of environmental elements that may instigate and influence the predator to attack. This makes it is difficult to control the experiences of any single animal. A deeper understanding of the individual intraguild predator’s internal state will enrich understanding of observed behaviour in the field as well as provide more accurate predictions of the ecological effects of intraguild predation. For example, prey avoidance of intraguild predators has been shown to be a critical constraint on species coexistence (Sommers & Chesson, 2019; Pringle et al., 2019). However, little is known about how the intraguild predator’s motivation influences its proclivity to attack intraguild prey, which in turn may influence level of prey avoidance.
We suggest that the laboratory study of a simple tripartite community module consisting of P. pacificus, C. elegans, and bacteria is ideal for elucidating the context-dependent motivations underlying intraguild predation. In contrast to vertebrate, the use of invertebrate prey circumvents ethical qualms of purposefully subjecting vertebrates to being painfully killed and eaten as prey. Additionally, P. pacificus and C. elegans have large brood sizes and short life cycles of only 3–4 days in optimal conditions (Byerly et al., 1976; Félix et al., 1999), allowing for fast quantification of fitness consequences. Both nematodes are cultivated in the laboratory using the same standard bacterial strain E. coli OP50 (Brenner, 1974; Sommer et al., 1996), although other bacterial strains can be fed to explore effects on diet and competition. Perhaps the most powerful advantage of studying the proposed tripartite system is the relative ease of applying genetic tools to P. pacificus, bacteria, and especially C. elegans. All three organisms conveniently produce genetically identical progeny: C. elegans and P. pacificus are self-fertilizing species (Brenner, 1974; Sommer et al., 1996), while bacteria reproduce asexually. Genetic modification methods such as RNAi, DNA-mediated transformation, and genome editing have been established for P. pacificus (Schlager et al., 2009; Cinkornpumin et al., 2011; Witte et al., 2015) and C. elegans (Dickinson & Goldstein, 2016; Nance & Frøkjær-Jensen, 2019). The genomes of the laboratory E. coli OP50 strain (May et al., 2009) as well as wild microbiomes from P. pacificus (Rae et al., 2008; Koneru et al., 2016; Akduman et al., 2018) and C. elegans (Dirksen et al., 2016; Samuel et al., 2016; Schulenburg & Félix, 2017) will allow for correlation of bacterial genetic components with resource-dependent perturbations of nematode behaviour. Furthermore, bacterial transformation methods (Sheth et al., 2016) can be used to engineer bacteria in order to causally identify which bacterial signals trigger nematode competitive responses.
This review is unconventional in that it is intended to provide a broad conceptual foundation for catalysing future laboratory experiments of nematode intraguild predation, which are currently non-existent in the published corpus of nematode literature. To begin to unravel aggressive and predatory motivational components of intraguild predation between P. pacificus and C. elegans, this review considers relevant key concepts, identifies guiding principles, and highlights approaches in aggression, predation, and intraguild predation. First, we establish definitions of aggression that are broadly applicable and discuss interspecific aggression. Second, predation is reviewed to explore which predatory behaviours allow the possibility for predatory attack to be intentionally harmful. Third, field observations and theoretical predictions of intraguild predation are outlined as a conceptual framework for future work. Finally, P. pacificus, C. elegans, and their trophic relationships with each other and bacteria are characterized as the focal intraguild predation community module of this review.
Aggression
“Aggression” is an unbound term used to refer to a subset of complex social interactions. Although numerous definitions of aggression have been proposed, none concisely encapsulate the behavioural diversity of aggression. Furthermore, many of these definitions are fraught with stipulations about motivations that are not readily observable. Despite lack of consensus, it is generally accepted that a hallmark feature of aggression is intentional harm or injury to others (Berkowitz, 1981). From this, a minimal definition of aggression can be framed as any behaviour that is intended to inflict harm to another individual (Berkowitz, 1993; Buss, 1961; Gendreau & Archer, 2005; Olivier & Young, 2002). This minimal definition inherently possesses little value for discriminating between aggressive behaviours and does not capture the multifaceted complexity of aggression. Several taxonomies have been developed to meaningfully characterize differences between aggressive behaviours and sort them into discrete subtypes. These classification systems vary in which dimensions of aggression they use to compare aggressive behaviours. These dimensions include behavioural expression, eliciting stimulus, motivation, functional value, and underlying neurophysiological mechanisms (Gendreau & Archer, 2005). Of these classification dimensions, motivation is the most difficult to evaluate because it must be inferred from the others.
In all aggression taxonomies, competition is the most representative and often defining function of aggression (Archer, 1988; Nelson, 2005). We will therefore introduce a more stringent definition of aggression that we will refer to as the competitive definition of aggression, which we define as any behaviour that is intended to 1) inflict harm to another individual and 2) deal with competition. It is important to note that this definition requires that both harm and competition be intentional. Aggression that conforms this is competitive definition of aggression include some of the most distinctive aggressive behaviours. For example, aggression associated with male-male competition for mates is often marked by conspicuous behavioural expression (ritual combat) that is specifically elicited (by male targets) for a singular observable function (access to mates) (Chen et al., 2002; Crane, 1966; Darwin, 1896; Huxley, 1996; Issa & Edwards, 2006; Kravitz & Huber, 2003; Moynihan & Moynihan, 1998). It has been suggested that ritualized aggression evolved as a way for social species to settle intraspecific contests without killing conspecifics (De Waal, 2000; Nelson, 2000). In the case of ritualized aggression, one-to-one mapping between behavioural expression, eliciting stimulus, and function provide unambiguous support that mate competition is the driving motivation of aggression.
Interspecific aggression
In contrast to mate competition that is necessarily intraspecific, territoriality is the most commonly studied form of agonistic interactions between species (Grether et al., 2009; Peiman & Robinson, 2010). Although first described in birds (Howard, 1920), territoriality evolved in many animals such as fish (Gerking, 1959), mammals (Burt, 1943), reptiles (Brattstrom, 1974), and insects (Baker, 1983). ‘Territory’ is any defended area in which a dominant individual or group has priority of access to resources (Kaufmann, 1983; Nice, 1941). Notably, this dominance must be achieved through social interaction, often with aggressive attacks and threats. Territorial aggression is adaptive only when the resource benefits outweighs the energetic costs of defending territory (Brown & Orians, 1970; MacLean & Seastedt, 1979). In general, territorial aggression serves to reduce intruder trespass by driving out intruders and inducing avoidance, which ensures future supply of resources for the aggressor.
Interspecific aggression as exerted by a focal species is frequently evaluated by comparing it to intraspecific aggression that occurs in that species. In the case of interspecific territoriality involving phylogenetically related species, this kind of comparison is particular useful for determining whether interspecific aggression is a by-product of misidentification of heterospecifics as conspecifics due to apparent similarity (Murray, 1981), or if it is a case of alpha-selection, in which interspecific territorial aggression is an adaptive response to resource overlap with another species and is selected for separately from intraspecific aggression (Gill, 1974). For example, a study of two species of reciprocally aggressive salamanders showed that one species likely misidentifies since it is equally aggressive to conspecifics and heterospecifics across levels of sympatry and interspecific competition, while the other species was equally aggressive to both heterospecifics and conspecifics only when interspecific competition was strong (Nishikawa, 1987). Comparison between intraspecific and interspecific terroriality is also useful for understanding evolution of associated phenotypes, also known as agonistic character displacement (Grether et al., 2009, 2013). A species that benefits from dealing with both conspecific and heterospecific intruders could do so most efficiently if competitor recognition cues were similar in both species, driving the convergence of characteristics in both species (Cody, 1969). Conversely divergence of characteristics may occur when interspecific aggression is maladaptive (Lorenz, 1966; Tynkkynen et al., 2004).
Predation
Little is known about how interspecific aggression evolved in animals that do not already possess intraspecific aggression. In cases like this, interspecific aggression can be compared to predator-prey encounters, since both are agonistic behaviours that often have similar motor or action patterns despite having different functions (King, 1973). In order to accurately relate predation to aggression, predation must first be explicitly defined. Predation at its broadest refers to an organism killing another organism for nutritional purposes (Taylor, 1984). This definition differs from the previously described minimal definition of aggression in three important ways: (1) harm is ideally lethal, (2) harm does not need to be intentional, and (3) nutrition is the function for behaviour. This last point is the main cause of contention regarding whether predatory killing should be included as a subtype of aggression. Moyer (1968) was first to outline a stimulus-based taxonomy of aggression, in which predatory aggression was defined as behaviour that is elicited by and targeted at prey. However, a subsequent classification scheme, based on function rather than eliciting stimulus, rejected predation as valid form of aggression because it did not fulfil any competitive, protective, or parental purpose (Archer, 1988). In discussing predation, we will not yet impose these exclusionary criteria based on function, though they should be acknowledged for their classification value. Instead, this section will explore how predation may overlap with aggression, based on the broad definition of predation and minimal definition of aggression. Specifically, this section will focus on the intentionality of the harm inflicted during predation, as well as how it fulfils a requirement of aggression.
Nonaggressive predatory behaviours
Predation likely first evolved when the first unicellular life forms appeared, and has since evolved independently many times across all domains and many kingdoms of life (Bengston, 2002). In contrast, aggression is typically considered to only occur between animals. A key factor for disqualifying simpler predators from aggression is whether predatory attack and feeding are simultaneous or occur in separate phases. Unicellular organisms, especially protozoa, can predate on each other by using phagocytosis to engulf a whole prey (Lancaster et al., 2019). Predatory phagocytosis is strongly implicated in the origin of mitochondria and chloroplasts as resident prokaryotes that survived engulfment (McFadden et al., 1994; Roger, 1999), leading to the origin of eukaryotes (Cavalier-Smith, 2009; Davidov & Jurkevitch, 2009). Engulfment is a simple and compressed form of predation in which killing and feeding are achieved simultaneously - there is no separate attack phase. In this case, killing of the engulfed prey is incidental to feeding on the prey and is generally not considered intentional harm, a requirement for aggression. A similar logic can also be applied to exclude multicellular suspension/filter feeders from being considered aggressive.
Other instances in which predator-prey interactions are not deemed aggressive concern the prey’s response. For example, herbivores that kill plant or algae in the process of grazing are not considered predators. Unlike engulfers and suspension/filter feeders, grazer-type herbivores can kill and feed in separate steps. For example, sea urchins can use its rasping teeth to incrementally carve away and feed on portions of kelp without necessarily killing it first (Harrold & Reed, 1985). In other words, sea urchins do not have to subjugate the kelp first to reap nutritional rewards. The kelp only dies when it receives a critical amount of damage, and once again, killing is a side effect of feeding, albeit delayed. Feeding without killing is possible when the prey is too large for engulfment and does not physically evade harm. Plants certainly can suffer from harm inflicted by herbivores and have accordingly evolved anti-herbivore defences, including chemical defences and tolerance to herbivory (Agrawal, 2011). However, these plant defences are largely passive or invisible to the herbivore, and therefore the predatory grazer lacks discernible cues for associating its own harmful actions with a correlated harm response from the prey. From an epistemological perspective, the predatory grazer cannot intend harm if it does not ‘know’ that its grazing is harmful to the prey. From an evolutionary perspective, the predatory grazer cannot intend harm if evolution did not select for it, particularly when the predator has no additional adaptive benefit from inflicting harm separately from feeding.
Potentially aggressive predatory behaviours
A predator receives feedback that its actions are harmful to prey when prey must be sufficiently maimed or killed before consumption. The potential for predation to be aggressive arises as prey become more difficult to kill and predation transforms from a simple process into a complex sequence of steps in which killing must precede feeding (Fig. 2). Predation exerts a stronger selective pressure on prey than on predators. Referred to as the ‘life–dinner principle’, failure costs the prey its life, whereas it only costs the predator a meal (Dawkins & Krebs, 1979). Mutations that are disadvantageous for predation survive longer in the predator gene pool than in the prey gene pool. This suggests that prey can quickly evolve antipredatory adaptations and accelerate co-evolution between predator and prey. Such antipredatory adaptations, such as increased size and speed, make prey more resistant to harm and ingestion and more able to escape. As prey become too big to swallow and motile instead of sessile, engulfment and grazing cease to be adequate predatory strategies. Instead of achieving harm and feeding in the same step, predation now requires considerably more effort to capture the prey before feeding can even commence. The predatory process leading to capture can be subdivided into a sequence of escalating steps: encounter, detection, pursuit, attack, and capture (Lima & Dill, 1990). The prey has the opportunity to escape at any of these points of escalation, placing selective pressure on the predator to develop efficient hunting skills. In this elongated predation process, harm is temporally separated from feeding.
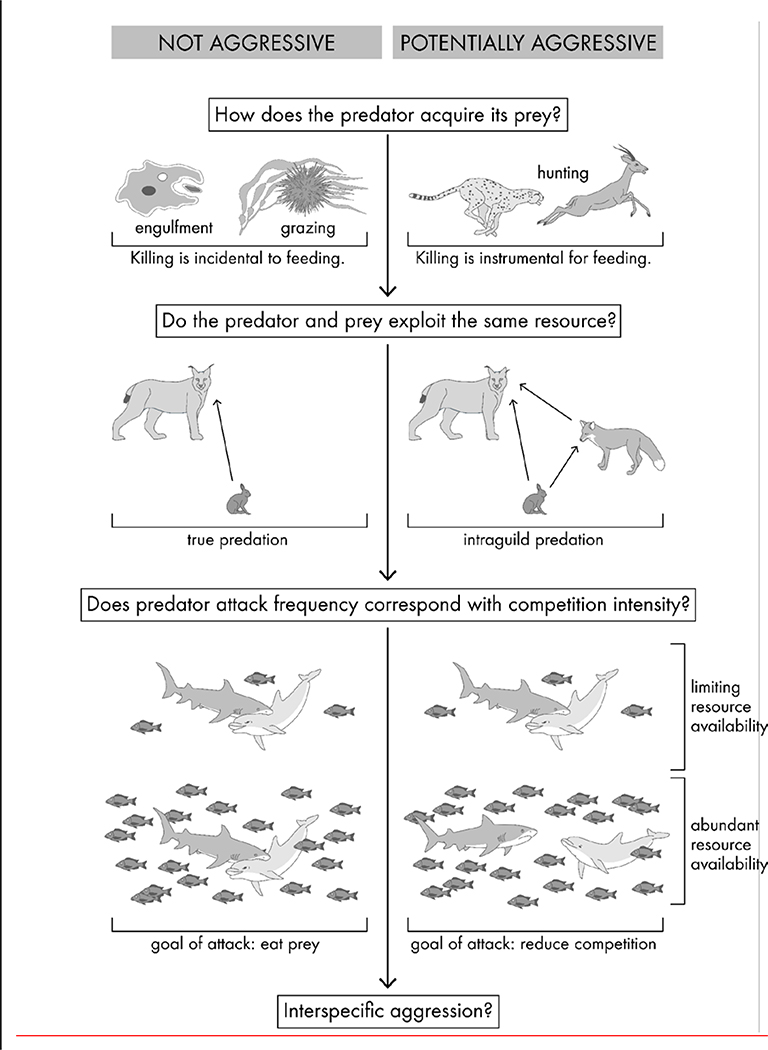
Figure 2.
Three key questions are critical for determining whether a particular predatory behaviour has the potential to be aggressive. The first question establishes whether harm in predation is intentionally inflicted. The second question identifies whether the prey can be a potential competitor. Finally, the third question explores whether the predator intentionally harms the prey for interference competition.
The particular temporal order of harm and feeding affects the degree to which intentionality of harm can be inferred. As previously described, it is difficult to disprove that harm is incidental to feeding when killing coincides with or follows feeding. In contrast, when killing precedes feeding, a causal relationship between the two becomes available as a possibility. More specifically, predatory attack may be vitally instrumental in capturing prey and contribute directly to the predator’s ability to feed on prey (Fig. 2, 1st question). In order to argue a case for predatory aggression, it must be demonstrated that harm inflicted by predatory attack is intentionally perpetrated. However, the close sequential proximity between killing and feeding insinuates that killing may be directly associated with feeding as part of a programmed feeding behavioural sequence, which would rule out aggressive intent. Predatory attack can fulfil the intentionality requirement of aggression only if it can operate separately from feeding. Therefore, studies that argue for an aggressive quality to predation have outlined ways in which predatory attack is a deliberate and separate behaviour that can operate in an uncoordinated way from feeding.
Behavioural evidence for an incongruous relationship between tendency to kill, tendency to feed, and hunger have existed for some time (Polsky, 1975). The most prominent indication comes from widespread observations that predators often kill prey in excess of what they need to fulfil their nutritional requirements, with numerous instances in which killed prey is abandoned without being consumed. Surplus killing behaviour has been readily observed in the wild for a variety of predators, including mammalian carnivores (Jedrzejewska & Jedrzejewski, 1989; Kruuk, 1972; Lincoln & Quinn, 2019; Rasa, 1973; Schaller, 2009; Zimmerman et al., 2015), rodents, (Boice & Schmeck, 1968; Desisto & Huston, 1970), birds (Nunn et al., 1976; Solheim, 1984), and insects (Lounibos et al., 2008). Experimental efforts to differentially influence killing and feeding behaviour largely come from studies of muricide by rats. Rats are known to predate on mice in the wild and in the laboratory (Karli, 1956; O’Boyle, 1974). When presented with mice, a small proportion of laboratory rats kill mice (Karli, 1956). Notably, rats that kill will only eat a portion of killed prey and with variable latency after killing. These ‘killers’ attack regardless of whether they are hungry or fully satiated. Further exploration into water deprivation, food deprivation, and time of testing relative to regular scheduled feeding time failed to show any significant effect on the tendency of killers to attack mice (Paul et al., 1971; Paul, 1972). Conversely, ‘nonkillers’ cannot be coerced into killing mice with extreme food deprivation - some rats were reported to have even starved to death in the presence of prey (Karli, 1956). Studies have also shown that the respective tendencies to kill and eat are not mutually reinforcing and do not follow each other as one is selectively repressed or promoted. For example, killing does not decrease when the rat is prevented from feeding on its prey (Myer, 1967, 1969, 1971), and killing experience is sufficient to promote killing tendency (Leyhausen, 1973). However, killing does not potentiate subsequent feeding. Rats presented with pre-killed prey were just as likely to feed as rats who were allowed to kill their own prey (Paul & Posner, 1973). Therefore, promoting killing does not always enhance feeding, nor is the inverse true. Altogether, this body of evidence suggests that predation does not always proceed as a unitary behavioural chain of killing and feeding. Rather, predatory attack can be influenced by factors other than those that influence feeding. Predatory attack may be more aptly described as an aggressive behavioural module that is intentionally, though not necessarily, deployed as a means to acquire prey.
Mouse killing is peculiarly situated in between two other rat behaviours that involve harming others: predation of other less-related species and aggression against conspecific intruders. Unlike mice prey, predation of phylogenetically distant species is characterized by much higher and more consistent rates of attack and subsequent feeding of prey such as frogs, turtles, chicks (Bandler, 1970; Desisto & Huston, 1970), and insects (Kemble & Davies, 1981). In one study, nearly all tested rats attacked frogs or turtles placed in the same cage, while only 17% of rats attacked mice in the same cage (Bandler, 1970). Killing of frog and turtle prey was almost always accompanied by eating of the corpse (Landry, 1970). Mouse killing therefore differs starkly from predation by rats’ most common prey food, and brings into question whether mouse killing possesses some non-predatory component. Since mice are phylogenetically close to rats, Blanchard et al. (1977) surmised that mouse killing shares aspects of conspecific aggression between rats. Rat colonies are known to attack strange intruder rats, and experience with intruders leads to increased attack behaviour (Blanchard et al., 1977). If mouse killing resembles intraspecific aggression against conspecific intruders, then the increase in aggression induced by exposure to conspecific rat intruders should also lead to an increase in aggression against heterospecific mice intruders. However, previous aggressive exposure to conspecifics failed to induce any change in the readiness of rats to attack either mouse targets or roach controls (Kemble & Davies, 1981). If predatory attack is indeed aggressive, it is not influenced by the same factors that govern intraspecific aggression. Thus, the behavioural signatures of intraspecific aggression cannot be referenced for identification and validation of predatory attack as a form of aggression.
In addition to behavioural evidence, hypothalamic stimulation studies in cats have shown that that feeding and killing are separable on the neuroanatomical level. While some hypothalamic sites can elicit both predatory attack and eating (Hutchinson & Renfrew, 1966), stimulation of a particular site in the lateral hypothalamus in cats has been shown to selectively elicit predatory attack (Siegel & Pott, 1988; Siegel & Brutus, 1990; Siegel & Shaikh, 1997). In order to ascertain that this lateral hypothalamic site is indeed specifically dedicated to the attack aspect of predation, Flynn and associates conducted an exhaustive set of behavioural experiments in which they attempted to coax eating behaviour out of cats while they were stimulated (Flynn, 1967; Flynn et al., 1970; Polsky, 1975). First, researchers increased stimulation to the hypothalamic site that reliably induces a cat to attack a rat, finding that even the highest intensities could not induce most tested cats to eat their captured rat prey. Similarly, persistent stimulation duration past the point of attack did not lead to consummatory feeding after predatory attack of a rat had already been evoked. Second, the researchers presented easily attainable non-prey food to reduce the effort needed to eat. When a dish of non-prey food was presented during stimulation, most cats attacked the dish but never consumed the food (Wasman & Flynn, 1962). When horsemeat is placed closer than an anesthetized rat prey in relation to a cat, stimulation induced most cats to pass over the horsemeat and attack the rat. Finally, the researchers increased motivation eat by starving cats for three days. The starved cats were then fed non-prey food and stimulated while eating. Amazingly, most of the cats halted eating of the non-prey food and proceeded to attack a nearby rat. Altogether, these cat studies indicate that a predatory attack site of the lateral hypothalamus exists that is functionally selective in influencing the attack component of predation, and is neuroanatomically distinct from other neighbouring sites that influence eating or the predatory process as a whole. Combined with previously described behavioural experiments of muricide by rats, a strong body of evidence suggests that predatory attack is dissociable from feeding, thus opening up the possibility for predatory attack to be applied for other functions, such as reducing competition.
Intraguild predation
While predatory attack as described above has been labelled as predatory aggression by a relatively small cohort of aggression researchers, consensus remains far out of reach. One explanation for this hesitancy is that it is unsatisfactory to only show that predatory attack can be dissociated from feeding - something else must replace feeding as the motivation for and function of the attack. For many, the most convincing motivation and function is competition (Archer, 1988; Nelson, 2005). We will henceforth adopt the competitive definition of aggression, which requires not only intentional harm, but also competition as the goal of that harm.
One class of interspecific interaction that can potentially satisfy both competitive motivation and function of predatory attack, and thus aggression in a more widely accepted sense, is intraguild predation (Fig. 2, 2nd question). In intraguild predation, a predator kills and sometimes eats a potential interspecific competitor (Polis, 1989). A guild consists of a group of species that exploit the same resource in a similar way (Simberloff & Dayan, 1991). From a food chain perspective, intraguild predation is the set of relationships between three trophic levels: the intraguild predator, the intraguild prey, and the shared resource. A basic model of intraguild predation has the following trophic structure: (1) Both the intraguild predator and intraguild prey exploit the same shared resource, and (2) the intraguild predator is facultative and can also eat the intraguild prey (Holt & Polis, 1997; Holt & Huxel, 2007; Polis, 1989). This type of intraguild predation is asymmetric, because only one of the guild species consistently predates on the other.
Two types of interspecific competition
Two general forms of competition, exploitation and interference, are involved in this basic form of intraguild predation. First is exploitative competition, in which two species indirectly negatively affect each other by consuming the same resource and thereby reducing resource abundance (Case & Gilpin, 1974; Tilman, 1982; Vance, 1984). If two species have the exact same resource needs and only engage in exploitative competition, the species that is more efficient at consuming the shared resource should theoretically emerge as the winner, while the less efficient consumer is driven to extinction or a different niche (Vance, 1984). In order for intraguild predation to be robust and its participating species to coexist, it must include a second form of competition, interference competition (Amarasekare, 2002; Hsu, 1982; Vance, 1984). In interference competition, one species reduces the ability of the other to exploit the shared resource (Case & Gilpin, 1974; Hsu, 1982; Vance, 1984). Intraguild predation involves a severe form of interference competition in which the competitor is killed. With these two forms of competition in mind, there are three key predictions of a simple model of stable intraguild predation (Holt & Polis, 1997; Holt & Huxel, 2007):
The intraguild prey is superior in exploiting the shared resource.
The intraguild predator should have greater fitness from predating on the intraguild prey than from competing on a purely exploitative level.
The intraguild predator, by reducing the population of the more efficient consumer species, indirectly increases the abundance of the shared resource at equilibrium.
Interference competition is the component of intraguild predation that is most relevant to demonstrating that predatory attack can be aggressive. By definition, predation of the intraguild prey eliminates competitors for a shared resource and thus fulfils a competitive function for the intraguild predator. Competitive motivation, on the other hand, is difficult to prove in intraguild predation. The set of interactions that comprise intraguild predation are difficult to disentangle. Predation and interference competition are especially difficult to delineate because they usually occur simultaneously, which add another dimension to intentionality: in addition to harm being intentionally inflicted, is competition also intentional? Or is it an accidental benefit that emerges from facultative generalists that consume multiple trophic levels? Unfortunately, most intraguild predation research focuses on the ecological effects on intraguild predation on community structure, rather than on the individual scale. Specifically, much of the interest in intraguild predation lies in understanding if and how intraguild predation promotes species coexistence and biodiversity, often with complex variations of the intraguild predation community module.
Uneaten killed prey
Meanwhile, little research has been done to dissect the motivations of an intraguild predator, even when field examples seem to conform to the simplest form of intraguild predation. When the intraguild predator successfully kills and eats the intraguild prey, nutrition and competition benefits are simultaneously achieved and thus the corresponding motivations are difficult to distinguish. However, when the intraguild predator does not consume a proportion of intraguild prey that it kills, an opportunity arises to use the percentage of uneaten intraguild prey as a proxy indicator of non-predatory motivation.
This idea is reminiscent of the aforementioned studies of mouse-killing by rats, in which some mouse prey are left uneaten after being killed (Karli, 1956). Since both rats and mice and are phylogenetically related, it was previously hypothesized and then rejected that perhaps the killing of mice mimicked intraspecific competition against invader rats (Kemble & Davies, 1981). Instead of intraspecific competition, phylogenetic relatedness may more strongly suggest that rats and mice have overlapping resource niches. Indeed, field studies indicate that rats and mice compete intensely for the same food resources and reciprocally affect each other’s population number (King et al., 1996; Ruscoe & Murphy, 2005). Rats have also been previously described as intraguild predators of competing mice (O’Boyle, 1975). Field studies of poisoned or trapped rats have shown that mice dramatically increase in abundance when rats are removed, even if mice were also being eradicated at the same rate (Brown et al., 1996; Innes, 1995; Miller & Miller, 1995). In what is sometimes referred to as ‘competitor release’, the increase in mouse population from rat removal is much higher than expected from exploitation competition alone and strongly implicates interference competition through predation (Brown et al., 1996; Caut et al., 2007; Stapp, 1997). In order to validate whether this interference competition against mice is intentional, or just simple predatory behaviour with incidental competitive benefits, Bridgman et al. (2013) looked for (1) threat and display features associated with intraspecific aggression, and (2) uneaten prey. Results taken from wild rats indicated a lack of threat and display features towards mice, and all well-fed and starved rats ate at least a portion of euthanized mice. These findings led the researchers to conclude that interference competition in this case was predatory behaviour and not intentionally competitive.
There are two important caveats to this conclusion. First, it is important to note that here, just as in aforementioned mouse-killing studies, the researchers used similarity to intraspecific competition as an indirect metric for whether interference competition is intentional. Similarity to intraspecific competition does not address competition in a definitional sense that directly accounts for resource motivations. Additionally, intraspecific competition, especially for mates, likely evolved display postures and ritualized fighting as a way to establish dominance without killing of conspecifics (De Waal, 2000; Nelson, 2000). These social methods of communicating threat and determining the winner may serve as species-preserving restrictions on the severity of harm, and as such may not be applicable to competition between recognizably different species. Second, it is known that wild rats consume most killed mice, while laboratory rats consume only a small portion of killed mice (Karli, 1956). Laboratory rats were used to demonstrate that feeding and killing were behaviourally dissociable components of predation. While wild rats are more pertinent for ecologically valid representation of an actual ecosystem, laboratory rats may have been more valuable for extricating competitive and predatory motivations for eating or not eating prey.
In contrast to the aforementioned studies of intraguild predation in wild rats, Sunde et al. (1999) investigated the motivation of the intraguild predator by comparing intraguild predation to conventional predation, rather than to intraspecific competition. In this study, lynxes are the intraguild predator and foxes are the intraguild prey. Lynxes and foxes both predate on smaller animals such as roe deer and mountain hares. Since they do not compete with lynxes, roe deer and mountain hares are referred to as ‘true’ prey species. Predation of true prey species is considered ‘true’ foraging, because it only serves nutritional purposes and does not confer competitive benefits. If nutritional need is the only factor motivating killing of foxes, then the proportion of uneaten fox corpses should closely match the proportions of uneaten roe deer and hares. On the other hand, if something other than nutrition also motivates killing of foxes, then killed foxes should be left uneaten more often than roe deer and hare. The latter prediction was vindicated: 37% of foxes killed by lynxes are uneaten, while 2% of roe deer and 0% of hares were uneaten. This finding is similar to the previously mentioned behaviour of lab rats that attack and eat almost all frog, turtle, or insect prey but only a small percentage of mice (Bandler, 1970; Desisto & Huston, 1970), and insects (Kemble & Davies, 1981). The notable difference between these rat-mouse-true prey studies and the lynx-fox-true prey study is that intraguild predation relationships were only explicitly described in the latter. This opens a line of questioning about interference competition, rather than intraspecific competition, as a potential ‘other’ factor for driving killing of the intraguild prey.
While it may be tempting to conclude that competition is the putative other factor that motivates lynxes to kill but only sometimes eat foxes, the field study was unable to account for relative abundance of foxes, roe deer, and hares. Specifically, they could not account for how often lynxes encountered foxes or true prey by coincidence. Even if the absolute population counts of true prey were large, they may be effectively scarce to lynxes if true prey are good at evading lynx detection. On the other hand, foxes may be effectively abundant if they were poor at evading lynx detection and lynxes encountered them more often by chance. In the latter case, lynxes may find that the extra immediate energy required to subdue a fox prey may be worthwhile if they do not require as much time and energy for prey search. In short, scarcity of true prey species should increase uneaten fox corpses, while abundance of foxes should increase eating of foxes. Without full control and understanding of the relative abundances of intraguild prey and shared resources, it is difficult to concretely attribute uneaten intraguild prey to competition. Firm evidence of competition must be acquired before competitive aggression can be argued for.
The use of percentage of uneaten killed prey does not clearly delineate between predatory and competitive motivations for attacking. Killing prey without immediately feeding can have advantages that indirectly promote predation, such as caching uneaten prey for possible later consumption, benefiting other members of same social unit, or gaining experience that may facilitate later kills (Kruuk, 1972). Therefore, some have narrowed the definition of ‘surplus killing’ to refer to cases in which the predator makes no use of the kills whatsoever (Mueller & Hastings, 1977). It has been suggested that selective consumption and discarding of killed prey is be an optimal foraging strategy when the focal prey is larger than can be consumed in one feeding or there is a high density of prey (Cook & Cockrell, 1978; Formanowicz, 1984; Sih, 1980; Zong et al., 2012). For example, bears discard killed salmon during high prey abundance and when prey are low in nutritional quality, which are consistent with a strategy to maximize energy intake (Lincoln & Quinn, 2019). Therefore, it is critical to consider the energetic costs, density, and nutritional differences between true prey and competing prey before the predation of both can be compared.
To get around the problems of interpreting uneaten killed competing prey, we suggest supplementing measure of uneaten killed prey with an attack-based metric to allow for more balanced and direct measurement of predatory and competitive motivations. Harm is integral and instrumental to both predation and aggression, but feeding on prey is only relevant to predation. Therefore, uneaten killed prey can only tell us that something other than immediately feeding on that prey is motivating the predator, but does not point to what that other motivation may be. Without a positive indicator of competitive motivation for attacking, it is difficult to rule out some distally predatory function for uneaten killed competing prey. To facilitate the equal detection of both predatory and competitive intent for attacking in intraguild predation, we suggest measuring how frequency of attack changes across resource contexts (Fig. 2, 3rd question). In the language of motivation, frequency of attack indicates intensity of pursuit, while how frequency of attack changes across resource conditions indicates whether predation or aggression is the goal of attacking.
A similar approach has been applied to determine the motivation for interspecific territorial aggression between phylogenetically related species. For example, Nishikawa (1987) measured how frequency of aggressive behaviour between two species of salamanders varied across different levels of sympatry and interspecific competition in order to answer whether interspecific aggression was due to misidentification of heterospecifics as conspecifics (Murray, 1981), or whether interspecific territoriality is adaptive interference (Gill, 1974). If the latter were true, then frequency of aggressive behaviour should increase as interspecific competition increases. This concept also applies to interspecific aggression in intraguild predation. Specifically, an intraguild predator motivated by interference competition should attack the competing prey more frequently when the shared resource is more scarce or valuable. In contrast, an intraguild predator motivated by predation should attack the competing prey most when the share resource is absent and the competing is the only available food option. Motivation directs behaviour by specifying a goal and setting the intensity with which to pursue that goal (Simpson & Balsam, 2016).
Nematode intraguild predation
While field studies can provide insight into the true mix of selective pressures that an animal faces in its natural life, the laboratory setting potentially offers greater control over the many variables that can affect the intraguild predator’s motivation for attacking a competing prey. To study intraguild predation in the lab in an efficient manner, we recommend the nematodes P. pacificus and C. elegans as intraguild predator and prey, respectively, with bacteria as the shared resource (Fig. 3). In this section, we will review literature about P. pacificus and C. elegans with relation to each other and to bacteria. The goal of this section is outline what is known about the participants and interactions that constitute this proposed nematode model of intraguild predation.
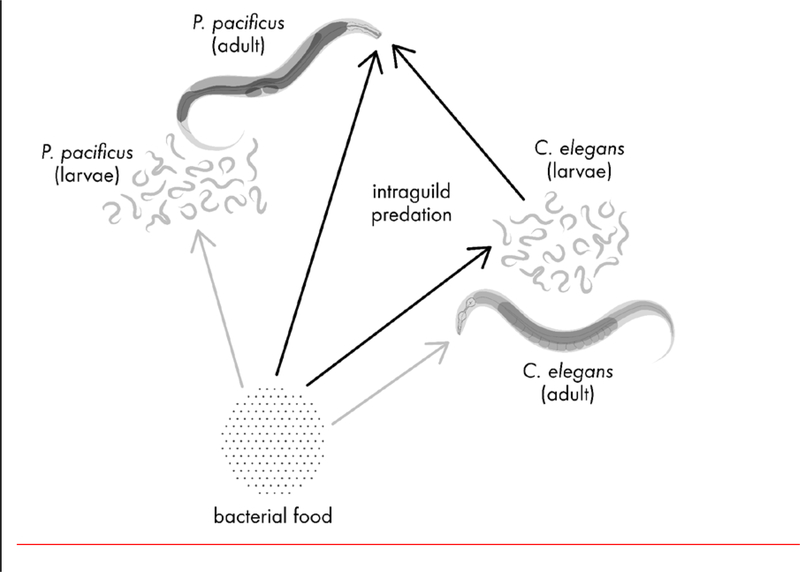
Figure 3.
This food web diagram shows the directions in which different types of food travel between P. pacificus, C. elegans, and a bacterial food that both species exploit. Arrows originate from a food source and point to the organism that eats that food. Black arrows lead between direct participants in intraguild predation, while grey arrows indicate feeding interactions that are indirectly involved. The intraguild predator is adult P. pacificus, which predates on larval C. elegans as its intraguild prey. Adult and larval stages of P. pacificus and C. elegans consume bacteria.
Intraguild predator: Pristionchus pacificus
Intraguild predators, including P. pacificus, are omnivores by definition. As a facultative predator, adult P. pacificus can derive nutrition from grazing on bacterial food and predating on nematode larva. The consumption of each of these food types flexibly engages different feeding rhythms that vary in rate of pharyngeal pumping and dorsal tooth movement (Wilecki et al., 2015). While eating bacteria, pharyngeal pumping is high and tooth movements are rare. When switching to predatory feeding, pharyngeal pumping decreases to about 66% of the bacterial rate and tooth movement increases dramatically until it matches pharyngeal pumping in a 1:1 ratio. Exogenous treatment of serotonin triggers predatory rhythms in the absence of prey, while interruption of serotonin synthesis and ablation of serotonergic neurons result in uncoordinated rhythms (Okumura et al., 2017; Wilecki et al., 2015).
P. pacificus seems to prefer bacterial food over nematode prey. When P. pacificus is presented with an excess of both larval C. elegans and bacteria, P. pacificus bite larval prey less often than when bacteria are absent (Wilecki et al., 2015). Reduced biting of larvae on bacteria suggests that predatory drive decreases when bacteria become available as an alternate food. Consistently, P. pacificus chemotaxes toward a source of E. coli OP50 bacteria when presented on the same plate as a source of larval C. elegans prey (Wilecki et al., 2015). Despite preference for naturally co-occurring bacteria over E. coli OP50, P. pacificus fecundity and survival is as high or better on a diet of E. coli (Akduman et al., 2018; Rae et al., 2008). In fact, sometimes this preference is displayed for pathogenic bacteria, such as those of the Serratia genus (Akduman et al., 2018). Overall, bacterial preference does not strongly correlate with the suitability of the food source (Akduman et al., 2018). It may also be that this discordance between nutrition and food preference may also extend to prey food that vary in species and life stage. Whether P. pacificus is more motivated to predate or compete for bacteria will likely depend on the relative valuation of the bacterial and prey foods selected for a particular intraguild predation experiment. In the previously mentioned study by (Wilecki et al., 2015) in which P. pacificus reduces biting of larval C. elegans, one could imagine that switching out E. coli OP50 to as undesirable bacteria may attenuate the reduction in biting and perhaps even elevate larval prey as the preferred food relative to the undesirable bacterial option.
It is important to note that the convention of feeding E. coli OP50 to P. pacificus in the laboratory setting was established out of convenience and a desire to ease the adoption of P. pacificus into existing C. elegans laboratories. Several studies have surveyed the microbiomes of the P. pacificus collected from natural settings (Akduman et al., 2018; Koneru et al., 2016; Meyer et al., 2017; Rae et al., 2008). Although P. pacificus can also be found in rotting plant material (Félix et al., 2018), these microbiome studies focused on the bacteria present alongside P. pacificus in scarab beetles. P. pacificus can have a necromenic association with scarab beetles, whereby they reside exclusively as dauer larvae inside the living beetle and resume development once the beetle starts to decay (Herrmann et al., 2007; Meyer et al., 2017; Ragsdale et al., 2015). Enterobaceriaceae was found in many of this studies to be the most abundant family of bacteria present P. pacificus harvested from beetles, although many other types of bacteria were also isolated (Koneru et al., 2016; Meyer et al., 2017). While E. coli is part of the Enterobaceriaceae family, Escherichia species were rarely encountered (Koneru et al., 2016). P. pacificus grown on E. coli OP50 preferred many of the bacteria isolated from beetles and soil, as measured by chemotaxis assays (Akduman et al., 2018; Koneru et al., 2016; Rae et al., 2008).
In additional to being bacterial generalists, P. pacificus are generalist predators of larvae of many nematode species (Lightfoot et al., 2019). P. pacificus uses highly specific small peptide-mediated self-recognition that allows them to discriminate between their own larvae and those of other species as well as different geographical isolates of P. pacificus (Lightfoot et al., 2019). This provides strong evidence that, if interspecific aggression indeed exists between P. pacificus and C. elegans, it is highly unlikely to be caused by misidentification of heterospecifics as conspecifics, especially since intraspecific aggression has yet to be seen between members of the same P. pacificus isolate strain.
Presented with an excess of larval C. elegans in the absence of bacteria, the standard P. pacificus laboratory strain PS312 readily bites larval prey, with about 34\% of bites resulting in killed corpses (Wilecki et al., 2015). However, the same study noted that only about half of larvae corpses were eaten and surmised that surplus killing by P. pacificus may be serve to eliminate competition. Although intraguild predation was not explicitly mentioned in (Wilecki et al., 2015), the metric of uneaten killed prey once again raises the question of whether a non-predatory component is behind the motivation for killing prey. It still remains to be demonstrated whether or not competition is in fact the non-predatory motivation in question. Recent findings reveal that the level of surplus killing by P. pacificus is influenced by the nutrient composition of its bacterial diet (Akduman et al., 2019). Specifically, B12 derived from the bacterial strain Novosphingobium L76 was found to double the killing efficiency of P. pacificus without co-ordinately increasing feeding rate. P. pacificus raised on a Novosphingobium L76 diet versus an E. coli OP50 diet exhibited differential expression of genes involved in fatty acid metabolism. Thus, in addition to bacterial preference, physiological changes induced by bacterial diet also affect predatory behaviour. In order to design contexts that may potentially discourage predation for eating prey and instead promote competition against C. elegans for bacteria, multiple bacterial variables such as abundance, preference, caloric value, and nutrient composition can be individually manipulated. How killing efficiency and surplus killing changes across bacterial conditions may provide answers to the question of whether competition can motivate P. pacificus to bite C. elegans in conditions that exacerbate competition for bacterial food.
Intraguild prey: Caenorhabditis elegans
Successful interspecific aggression often depends on the response of the target, which is C. elegans in the proposed nematode intraguild predation model. The C. elegans response to P. pacificus has not been studied in depth, which is likely due to the fact that the smallest larval stage (L1) of C. elegans are most often used to assay P. pacificus predatory behaviour. Often, the larval C. elegans is killed immediately upon contact with P. pacificus nose, thereby precluding any subsequent C. elegans response. A recent study removed the danger of live P. pacificus by instead using an extract of excretions collected from live P. pacificus animals (Liu et al., 2018). Interestingly, adult C. elegans immediately avoided this ‘predator cue’ when it was collected from starved P. pacificus, but not when the cue was collected from well-fed P. pacificus. This suggest that P. pacificus may be a more serious threat to C. elegans when bacteria are absent as a preferred food source for P. pacificus.
The concept of intentionality in motivated behaviour is useful not only for exploring aggression in P. pacificus, but also for designing experiments to characterize risk-taking and fear in C. elegans responses in intraguild predation interactions. By first understanding how environmental and internal conditions modulate P. pacificus motivation to attack C. elegans, P. pacificus can then be deliberately manipulated to pose particular levels of risk toward C. elegans. With additional manipulation of bacterial variables, C. elegans responses in intraguild predation interactions can be measured as a reflection of internal balancing of appetitive bacterial factors and aversive P. pacificus factors, the latter acquiring more weight with induced fear. More broadly, the construction of behavioural experiments to probe C. elegans intentionality opens the way for analysing more complex computations and cognitive processes underlying motivation and decision-making.
In contrast to C. elegans behavioural responses to P. pacificus, much more is known about the relationship between C. elegans and bacteria. Importantly, this knowledge may inform homology-based hypotheses about how P. pacificus senses and responds to bacteria. It is known that P. pacificus and C. elegans have disparate responses to the same set of odorants, with some odorants that are attractive to one and repulsive to the other (Hong & Sommer, 2006a; Hong, 2015). Therefore, any discussion of potential conserved bacteria responses and underlying mechanisms will have to involve direct sensation of bacteria and not of proxy odorants, such as benzaldehyde and diacetyl, that putatively represent bacteria in C. elegans. The first notable change in behaviour that C. elegans exhibits upon finding a bacterial lawn is to decrease its locomotory rate (Sawin et al., 2000). This basal slowing response requires dopamine, as dopamine synthesis mutants continue moving through bacteria at the same rate as when bacteria is absent (Sawin et al., 2000).
In addition to binary detection of the presence or absence of bacteria, C. elegans is also able to distinguish and seek out the boundary of a bacterial lawn from its circumscribed region. Some social wild strains of C. elegans and npr-1 mutants that lack the neuropeptide Y receptor naturally migrate to and aggregate at the border of a bacterial lawn, where bacteria is thickest (De Bono & Bargmann, 1998). This bordering tendency involves oxygen sensing by guanylate cyclase, which promotes aerotaxing away from regions of higher oxygen levels towards areas of lower oxygen levels in both wildtype and npr-1 mutants (Gray et al., 2004). Thick E. coli OP50 bacterial lawns consume oxygen more quickly than can be replenished by ambient diffusion, and borders with the highest concentration of bacteria were observed to have lower effective oxygen concentrations (Gray et al., 2004). Acute reduction of ambient oxygen levels abolished bordering behaviour in C. elegans (Gray et al., 2004), as well as in P. pacificus (Moreno et al., 2016). Therefore, C. elegans, as well as P. pacificus, may use relative lower oxygen concentrations to find and demarcate the lawn edge. The ability to detect the edge of a lawn opens up the possibility of estimating the size of the lawn. Indeed, guanylate cyclase C. elegans mutants were unable to distinguish between small and large lawns of bacteria (Calhoun et al., 2015). The mechanism for computing lawn size experience depends on the variability in bacteria levels that C. elegans senses during its exploration of the lawn. The thick edge relative to the thinner interior of the lawn means that C. elegans will experience changing bacteria levels more often in a small lawn, where the animal will encounter the edge at a higher rate. Large bacterial variability is sensed by ASI and ASK neurons and result in downstream dopamine release.
C. elegans has been cultivated in the laboratory setting with E. coli, OP50 since its debut as a model organism (Brenner, 1974). However, like P. pacificus, C. elegans is found in nature with a variety of other bacteria species, with Enterobacteriaceae and Acetobacteraceae species associated with high proliferation (Dirksen et al., 2016; Samuel et al., 2016; Schulenburg & Félix, 2017). C. elegans also displays preference for bacterial species other than E. coli OP50, particularly if the other bacteria is higher quality food, as measured by growth rate (Shtonda & Avery, 2006). Furthermore, C. elegans raised on higher quality bacteria leave mediocre bacteria more often (Shtonda & Avery, 2006). One such high quality bacterial strain is Comamonas sp., which was isolated from a soil environment (Avery & Shtonda, 2003). Interestingly, the list of bacteria naturally found with and preferred by P. pacificus also includes the Comamonadaceae family (Akduman et al., 2018; Koneru et al., 2016). Additionally, a Comamonas sp. DA1877 diet has been shown to increase surplus killing in P. pacificus via increased the same B12 mechanism as in a Novosphingobium L76 diet (Akduman et al., 2019). Therefore, Comamonas sp. may be useful in mutually exacerbating competition between P. pacificus and C. elegans.
Concluding remarks
The aim of this review was to outline key concepts about interspecific interactions and specifically identify feeding-related nematode literature that are relevant to answering whether P. pacificus biting of C. elegans may be a form of interspecific aggression derived from intraguild predation. In particular, establishing whether the goal of biting is to kill prey for consumption or to defend bacterial resources will be critical to answering this question.
Interspecific aggression between nematodes has been previously observed between Steinernema species that compete for host resources (O’Callaghan et al., 2014), but these parasitic nematodes also exhibit intraspecific aggression between males (Zenner et al., 2014). If interspecific aggression exists in P. pacificus, it likely arose de novo as a modification of some non-aggressive behaviour. Without intraspecific aggression as a point of comparison, we suggest contrasting potential interspecific aggression between P. pacificus and C. elegans to an agonistic interaction that already exists between the two species, predation. Second, while inter- and intraspecific aggression necessarily involve different targets of different species, C. elegans is the same target regardless of whether P. pacificus is motivated by predatory or competitive goals. To obtain interspecific aggression from intraspecific aggression, an animal only needs to change how they recognize competitors to include both conspecific and heterospecific targets. In contrast, to achieve interspecific aggression from intraguild predation, P. pacificus must be able to flexibly change which goals motivates it to harm C. elegans, either to eat or compete with it for bacteria. We hope that our proposed nematode intraguild predation model may provide insight into how a behaviour as complex as interspecific aggression can arise in a simple nematode without having intraspecific aggression as a convenient behavioural substrate.
We have presented a series of relevant concepts in aggression, predation, and intraguild predation that together provide one possible approach for determining the motivation driving the intraguild predator P. pacificus when it kills its intraguild prey C. elegans. This approach begins with establishing core criteria for aggression, which we distil into two components: intentional harm and a competitive goal for harm. More criteria can be added to achieve more face validity with aggressive behaviour as it is typically considered in the field. Since members of different species do not compete for mates, territorial aggression for the defence of overlapping resources is the most probable form of interspecific aggression. The next step is to assess whether the predatory behaviour is potentially aggressive, which we take to mean intentionally harmful. We disqualify engulfing and grazing because harm is incidental to feeding actions. Instead, we suggest that harm that precedes feeding and directly contributes to the capture and killing of prey can be intentional. Once intentional harm is established in predatory behaviour, the major task at hand is to demonstrate that a competitive goal for harm can increase P. pacificus attack frequency in conditions in which competition for bacteria in intensified. For intraguild predation, this requires assessment of both exploitative and interference competition. Careful consideration of multiple bacterial and prey factors will be crucial for designing conditions and experiments that are informative about how P. pacificus food experience and relative valuation of bacterial and prey food factor into its motivation for attacking C. elegans.
References
- Agrawal AA (2011). Current trends in the evolutionary ecology of plant defence. Functional Ecology, 25(2), 420–432. [Google Scholar]
- Akduman N, Rödelsperger C, & Sommer RJ (2018). Culture-based analysis of Pristionchus-associated microbiota from beetles and figs for studying nematode-bacterial interactions. PloS one, 13(6), e0198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akduman N, Lightfoot JW, Röseler W, Witte H, Lo WS, Rödelsperger C, & Sommer RJ (2019). Bacterial derived vitamin B12 enhances predatory behaviors in nematodes. bioRxiv, 797803. [Google Scholar]
- Amarasekare P (2002). Interference competition and species coexistence. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1509), 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J (1988). The behavioral biology of aggression. Cambridge University Press. [Google Scholar]
- Arim M, & Marquet PA (2004). Intraguild predation: a widespread interaction related to species biology. Ecology Letters 7(7), 557–564. [Google Scholar]
- Avery L, & Shtonda BB (2003). Food transport in the C. elegans pharynx. Journal of Experimental Biology, 206(14), 2441–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RR (1983). Insect territoriality. Annual Review of Entomology, 28(1), 65–89. [Google Scholar]
- Bandler RJ Jr (1970). Animals spontaneously attacked by rats. Commun Behav Biol, 5, 177–182. [Google Scholar]
- Bengtson S (2002). Origins and early evolution of predation. The Paleontological Society Papers, 8, (pp. 289–318). [Google Scholar]
- Bento G, Ogawa A, & Sommer RJ (2010). Co-option of the hormone-signalling module dafachronic acid–DAF-12 in nematode evolution. Nature, 466(7305), 494–497. [DOI] [PubMed] [Google Scholar]
- Berkowitz L (1981). The concept of aggression In Brain PE & Benton D (Eds.), Multidisciplinary approaches to aggression research (pp. 3–15). Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Berkowitz L (1993). Aggression: Its causes, consequences, and control. New York, NY: McGraw-Hill. [Google Scholar]
- Blanchard RJ, Takahashi LK, & Blanchard DC (1977). The development of intruder attack in colonies of laboratory rats. Animal Learning & Behavior, 5(4), 365–369. [Google Scholar]
- Boice R, & Schmeck RR (1968). Predatory behaviors of grasshopper mice (Onychomys leucogaster). American Zoologist, 8(4), 751. [Google Scholar]
- Brattstrom BH (1974). The evolution of reptilian social behavior. American Zoologist, 14(1), 35–49. [Google Scholar]
- Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman LJ, Innes J, Gillies C, Fitzgerald NB, Miller S, & King CM (2013). Do ship rats display predatory behaviour towards house mice?. Animal Behaviour, 86(2), 257–268. [Google Scholar]
- Brown JL, & Orians GH (1970). Spacing patterns in mobile animals. Annual review of ecology and systematics, 1(1), 239–262. [Google Scholar]
- Brown KP, Moller H, Innes J, & Alterio N (1996). Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. New Zealand Journal of Ecology, 271–275. [Google Scholar]
- Bumbarger D, Riebesell M, & Sommer R (2013). System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Cell, 152(1–2), 109–119. [DOI] [PubMed] [Google Scholar]
- Burt WH (1943). Territoriality and home range concepts as applied to mammals. Journal of mammalogy, 24(3), 346–352. [Google Scholar]
- Buss AH (1961). The psychology of aggression. New York, NY: Wiley. [Google Scholar]
- Byerly L, Cassada RC, & Russell RL (1976). The life cycle of the nematode Caenorhabditis elegans: I. Wild-type growth and reproduction. Developmental biology, 51(1), 23–33. [DOI] [PubMed] [Google Scholar]
- Calhoun AJ, Tong A, Pokala N, Fitzpatrick JA, Sharpee TO, & Chalasani SH (2015). Neural mechanisms for evaluating environmental variability in Caenorhabditis elegans. Neuron, 86(2), 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case TJ, & Gilpin ME (1974). Interference competition and niche theory. Proceedings of the National Academy of Sciences, 71(8), 3073–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caut S, Casanovas JG, Virgos E, Lozano J, Witmer GW, & Courchamp F (2007). Rats dying for mice: modelling the competitor release effect. Austral Ecology, 32(8), 858–868. [Google Scholar]
- Cavalier-Smith T (2009). Predation and eukaryote cell origins: a coevolutionary perspective. The international journal of biochemistry & cell biology, 41(2), 307–322. [DOI] [PubMed] [Google Scholar]
- Chen S, Lee AY, Bowens NM, Huber R, & Kravitz EA (2002). Fighting fruit flies: a model system for the study of aggression. Proceedings of the National Academy of Sciences, 99(8), 5664–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinkornpumin JK, & Hong RL (2011). RNAi mediated gene knockdown and transgenesis by microinjection in the necromenic nematode Pristionchus pacificus. JoVE (Journal of Visualized Experiments), (56), e3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody ML (1969). Convergent characteristics in sympatric species: a possible relation to interspecific competition and aggression. Condor, 71(3), 223–239. [Google Scholar]
- Cook RM, & Cockrell BJ (1978). Predator ingestion rate and its bearing on feeding time and the theory of optimal diets. The Journal of Animal Ecology, 47(2), 529–547. [Google Scholar]
- Crane J (1966). Combat, display and ritualization in fiddler crabs (Ocypodidae, genus Uca). Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 251(772), 459–472. [Google Scholar]
- Darwin C (1896). Charles Darwin’s Works: The descent of man and selection in relation to sex (Vol. 9). New York: D. Appleton. [Google Scholar]
- Davidov Y, & Jurkevitch E (2009). Predation between prokaryotes and the origin of eukaryotes. BioEssays, 31(7), 748–757. [DOI] [PubMed] [Google Scholar]
- Dawkins R, & Krebs JR (1979). Arms races between and within species. Proceedings of the Royal Society of London. Series B. Biological Sciences, 205(1161), 489–511. [DOI] [PubMed] [Google Scholar]
- De Bono M, & Bargmann CI (1998). Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell, 94(5), 679–689. [DOI] [PubMed] [Google Scholar]
- De Waal FB (2000). Primates---a natural heritage of conflict resolution. Science, 289(5479), 586–590. [DOI] [PubMed] [Google Scholar]
- Desisto MJ, & Huston JP (1970). Effect of territory on frog-killing by rats. The Journal of general psychology, 83(2), 179–184. [DOI] [PubMed] [Google Scholar]
- Dickinson DJ, & Goldstein B (2016). CRISPR-based methods for Caenorhabditis elegans genome engineering. Genetics, 202(3), 885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich C, Clifton S, Schuster LN, Chinwalla A, Delehaunty K, Dinkelacker, … Sommer RJ (2008). The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nature Genetics, 40, 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen P, Marsh SA, Braker I, Heitland N, Wagner S, Nakad R, … & Félix MA (2016). The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC biology, 14(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix MA, Hill RJ, Schwarz H, Sternberg PW, Sudhaus W, & Sommer RJ (1999). Pristionchus pacificus, a nematode with only three juvenile stages, displays major heterochronic changes relative to Caenorhabditis elegans. Proceedings of the Royal Society of London. Series B: Biological Sciences, 266(1429), 1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M-A, Ailion M, Hsu JC, Richaud A, & Wang J (2018). Pristionchus nematodes occur frequently in diverse rotting vegetal substrates and are not exclusively necromenic, while Panagrellus redivivoides is found specifically in rotting fruits. PloS one, 13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JP (1967). The neural basis of aggression in cats In Glass DC (Ed.), Neurophysiology and emotion, (pp. 40–60). New York: Rockefeller University Press and Russell Sage Foundation. [Google Scholar]
- Flynn JP, Vanegas H, Foote W, & Edwards S (1970). Neural mechanisms involved in a cat’s attack on a rat In The neural control of behavior (pp. 135–173). Academic Press. [Google Scholar]
- Flynn JP (1976). Neural basis of threat and attack. Biological foundations of psychiatry, 1, 273–295. [Google Scholar]
- Formanowicz DR Jr (1984). Foraging tactics of an aquatic insect: partial consumption of prey. Animal Behaviour, 32(3), 774–781. [Google Scholar]
- Gendreau PL & Archer J (2005). Subtypes of Aggression in Humans and Animals In R. Tremblay E, Hartup WW, & Archer J (Eds.), Developmental origins of aggression, (pp. 25–46). New York: Guilford Press. [Google Scholar]
- Gerking SD (1959). The restricted movement of fish populations. Biological reviews, 34(2), 221–242. [Google Scholar]
- Gill DE (1974). Intrinsic rate of increase, saturation density, and competitive ability. II. The evolution of competitive ability. The American Naturalist, 108(959), 103–116. [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, … & Bargmann CI (2004). Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature, 430(6997), 317–322. [DOI] [PubMed] [Google Scholar]
- Grether GF, Losin N, Anderson CN, & Okamoto K (2009). The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biological Reviews, 84(4), 617–635. [DOI] [PubMed] [Google Scholar]
- Grether GF, Anderson CN, Drury JP, Kirschel AN, Losin N, Okamoto K, & Peiman KS (2013). The evolutionary consequences of interspecific aggression. Annals of the New York Academy of Sciences, 1289(1), 48–68. [DOI] [PubMed] [Google Scholar]
- Harrold C, & Reed DC (1985). Food availability, sea urchin grazing, and kelp forest community structure. Ecology, 66(4), 1160–1169. [Google Scholar]
- Herrmann M, Mayer WE, Hong RL, Kienle S, Minasaki R, & Sommer RJ (2007). The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zoological science, 24(9), 883–889. [DOI] [PubMed] [Google Scholar]
- Holt RD, & Polis GA (1997). A theoretical framework for intraguild predation. The American Naturalist, 149(4), 745–764. [Google Scholar]
- Holt RD, & Huxel GR (2007). Alternative prey and the dynamics of intraguild predation: theoretical perspectives. Ecology, 88(11), 2706–2712. [DOI] [PubMed] [Google Scholar]
- Hong RL, & Sommer RJ (2006a). Chemoattraction in Pristionchus nematodes and implications for insect recognition. Current Biology, 16(23), 2359–2365. [DOI] [PubMed] [Google Scholar]
- Hong RL, & Sommer RJ (2006b). Pristionchus pacificus: a well-rounded nematode. Bioessays, 28(6), 651–659. [DOI] [PubMed] [Google Scholar]
- Hong RL (2015). Pristionchus pacificus olfaction In Sommer RJ (Ed.), Pristionchus pacificus: a nematode model for comparative and evolutionary biology, (pp. 331–352). Leiden, Netherlands: Brill. [Google Scholar]
- Hong RL, Riebesell M, Bumbarger DJ, Cook SJ, Carstensen HR, Sarpolaki T, … & Hobert O (2019). Evolution of neuronal anatomy and circuitry in two highly divergent nematode species. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard HE (1920). Territory in bird life. New York: EP. Dutton & Company. [Google Scholar]
- Hsu SB (1982). On a resource based ecological competition model with interference. Journal of Mathematical Biology, 12(1), 45–52. [Google Scholar]
- Hutchinson RR, & Renfrew JW (1966). Stalking attack and eating behaviors elicited from the same sites in the hypothalamus. Journal of Comparative and Physiological Psychology, 61(3), 360. [DOI] [PubMed] [Google Scholar]
- Huxley J (1966). Introduction: A discussion on ritualization of behaviour in animals and man. Philosophical Transactions of the Royal Society of London, Series B}, 251, 249–271. [Google Scholar]
- Innes J, Warburton B, Williams D, Speed H, & Bradfield P (1995). Large-scale poisoning of ship rats (Rattus rattus) in indigenous forests of the North Island, New Zealand. New Zealand journal of ecology, 5–17. [Google Scholar]
- Issa FA, & Edwards DH (2006). Ritualized submission and the reduction of aggression in an invertebrate. Current Biology, 16(22), 2217–2221. [DOI] [PubMed] [Google Scholar]
- Jedrzejewska B, & Jedrzejewski W (1989). Seasonal surplus killing as hunting strategy of the weasel Mustela nivalis-test of a hypothesis. Acta Theriologica, 34(12–28), 347–360. [Google Scholar]
- Karli P (1956). The Norway rat’s killing response to the white mouse: an experimental analysis. Behaviour, 1(2) 81–103. [Google Scholar]
- Kaufmann JH (1983). On the definitions and functions of dominance and territoriality. Biological Reviews, 58(1), 1–20. [Google Scholar]
- Kemble ED, & Davies VA (1981). Effects of prior environmental enrichment and amygdaloid lesions on consumatory behavior, activity, predation, and shuttlebox avoidance in male and female rats. Physiological Psychology, 9(4), 340–346. [Google Scholar]
- King JA (1973). The ecology of aggressive behavior. Annual review of ecology and systematics, 4(1), 117–138. [Google Scholar]
- King CM, Innes JG, Flux M, Kimberley MO, Leathwick JR, & Williams DS (1996). Distribution and abundance of small mammals in relation to habitat in Pureora Forest Park. New Zealand Journal of Ecology, 215–240. [Google Scholar]
- Koneru SL, Salinas H, Flores GE, & Hong RL (2016). The bacterial community of entomophilic nematodes and host beetles. Molecular ecology, 25(10), 2312–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA, & Huber R (2003). Aggression in invertebrates. Current opinion in neurobiology, 13(6), 736–743. [DOI] [PubMed] [Google Scholar]
- Kruuk H (1972). Surplus killing by carnivores. Journal of Zoology, 166(2), 233–244. [Google Scholar]
- Lancaster CE, Ho CY, Hipolito VE, Botelho RJ, & Terebiznik MR (2019). Phagocytosis: what’s on the menu?. Biochemistry and Cell Biology, 97(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Landry SO Jr (1970). The Rodentia as omnivores. The Quarterly Review of Biology, 45(4), 351–372. [DOI] [PubMed] [Google Scholar]
- Leyhausen P (1973). On the function of the relative hierarchy of moods (as exemplified by the phylogenetic and ontogenetic development of prey-catching in carnivores) In Lorenz K and Leyhausen P (Eds.), Motivation of Human and Animal Behavior, (pp. 144–247). London: Van Nostrand Reinhold. [Google Scholar]
- Lightfoot JW, Wilecki M, Rödelsperger C, Moreno E, Susoy V, Witte H, & Sommer RJ (2019). Small peptide–mediated self-recognition prevents cannibalism in predatory nematodes. Science, 364(6435), 86–89. [DOI] [PubMed] [Google Scholar]
- Lima SL, & Dill LM (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Canadian journal of zoology, 68(4), 619–640. [Google Scholar]
- Lincoln AE, & Quinn TP (2019). Optimal foraging or surplus killing: selective consumption and discarding of salmon by brown bears. Behavioral Ecology, 30(1), 202–212. [Google Scholar]
- Liu Z, Kariya MJ, Chute CD, Pribadi AK, Leinwand SG, Tong A, … & Chalasani SH (2018). Predator-secreted sulfolipids induce defensive responses in C. elegans. Nature communications, 9(1), 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K (1966). On aggression. New York: Harcourt, Brace and World. [Google Scholar]
- Lounibos LP, Makhni S, Alto BW, & Kesavaraju B (2008). Surplus killing by predatory larvae of Corethrella appendiculata: prepupal timing and site-specific attack on mosquito prey. Journal of insect behavior, 21(2), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean SF Jr, & Seastedt TR (1979). Avian territoriality: sufficient resources or interference competition. The American Naturalist, 114(2), 308–312. [Google Scholar]
- May RC, Loman NJ, Haines AS, Pallen MJ, Boehnisch C, Penn CW, … & Kim J (2009). The genome sequence of E. coli OP50. Worm Breed. Gaz, 18, 24. [Google Scholar]
- McFadden GI, Gilson PR, Hofmann C, Adcock GJ, & Maier U-G. (1994). Evidence that an amoeba acquired a chloroplast by retaining part of an engulfed eukaryotic alga. Proceedings of the National Academy of Sciences, 91(9), 3690–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Baskaran P, Quast C, Susoy V, Rödelsperger C, Glöckner FO, & Sommer RJ (2017). Succession and dynamics of Pristionchus nematodes and their microbiome during decomposition of Oryctes borbonicus on La Réunion Island. Environmental microbiology, 19(4), 1476–1489. [DOI] [PubMed] [Google Scholar]
- Miller CJ, & Miller TK (1995). Population dynamics and diet of rodents on Rangitoto Island, New Zealand, including the effect of a 1080 poison operation. New Zealand Journal of Ecology, 19–27. [Google Scholar]
- Moreno E, McGaughran A, Rödelsperger C, Zimmer M, & Sommer RJ (2016). Oxygen-induced social behaviours in Pristionchus pacificus have a distinct evolutionary history and genetic regulation from Caenorhabditis elegans. Proceedings of the Royal Society B: Biological Sciences, 283(1825), 20152263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer KE (1968). Kinds of aggression and their physiological basis. Communications in Behavioral Biology, 2(2), 65–87. [Google Scholar]
- Moynihan M, & Moynihan M (1998). The social regulation of competition and aggression in animals. Washington DC: Smithsonian Institution Press. [Google Scholar]
- Mueller DL, & Hastings BC (1975). A clarification of “surplus killing”. Animal Behaviour, 25, 1065. [Google Scholar]
- Murray BG (1981). The origins of adaptive interspecific territorialism. Biological Reviews, 56(1), 1–22. [Google Scholar]
- Myer JS (1967). Prior killing experience and the suppressive effects of punishment on the killing of mice by rats. Animal behaviour, 15(1), 59–61. [DOI] [PubMed] [Google Scholar]
- Myer JS (1969). Early experience and the development of mouse killing by rats. Journal of Comparative and Physiological Psychology, 67(1), 46. [DOI] [PubMed] [Google Scholar]
- Myer JS (1971). Experience and the stability of mouse killing by rats. Journal of Comparative and Physiological Psychology, 75(2), 264. [DOI] [PubMed] [Google Scholar]
- Namdeo S, Moreno E, Rödelsperger C, Baskaran P, Witte H, & Sommer RJ (2018). Two independent sulfation processes regulate mouth-form plasticity in the nematode Pristionchus pacificus. Development, 145(13), dev166272. [DOI] [PubMed] [Google Scholar]
- Nance J, & Frøkjær-Jensen C (2019). The Caenorhabditis elegans transgenic toolbox. Genetics, 212(4), 959–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ (2000). Affiliative and aggressive behavior In An introduction to behavioral endocrinology (3rd ed., pp. 395–445). Sunderland, MA: Sinauer. [Google Scholar]
- Nelson RJ (Ed.). (2005). Biology of aggression. Oxford University Press. [Google Scholar]
- Nice MM (1941). The role of territory in bird life. American Midland Naturalist,26(3), 441–487. [Google Scholar]
- Nishikawa KC (1987). Interspecific aggressive behaviour in salamanders: species-specific interference or misidentification?. Animal Behaviour, 35(1), 263–270. [Google Scholar]
- Nunn GL, Klem D Jr, Kimmel T, & Merriman T (1976). Surplus killing and caching by American kestrels (Falco sparverius). Animal Behaviour, 24(4), 759–763. [Google Scholar]
- O’Boyle M (1974). Rats and mice together: The predatory nature of the rat’s mouse-killing response. Psychological bulletin, 81(4), 261. [DOI] [PubMed] [Google Scholar]
- O’Boyle M (1975). The rat as a predator. Psychological Bulletin, 82, 460–462 [Google Scholar]
- O’Callaghan KM, Zenner AN, Hartley CJ, & Griffin CT (2014). Interference competition in entomopathogenic nematodes: male Steinernema kill members of their own and other species. International journal for parasitology, 44(13), 1009–1017. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Streit A, Antebi A, & Sommer RJ (2009). A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Current Biology, 19(1), 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M, Wilecki M, & Sommer RJ (2017). Serotonin drives predatory feeding behavior via synchronous feeding rhythms in the nematode Pristionchus pacificus. G3: Genes, Genomes, Genetics, 7(11), 3745–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B & Young LJ (2002). Animal models of aggression In Davis KL, Charney D, Coyle JT, & Nemeroff C, Neuropsychopharmacology: The Fifth Generation of Progress, (pp. 1699–1706). Philadelphia, PA: Lippincott. [Google Scholar]
- Paul L, Miley WM, & Baenninger R (1971). Mouse killing by rats: Roles of hunger and thirst in its initiation and maintenance. Journal of Comparative and Physiological Psychology, 76(2), 242. [DOI] [PubMed] [Google Scholar]
- Paul L (1972). Predatory attack by rats: Its relationship to feeding and type of prey. Journal of Comparative and Physiological Psychology, 78(1), 69. [DOI] [PubMed] [Google Scholar]
- Paul L, & Posner I (1973). Predation and feeding: Comparisons of feeding behavior of killer and nonkiller rats. Journal of Comparative and Physiological Psychology, 84(2), 258. [DOI] [PubMed] [Google Scholar]
- Peiman K, & Robinson B (2010). Ecology and evolution of resource-related heterospecific aggression. The Quarterly Review of Biology, 85(2), 133–158. [DOI] [PubMed] [Google Scholar]
- Polis GA, Myers CA, & Holt RD (1989). The ecology and evolution of intraguild predation: potential competitors that eat each other. Annual review of ecology and systematics, 20(1), 297–330. [Google Scholar]
- Polsky RH (1975). Hunger, prey feeding, and predatory aggression. Behavioral Biology, 13(1), 81–93. [DOI] [PubMed] [Google Scholar]
- Rae R, Riebesell M, Dinkelacker I, Wang Q, Herrmann M, Weller AM, … & Sommer RJ (2008). Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. Journal of Experimental Biology, 211(12), 1927–1936. [DOI] [PubMed] [Google Scholar]
- Ragsdale EJ, Müller MR, Rödelsperger C, & Sommer RJ (2013). A developmental switch coupled to the evolution of plasticity acts through a sulfatase. Cell, 155(4), 922–933. [DOI] [PubMed] [Google Scholar]
- Ragsdale EJ, Kanzaki N, & Herrmann M (2015). Taxonomy and natural history: the genus Pristionchus In Sommer RJ (Ed.), Pristionchus pacificus: a nematode model for comparative and evolutionary biology, (pp. 77–120). Leiden, Netherlands: Brill. [Google Scholar]
- Rasa OAE (1973). Prey capture, feeding techniques, and their ontogeny in the African dwarf mongoose, Helogale undulata rufula. Zeitschrift für Tierpsychologie, 32(5), 449–488. [DOI] [PubMed] [Google Scholar]
- Roger AJ (1999). Reconstructing early events in eukaryotic evolution. The American Naturalist, 154(S4), S146–S163. [DOI] [PubMed] [Google Scholar]
- Ruscoe WA, & Murphy EC (2005). House mouse. In King CM (Ed.), The Handbook of New Zealand Mammals, (pp. 204–221). [Google Scholar]
- Samuel BS, Rowedder H, Braendle C, Félix MA, & Ruvkun G (2016). Caenorhabditis elegans responses to bacteria from its natural habitats. Proceedings of the National Academy of Sciences, 113(27), E3941–E3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, & Horvitz HR (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron, 26(3), 619–631. [DOI] [PubMed] [Google Scholar]
- Schaller GB (2009). The Serengeti lion: a study of predator-prey relations. University of Chicago Press. [Google Scholar]
- Schlager B, Wang X, Braach G, & Sommer RJ (2009). Molecular cloning of a dominant roller mutant and establishment of DNA-mediated transformation in the nematode Pristionchus pacificus. genesis, 47(5), 300–304. [DOI] [PubMed] [Google Scholar]
- Schulenburg H, & Félix MA (2017). The natural biotic environment of Caenorhabditis elegans. Genetics, 206(1), 55–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serobyan V, Ragsdale EJ, & Sommer RJ (2014). Adaptive value of a predatory mouth-form in a dimorphic nematode. Proceedings of the Royal Society B: Biological Sciences, 281(1791), 20141334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth RU, Cabral V, Chen SP, & Wang HH (2016). Manipulating bacterial communities by in situ microbiome engineering. Trends in Genetics, 32(4), 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtonda BB, & Avery L (2006). Dietary choice behavior in Caenorhabditis elegans. Journal of experimental biology, 209(1), 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, & Pott CB (1988). Neural substrates of aggression and flight in the cat. Progress in neurobiology, 31(4), 261–283. [DOI] [PubMed] [Google Scholar]
- Siegel A, & Brutus M (1990). Neural substrates of aggression and rage in the cat. Progress in psychobiology and physiological psychology, 14, 135–233. [Google Scholar]
- Siegel A, & Shaikh MB (1997). The neural bases of aggression and rage in the cat. Aggression and Violent Behavior, 2(3), 241–271. [Google Scholar]
- Sih A (1980). Optimal foraging: partial consumption of prey. The American Naturalist, 116(2), 281–290. [Google Scholar]
- Simberloff D, & Dayan T (1991). The guild concept and the structure of ecological communities. Annual review of ecology and systematics, 22(1), 115–143. [Google Scholar]
- Simpson EH, & Balsam PD (Eds.). (2016). Behavioral neuroscience of motivation, 1–12. New York: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim R (1984). Caching behaviour, prey choice and surplus killing by Pygmy Owls Glaucidium passerinum during winter, a functional response of a generalist predator. Annales Zoologici Fennici, 21(3), 301–308. [Google Scholar]
- Sommer RJ, Carta LK, Kim S. y., & Sternberg PW (1996). Morphological, genetic and molecular description of pristionchus pacificus sp. n.(nematoda: Neodiplogasteridae). Fundamental and applied Nematology, 19, 511–522. [Google Scholar]
- Sommer RJ (Ed). (2015). Pristionchus pacificus: a nematode model for comparative and evolutionary biology. Leiden, Netherlands: Brill. [Google Scholar]
- Srinivasan J, Durak O, & Sternberg PW (2008). Evolution of a polymodal sensory response network. BMC biology, 6(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapp P (1997). Community structure of shortgrass-prairie rodents: competition or risk of intraguild predation?. Ecology, 78(5), 1519–1530. [Google Scholar]
- Sunde P, Overskaug K, & Kvam T (1999). Intraguild predation of lynxes on foxes: evidence of interference competition?. Ecography, 22(5), 521–523. [Google Scholar]
- Taylor RJ (1984). Predation. London, England: Chapman and Hall. [Google Scholar]
- Tilman D (1982). Resource competition and community structure. Princeton university press. [PubMed] [Google Scholar]
- Tynkkynen K, Rantala MJ, & Suhonen J (2004). Interspecific aggression and character displacement in the damselfly Calopteryx splendens. Journal of evolutionary biology 7(4), 759–767. [DOI] [PubMed] [Google Scholar]
- Vance RR (1984). Interference competition and the coexistence of two competitors on a single limiting resource. Ecology, 65(5), 1349–1357. [Google Scholar]
- von Lieven AF, & Sudhaus W (2000). Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. Journal of Zoological Systematics and Evolutionary Research, 38(1), 37–63. [Google Scholar]
- von Lieven AF (2005). The embryonic moult in diplogastrids (Nematoda)–homology of developmental stages and heterochrony as a prerequisite for morphological diversity. Zoologischer Anzeiger-A Journal of Comparative Zoology, 244(1), 79–91. [Google Scholar]
- Wasman M, & Flynn JP (1962). Directed attack elicited from hypothalamus. Archives of Neurology, 6(3), 220–227. [DOI] [PubMed] [Google Scholar]
- Wilecki M, Lightfoot JW, Susoy V, & Sommer RJ (2015). Predatory feeding behaviour in Pristionchus nematodes is dependent on phenotypic plasticity and induced by serotonin. Journal of Experimental Biology, 218(9), 1306–1313. [DOI] [PubMed] [Google Scholar]
- Zenner AN, O’Callaghan KM, & Griffin CT (2014). Lethal fighting in nematodes is dependent on developmental pathway: male-male fighting in the entomopathogenic nematode Steinernema longicaudum. PLoS One, 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B, Sand H, Wabakken P, Liberg O, & Andreassen HP (2015). Predator-dependent functional response in wolves: From food limitation to surplus killing. Journal of Animal Ecology, 84(1), 102–112. [DOI] [PubMed] [Google Scholar]
- Zong C, Wauters LA, Rong K, Martinoli A, Preatoni D, & Tosi G (2012). Nutcrackers become choosy seed harvesters in a mast-crop year. Ethology Ecology & Evolution, 24(1), 54–61. [Google Scholar]