To the Editor:
Autoimmune bullous diseases (AIBDs) are a group of blistering conditions the management of which is mostly based on immunosuppressive drugs, and evidence on their outcomes is limited in the COVID-19 era.1
This retrospective cohort study on 704 AIBD patients was conducted in a dermatology referral hospital in Tehran, Iran, from April 17 to May 29, 2020. After ethics approval, history of COVID-19 and characteristics and history of AIBD treatments (ie, rituximab and prednisolone) were collected from 704 AIBD patients by an online survey, face-to-face visits, or phone calls.
The diagnosis of COVID-19 was based on typical clinical findings and positive real time (RT) polymerase chain reaction (PCR) for SARS-CoV-2 or lung involvement compatible with COVID-19 on chest computed tomography (CT) scan, as suggested by World Health Organization guidelines.2 Patients with typical signs and symptoms of COVID-19 not confirmed by RT PCR or CT scan, were defined as highly suspicious.
Results are expressed as relative risk (RR) with 95% confidence intervals (CI). After univariate log-binomial models, inverse probability weights (IPW) were calculated to minimize the effect of confounding factors. The individual predicted probabilities of rituximab (RTX) and prednisolone history were estimated with a multivariable logistic regression model, and weight was assigned for each subject. The effect of each variable was estimated using the multivariable log-binomial model.
Among 704 patients, 21 (2.98%) had COVID-19; 15 of them had been hospitalized and 7 needed intensive care facilities (including high flow or mechanical ventilation), of which, 3 (14.28%) died. All had pulmonary involvement on CT. SARS-CoV-2 was detected in 13 (61.9%) patients by RT PCR and was negative in 2 (9.6%) patients. Fourteen (66.7%) had received RTX during the last 12 months. The median time from the last RTX infusion to COVID-19 diagnosis was 3.5 (interquartile range [IQR]:1.8-5.0) months. Ten (47.6%) patients were receiving prednisolone doses greater than 10 mg/d, 8 (38.1%) were on 10 mg/d or less, and 3 (14.3%) were off prednisolone. Additionally, 35 cases were highly suspicious of COVID-19 (Table I ).
Table I.
Demographic and disease characteristics of patients with AIBDs
| Demographics and disease characteristics of AIBDs patients | All AIBDs patients (n = 704) | Total suspicious and diagnosed COVID-19 patients (n = 56) |
|
|---|---|---|---|
| Highly suspicious COVID-19 (n = 35)∗ | Diagnosed COVID-19 by PCR/chest CT (n = 21) | ||
| Mean age ± SD, y | 48.8 ± 13.4 | 46.2 ± 11.4 | 47.7 ± 11.6 |
| <45 y | 291 (41.3) | 17 (48.6) | 8 (38.1) |
| ≥45 y | 413 (58.7) | 18 (51.4) | 13 (61.9) |
| Male: Female | 314: 390 | 15: 20 | 8: 13 |
| Median body mass index [IQR], kg/m2 | 26.6 [24.1-29.8] | 25.6 [24.5-30.1] | 26.6 [25.0-27.7] |
| Smoking- no. (%) | 70 (9.9) | 4 (11.4) | 1 (4.8) |
| Suspicious contact history,† n (%) | 61 (8.7) | 14 (40) | 6 (28.6) |
| Bullous disease type, n (%) | |||
| Pemphigus | 620 (88.1) | 32 (91.4) | 20 (95.2) |
| Bullous pemphigoid | 54 (7.7) | 1 (2.9) | 0 (0) |
| Mucous membrane pemphigoid | 24 (3.4) | 1 (2.9) | 1 (4.8) |
| Linear IgA disease | 3 (0.4) | 0 (0) | 0 (0) |
| Epidermolysis bullosa acquisita | 2 (0.3) | 1 (2.9) | 0 (0) |
| Gestational pemphigoid | 1 (0.1) | 0 (0) | 0 (0) |
| Median duration bullous disease [IQR], y | 4.0 [2.0-8.0] | 4.0 [2.0-7.0] | 3.0 [1.0-8.0] |
| Comorbidities, n (%) | |||
| Hypothyroidism | 81 (11.5) | 6 (17.1) | 2 (9.5) |
| Obesity (BMI>30) | 172 (24.4) | 9 (25.7) | 1 (4.8) |
| Diabetes | 105 (14.9) | 5 (14.3) | 2 (9.5) |
| Cardiovascular disease | 150 (21.3) | 6 (17.1) | 7 (33.3) |
| Pulmonary disease | 20 (2.8) | 1 (2.9) | 0 (0) |
| Bullous disease status, n (%) | |||
| No relapse | 380 (54) | 13 (37.1) | 11 (52.4) |
| Bullae ≤ 7 d | 148 (21) | 12 (34.3) | 3 (14.3) |
| Bullae > 7 d | 176 (25) | 10 (28.6) | 7 (33.3) |
| History of rituximab use, n (%) | 571 (81.1) | 29 (82.9) | 17 (81) |
| From April 2019, n (%) | 337 (47.9) | 15 (42.9) | 14 (66.7) |
| From October 2019, n (%) | 225 (32) | 11 (31.4) | 13 (61.9) |
| Daily prednisolone dosage last 3 months, n (%) | |||
| ≤10 mg | 578 (82.1) | 27 (77.1) | 11 (52.4) |
| >10 mg | 126 (17.9) | 8 (22.9) | 10 (47.6) |
BMI, Body mass index.
Highly suspicious cases: Typical clinical findings of COVID-19 without PCR or chest CT scan.
Using χ2 analysis, there was a significant relationship between suspicious contact history with total COVID-19 (P < .001) and confirmed COVID-19 (P = .002) after excluding highly suspicious cases.
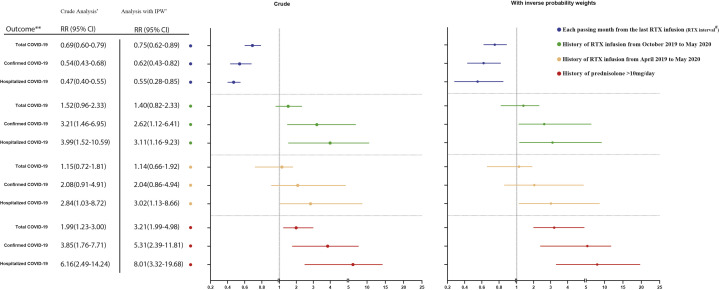
Multivariable analysis with IPW found an RR of 5.31 for subjects on greater than 10 mg/d prednisolone in cases diagnosed as COVID-19 (95% CI, 2.39-11.81) and 8.01 in the hospitalized group (95% CI, 3.32-19.68). Furthermore, the RR of getting COVID-19 and being hospitalized decreased by 38% (95% CI, 18%-57%) and 45% (95% CI, 15%-72%) with each passing month from the last RTX infusion, respectively. Including patients with highly suspicious COVID-19 in our analysis yielded similar results (Fig 1 ).
Fig 1.
Univariate and multivariate analysis with IPW. Association between prednisolone and rituximab infusion with COVID-19 in patients with autoimmune bullous diseases. Asterisk indicates all 704 patients were included in the total COVID-19 analysis. For the diagnosed COVID-19 analysis, highly suspicious cases were excluded from the cohort. Likewise, both highly suspicious and nonhospitalized COVID-19 cases were excluded from the cohort in the hospitalized COVID-19 analysis. Double asterisk indicates outcomes: Total COVID-19 including diagnosed and highly suspicious cases; diagnosed COVID-19 cases; hospitalized COVID-19 cases. Hashtag indicates RTX interval was analyzed for patients who received RTX after April 2019 and was defined as the interval from the last dose of RTX to either the date of contracting COVID-19 or May 2020. The blue line shows the relative risk of outcomes with each passing month from the last RTX infusion with a 95% CI.
By reviewing the literature, prednisone dose greater than 10 mg was suggested as a risk factor for hospitalization and mortality of COVID-19,3 whereas a randomized, controlled trial in the United Kingdom found low-dose systemic dexamethasone decreased the mortality rate in patients on a ventilator or oxygen.4 Similarly, opinions regarding the safety of RTX in COVID-19 are contradictory partly due to the controversies about the role of B cells in defending against SARS-CoV-2.5
The retrospective design, patient admissions in different hospitals, and undetected mild cases were our limitations. We found a higher risk of COVID-19 and hospitalization with prednisolone doses of greater than 10 mg/d. In addition, we showed each passing month from the last dose of RTX decreased these risks. Therefore, patients on long-term prednisolone and recent RTX should be monitored closely. Moreover, physicians should be more vigilant when deciding for RTX administration.
Conflicts of interest
None disclosed.
Footnotes
Drs Mahmoudi, Farid, and Nili contributed equally to this article.
Funding sources: This research was supported by Tehran University of Medical Sciences and Health Services grant number 99-1-161-47611.
IRB approval status: IR.TUMS.VCR.REC.1399.189.
References
- 1.Kasperkiewicz M., Schmidt E., Fairley J., et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization W.H. World Health Organization; 2020. Use of chest imaging in COVID-19: a rapid advice guide, 11 June 2020. [Google Scholar]
- 3.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. Published online July 17, 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 5.Guilpain P., Le Bihan C., Foulongne V., et al. Response to: ‘Severe COVID-19 associated pneumonia in 3 patients with systemic sclerosis treated with rituximab’ by Avouac et al. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217955. [DOI] [PubMed] [Google Scholar]