Abstract
The duration of viral shedding of SARS-CoV-2 is usually less than 10 days. We experienced a COVID-19 case with prolonged viral shedding for 2 months. His cell mediated immunity has been depressed (CD4+T cell <100/μl) due to advanced malignant lymphoma and chemotherapy which had been completed 4 months prior to the onset of symptoms of COVID-19. We administered several treatments against COVID-19, however the results of Polymerase Chain Reaction (PCR) from nasopharyngeal specimens remained positive to SARS-CoV-2 for 2 months. Moreover, virus isolation assays performed on Day 59 also remained positive. He was finally discharged on Day 69 with two consecutive negative PCR results for SARS-CoV-2. Immunocompromised status may prolong viral shedding and it is therefore important for the clinician to take into account this when assessing such patients.
Keywords: Viral replication, SARS-CoV-2 PCR, Follicular lymphoma, Bendamustine, Immunocompromised state
1. Introduction
Even though it has been reported that the viral burden of SARS-CoV-2 measured in upper respiratory specimens declines after onset of illness and replication competent virus has not been successfully cultured more than 9 days after onset of illness1), the actual duration of viral shedding and viral infectivity is still unclear.
We report a case of immunocompromised patient with malignant lymphoma, who had consecutive positive PCR results from his nasopharyngeal specimens (NPS) for two months and positive virus isolation at 59 days after the onset of symptoms.
2. Case report
A 47 y/o male developed a fever, sore throat and dysosmia and visited a local clinic on Day 10 after onset of symptoms. He had been previously diagnosed as a follicular lymphoma (FL) which was under complete remission after 6-courses of induction therapy with obinutuzumab plus bendamustine (GB). He had started to receive obinutuzumab bimonthly as a maintenance therapy two months prior to the onset of symptoms. He had also been receiving continuous prophylactic treatment with acyclovir and atovaquone. He doesn't drink alcohol and is a non-smoker.
Because of his suspected immunocompromised status, a chest computed tomography (CT) was performed at the clinic. Imaging revealed multiple patchy ground-glass opacities (GGO) in bilateral subpleural areas, findings consistent with typical CT findings of COVID-19. A nasopharyngeal specimen (NPS) for SARS-CoV-2 PCR assay was collected, and the patient returned home without hospitalization, as his oxygen saturation level was normal. A positive result for PCR was confirmed on the following day and the patient was isolated in his own home under remote inspection by the local public health center. During the observational period, his body temperature fluctuated. A second PCR assay was performed on Day 26, as the patient was to receive obinutuzumab monotherapy. The PCR test result still remained positive.
Although the patient did not have any further complaints, he visited our hospital on Day 28 under advisement of the local public health center. His body temperature was 36.9 °C and his oxygen saturation of peripheral artery was normal. Blood analysis showed mild elevation of LDH (313 U/L) and CRP (1.31 mg/dl). His chest radiograph showed ground glass opacity (GGO) of the right lower lobe. He refused to be admitted to the hospital because of a lack of serious subjective symptoms. Another NPS was collected and the result of PCR assay still remained positive.
On Day 31, he finally accepted hospitalization in our airborne-isolation ward to undergo antiviral therapy. Treatment with favipiravir was commenced on the day of admission, but was discontinued on the 6th day due to abrupt elevation of uric acid and skin rash. Treatment with ciclesonide was started on Day 40. On the 6th day of the treatment (Day 45), PCR assay was performed again. The result was still positive with high viral load (VL)/assay (Table 1 ). Assessment of his immunological status at this point revealed his CD4+Tcell count was 96/μl (CD4+/CD8+ ratio: 0.17) and IgG level was 760IU/ml. On Day 46, lopinavir/ritonavir was added to the treatment with ciclesonide in order to enhance the reduction of the viral burden. However, the patient suffered from diarrhea associated with the HIV protease inhibitor and refused to continue the treatment. The result of PCR assay was still positive on Day 55. Even though his body temperature was consistently high, he refused to undergo any other adjunct therapy. Evaluation of the etiology of this high fever was difficult as his infection with SARS-CoV-2 was a strong limitation for any detailed examination. Taking the patient outside the ward had to be limited to the minimum from the perspective of infection control. CT scan of whole body on Day 52, demonstrated old and new GGO in both lungs (Fig. 1 ). Blood analysis revealed an elevated ferritin (947.6ng/ml), sIL-2 receptor (789U/ml) and IL-6 (15.8pg/ml) on Day 59. Cytomegalovirus antigenemia assay, (1 → 3)-β-D-glucan test and interferon-gamma release assay for tuberculosis were all negative. We concluded that the persistent lesion was possibly organizing pneumonia as sequelae of COVID-19. The patient continued to be under close observation, corticosteroid medication was not administered as systemic administration of corticosteroid was contraindicated in his condition.
Table 1.
The dynamics of nasopharyngeal swab test of SARS-CoV-2 RNA.
| Date | Day 10 | Day 26 | Day 28 | Day 41 | Day 45 | Day 55 | Day 65 | Day 67 |
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 RNA (copies/assay) | 4.6 × 106 | 3.0 × 103 | 8.0 × 103 | positivea | 3.5 × 106 | 1.2 × 104 | UDb | UDb |
| Maximum body temperature (°C) | _ | _ | 36.9 | 38.1 | 37.2 | 37.8 | 38.2 | 37.9 |
PCR assay was conducted at Kawasaki City Institute for Public Health.
measured by a qualitative method.
undetectable.
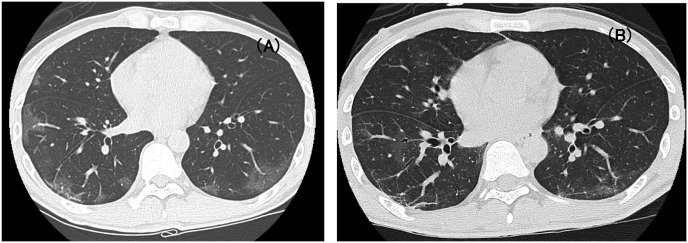
Fig. 1.
Chest CT findings on Day 10 and Day 56.
The multiple GGOs were observed on bilateral lower lungs on Day 10(Panel A). GGOs disappeared leaving linear shadow with collapse (Panel B), however several new GGOs were found on Day 56.
On Day 59, we requested the Department of Virology Ⅲ, National Institute of Infectious Diseases to perform isolation of SARS-CoV-2 with his NPS. The result revealed that SARS-CoV-2 had been isolated from the sample even though the VL was very low in the specimen. On Day 65, the PCR result turned negative. The consecutive PCR on Day 67 was also negative for SARS-CoV-2. The patient was finally discharged on Day 69.
The patient's NPS were again confirmed to be negative by PCR and antigen test (EsplineⓇSARS-CoV-2, Fuji Rebio) for SARS-CoV-2 on Day 82 at outpatient clinic. However, neither anti-IgG nor anti-IgM for SARS-CoV-2 antibody (GenBody COVID-19 IgM/IgG, GenBody and ElecsysⓇ Anti-SARS-CoV-2, Roche Diagnostics) was detected in his serum sample on the same day.
3. Discussion
In subjects with SARS-CoV-2, infectious virus has been isolated from samples derived from throat, lungs or ocular fluids [1]. In stool, PCR remained positive for SARS-CoV-2 for up to 13 days after pharyngeal samples were negative [2], however infectious virus was not isolated from such stool samples [3]. Blood and urine samples never yield virus [3].
The viral shedding of SARS-CoV-2 from upper respiratory specimens declines after onset of symptoms [4] and approaches zero by 10 days. However, some patients produced PCR-positive specimens for up to 6 weeks [5]. The duration of viral replication is uncertain, especially in case of immunocompromised population.
Regarding the remaining GGO on his chest CT, we considered the possibility that active virus was still present in his lungs, as angiotensin-converting enzyme-2 (ACE-2), identified as the cell entry receptor of SARS-CoV-2, is highly expressed in the lungs compared to the upper respiratory tract. Results of PCR from NPS test may be affected by many factors [6]and PCR assay of specimens from bronchoalveolar lavage are considered more accurate but pose a higher exposure risk. We could not obtain his sputum specimen for SARS-CoV-2 assay as he didn't bring up any sputum.
This patient was deemed to be immunocompromised as he had previously been received GB (obinutuzumab-bendamustine) treatment for progressed FL. Obinutuzumab is a glycol-engineered type Ⅱhumanized, anti-CD20 monoclonal antibody which affects mainly B cells. Bendamustine is an alkylating agent which causes prolonged lymphocytopenia, especially decreases CD4+ T cells [[7], [8], [9]]. The median time from the end of GB to B cell recovery has been reported to be 24 months and time for CD4+ T cell recovery to be 7–9 months.
The effect of low CD4+ T cell count on the course of COVID-19 is unclear. Chen J. et al. reported that CD4+ T cell count was independently associated with ICU admission of COVID-19 patients [10], however the influence of low CD4+ T cell count on the duration of viral shedding has not been elucidated. In our case, delayed CD4+ T cell count recovery from previous chemotherapy against FL which was observed. This may be a factor in the prolonged viral shedding observed in this patient despite symptoms of COVID-19 being mild.
We report a case of COVID-19 with a prolonged viral replication for 2 months. This unusually prolonged viral shedding is likely to be due to drug-induced immunosuppression. The clinicians should therefore take utmost care when assessing COVID-19 patients in immunocompromised state as such patients may be still positive for active virus even at 2 months after onset.
Author statement
All authors meet the ICMJE authorship criteria; Yukiko Nakajima was responsible for the conception of the work, interpretation of data and draft the work. Asuca Ogai and Karin Furukawa were responsible for the acquisition and analysis of the data. Ryosuke Arai, Ryusuke Anan, Yasushi Nakano and Yuko Kurihara were responsible for the design of the work. Hideaki Shimizu, Takako Misaki and Nobuhiko Okabe were responsible for the analysis of data. All authors revised this work critically and contributed to the writing of the final manuscript. All authors agreed to be accountable for all aspects of the work.
Declaration of competing interest
No reported conflicts of interest.
Acknowledgements
The authors acknowledge Shutoku Matsuyama and Makoto Takeda from National Institute of Infectious Diseases for his kind assistance with the isolation and detection assay of SARS-CoV-2.
References
- 1.Colavita F., Lapa D., Carletti J., et al. SARS-CoV-2 isolation from Ocular Secretions of a patient with COVID-10 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020 Apr 17:M20–M1176. doi: 10.7326/M20-1176. Published online 2020 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C., Gao G., Xu Y., et al. SARS CoV-2-positive sputum and feces after conversion of pharyngeal samples in patient with COVID-19. Ann Intern Med. 2020 Mar 30 doi: 10.7326/M20-0991. M20-0991. Published online 2020 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 4.Yen H.L., Peiris M., Wu J., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia A.T., Tong X.Y., Zhang S. Profile of RT-PCR for SARS-CoV-2: preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020:ciaa460. doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D., Xu W., Lei Z., et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gafter-Gvili A., Polliack A. Bendamustine associated immune suppression and infection during therapy of hematological malignancies. Leuk Lymphoma. 2016;57(3):512–519. doi: 10.3109/10428194.2015.1110748. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsumi Y., Ito S., Ohigashi H., et al. Sustained CD4 and CD8 lymphopenia after rituximab maintenance therapy following bendamustine and rituximab combination therapy for lymphoma. Leuk Lymphoma. 2015;56(11):3216–3218. doi: 10.3109/10428194.2015.1026818. [DOI] [PubMed] [Google Scholar]
- 9.Grigg A., Dyer M.J.S., Gonzalez Diaz M., et al. Safety and efficacy of obinutuzumab with CHOP or bendamustine in previously untreated follicular lymphoma. Haematologica. 2017;102(4):765–772. doi: 10.3324/haematol.2016.152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J., Qi T., Ling Y., et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]