Abstract
The current outbreak of novel COVID-19 challenges the development of an efficient treatment plan as soon as possible. Several promising treatment options stand out as potential therapy of COVID-19, including plasma-derived drugs, monoclonal antibodies, antivirals, antimalarial, cell therapy, and corticosteroids. Dexamethasone an approved corticosteroid medication, acting as an anti-inflammatory and immunosuppressant agent. In the current pandemic, dexamethasone is declared a “major development” in the fight against COVID-19. Steroidal dexamethasone was presented as the recent advancement that significantly reduces the mortality rate among severe COVID-19 cases. This review summarizes the preliminary opinion about the dexamethasone outbreak, therapeutic potential, risks, and strategies during the COVID-19 pandemic.
Keywords: Dexamethasone, Corticosteroid, SARS-CoV-2, COVID-19 therapeutics, Acute respiratory distress syndrome (ARDS), Immunosuppressant
1. Introduction
Since the emergence of COVID-19, researchers and healthcare providers are continually trying to find treatment options for the novel coronavirus (2019-nCoV). COVID-19 is a zoonotic virus that originated from bats with signs and symptoms of persistent fever, dry cough, myalgia, malaise, chills, dyspnea, loss of taste/smell, and headache in humans (Ye, Z.-W. et al., 2020). The World Health Organization issued general guidelines for dealing with SARS-COV-2 disease (COVID-19) on February 11, 2020. COVID-19 is either asymptomatic or includes a high risk of acute respiratory distress syndrome (ARDS), devastating pneumonia with bilateral lung infiltrates, cytokine storm syndrome, shock, and death (News, 2020a, Chen and Li, 2020). Currently, there are very few or no treatment choices to prevent COVID-19 infection (Sahin, A.R. et al., 2020). However, management strategies are functioning, which include palliative care parallel to different viral types of pneumonia: antibiotics for infections, advanced airway, ventilatory support, and supervision of ARDS (Villar, J. et al., 2020a). Since the advancement of treatment scope, additional consideration is directed toward available treatment strategies (Khan, M.M. et al., 2020) besides vaccines (Zhang, W. et al., 2020). Hence poly-pharmacology and drug repurposing open novel avenues to design and identify drugs for the COVID-19 pandemic rationally. Drug repurposing is the identification and testing, whether existing drugs used for other infections could also be useful in treating COVID-19 (Wang, J., 2020). Identification of the crystal structure of COVID-19 triggers the experts to start fundamental research for the therapy of novel corona infection to identify the drugs that might interact with this protein target (Liu, X. et al., 2020; Popov, D., 2020).
Over the last decade, corticosteroids (CS) have emerged as steroidal medicine to reduce inflammation in various disorders, including rheumatoid arthritis, systemic lupus erythematosus, asthma, and certain cancers by mimicking anti-inflammatory hormones produced by the body (Health, N.I.o., 2017; Bethesda, 2012; Rhen, T. and Cidlowski, J.A., 2005). Since 1977, dexamethasone listed on the world health organization (WHO) Model List of Essential Medicines in multiple formulations and presently available in most countries at reasonable cost and off-patent (Broccoli, M.C. et al., 2018). In the fight against corona infection, the body's immune system is activated and triggers inflammation as the body's immune response. However, occasionally the immune system becomes overdrive (a cytokine storm), and causes fetal reactions, and starts attacking the body's cells. Coronavirus further provokes respiratory failure, coagulopathy, and end-organ disease (Gulick, R.M. et al., 2020). CS use is linked to the suppression of the immune system; that is why their use has not been encouraged in the early phase of COVID-19 infection (Isidori et al., 2020, Singh, A.K. et al., 2020).
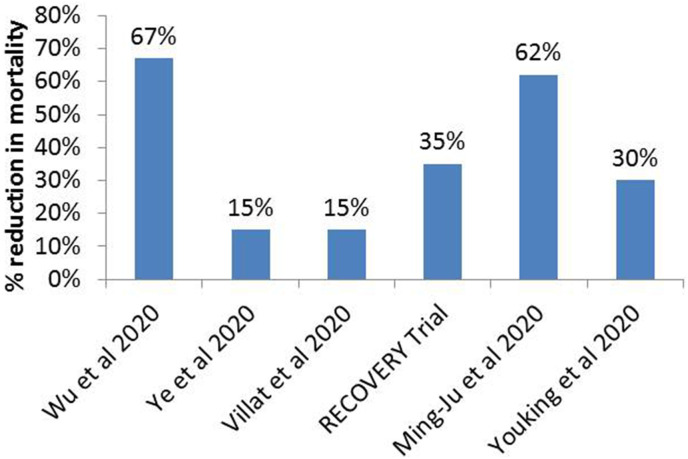
Their use in the early days of infection would result in viral replication, thus compromising the body's innate immunity (Isidori et al., 2020b). After several clinical trials (Fig. 1 ) on CS among individuals with COVID-19, it has been concluded that steroidal therapy is supposed to deal with the best possible subsistence for SARS-COV-2 cases that continues to severe disease (Huang, C. et al., 2020; Mahase, E., 2020a; Hasan, S.S. et al., 2020; Villar, J. et al., 2020b; Zha, L. et al., 2020).
Fig. 1.
%Age reduction in mortality rate with use of corticosteroids in COVID-19 associated ARDS (Villar, J. et al., 2020, Wu, C. et al., 2020, Ye, Zha et al., 2020, Zhou, Ye et al., 2020, Oxford, U.o., 2020).
Low-dose CS (dexamethasone), a breakthrough against COVID-19, reduces the risk of mortality by one-third for the patients on ventilators and one-fifth for those on oxygen (News, 2020c, Narrandes, N., 2020; Aljazeera, 2020). A different consideration of CS is the convenience of dexamethasone drug, and luckily its mechanism allowing us to conduct this drug repurposes screening. This drug works by dampening down the body's immune system. Moreover, it helps treat different diseases, including inflammation or swelling in the body, such as in allergic and asthmatic patients where inflammation is in the airways and lungs, or among patients with sore and inflamed joints (Jiang, K. et al., 2017). Other benefits include that dexamethasone has a long-lasting effect, allowing for a once-a-day regimen with the low-cost drug, which already exists and is available in bulk (Meijvis, S.C. et al., 2011). In this paper, we summarize the life-saving dexamethasone outbreak in treating novel COVID-19 with a particular focus on its mechanism and clinical trials exploring survival at a low dose as well as risks and future projections.
3. Cytokine storm and corticosteroids therapy
Research has identified that the excessive and uncontrolled production of soluble inflammatory markers known as ‘cytokine storm’ is a significant reason for ARDS in COVID-19 patients (Coperchini, F. et al., 2020; Wang, F. et al., 2020). ARDS, the primary cause of mortality in COVID-19 defined by infiltration of immune cells in both lungs and hypoxemia. In ARDS, alveolar-capillary membranes are injured due to inflammation, leading to more lung permeability and exudation of high protein edematous fluid into air sacs (Bhatia, M. et al., 2012). Previous research on SARS and MERS indicated the involvement of pro-inflammatory cytokines (IL-6, IL-12, interferon-gamma) and chemokines (CXCL10, CCL2) in the pulmonary inflammation associated with ARDS (Channappanavar, R. and Perlman, S., 2017). A recent investigation on SARS-CoV-2 by Huang et al. (2020) reported that pro-inflammatory cytokines and chemokines are elevated in infected patients (Huang, C. et al., 2020). Storm of pro-inflammatory cytokines and chemokines leads to the activation of T-helper-1(Th1) immune cells. Th1cell activation causes recruitment of IL-4 and IL-10, whose primary function is to reduce inflammation. This idea was initiated from the observation that patients seeking ICU admissions have more inflammatory mediators levels compared to patients with less severe infection (Chi Zhang, M. et al., 2020). Another recent study by Wong et al. (2004) described an increase in Th1 cytokine interferon-gamma, IL-1, IL-6, and IL-12 after two weeks of disease onset (Wong, C. et al., 2004). Cytokine storm is immediately followed by immune system activation and attacking the body's cells, which will ultimately cause ARDS, multi-organ failure, and death in severe cases of infection (Xu, Z. et al., 2020). Like all other severe respiratory tract infections, there is significant interest and debate regarding the use of corticosteroids to treat severe pneumonia due to coronaviruses. China's National health commission issued the fifth trial version of diagnosis and treatment for patients suffering from COVID-19 associated pneumonia in February 2020. Systemic corticosteroid therapy for severe cases of COVID-19 associate pneumonia approved for 3–5 days. Methylprednisolone dose of <2–3 mg/kg suggested as adjuvant therapy of COVID-19 (Zhou, W. et al., 2020).
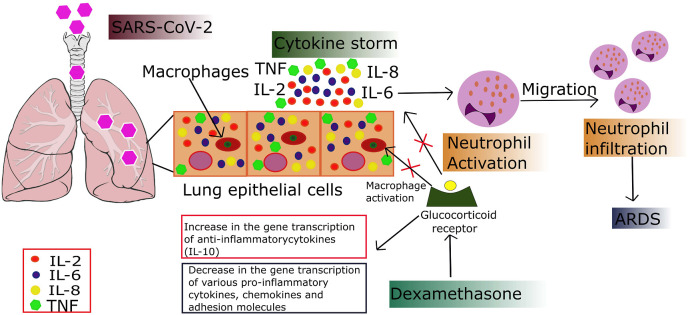
The potential role of corticosteroids in blocking the inflammatory pathway in critical conditions must be carefully monitored due to the chances of secondary infections, untoward effects, and other difficulties associated with corticosteroids usage (McCreary, E.K. and Pogue, J.M., 2020). Pathogenesis of COVID-19 involves pro-inflammatory cytokine production by macrophages in lung alveoli (Armitage, L.C. and Brettell, R., 2020). The use of corticosteroids is to suppress the host inflammatory reactions in the lungs, which may lead to acute lung injury and ARDS (Sanders, J.M. et al., 2020). One small randomized controlled trial has reported improved clinical outcomes in patients with COVID-19 who were given a short course of methylprednisolone (Corral, L. et al., 2020). Dexamethasone (a synthetic glucocorticoid) has previously been used to treat asthma, allergic reactions, arthritis, and other autoimmune diseases. It acts through the blockade of two pathways of inflammation; vasodilation and immune cell migration. Dexamethasone crosses the host cell membrane and binds to glucocorticoid receptors present in the cell cytoplasm, which initiates a series of immune cell responses that lead to pro-inflammatory suppression cytokines IL-1, IL-2, IL-6, IL-8, TNF, and IFN-γ through a decrease in gene transcription (Patel, S.K. et al., 2020). Out of these pro-inflammatory cytokines, five are associated with COVID-19 progression (Yong, S.J., 2020b). It also increases the gene expression of IL-10, which is an anti-inflammatory cytokine mediator (Azimi, S. et al., 2020) and inhibits neutrophil adhesion to endothelial cells, thus preventing the release of lysosomal enzymes and prevent chemotaxis at the site of inflammation (Coutinho, A.E. and Chapman, K.E., 2011). They also inhibit macrophages' activation, one of the significant perpetrators of cytokine storm in COVID-19 infected individuals (Youssef, J. et al., 2016) (Fig. 2 ).
Fig. 2.
Cytokine storm in COVID-19 associated ARDS and mechanism of action of dexamethasone.
4. Dexamethasone; a breakthrough in Covid-19 therapy
A ground-breaking development in the fight against COVID-19 came from the Randomized Evaluation of COVID-19 therapy Trial on June 16, 2020. This randomized trial was started in March 2020 as a randomized clinical trial to evaluate potential therapeutic options for COVID-19. More than 11,500 confirmed COVID-19 patients had been enrolled from 175 NHS hospitals in the UK (Oxford, U.o., 2020). The Oxford RECOVERY Trial tested low dose dexamethasone, lopinavir-ritonavir, hydroxychloroquine, and azithromycin in a randomized manner. The only dexamethasone succeeded in reducing COVID-19 associated mortality rate. The use of dexamethasone at a dosing rate of 6 mg per day was started on June 8, 2020, for ten days to evaluate the clinical effectiveness in patients compared to 4321 patients with usual care alone (Group, R.C., 2020; Horby, P. et al., 2020). Dexamethasone reduced the death rate by one third in patients on a ventilator and by one fifth in patients on oxygen support (Rees, V., 2020). Early findings showed that dexamethasone decreased the mortality risk from 40% to 28% in ventilated patients and 25%–20% for patients on oxygen therapy over 28 days. Dexamethasone use did not cause any significant side effects and was ineffective in mild cases (Yong, S.J., 2020a). However, The Oxford RECOVERY Trial has limitations like the results for key secondary outcomes, potential adverse effects, and efficacy of dexamethasone in patients with comorbidities have not been reported. The current administration of corticosteroids should be limited to patients with severe conditions related to cytokine storm, including ARDS, renal failure, acute cardiac injury, and elevated serum levels of D-dimers (Soy, M. et al., 2020).
A study conducted in Wuhan, China, demonstrated that corticosteroid use in patients with ARD reduced death risk, and 23 out of 50 (46%) patients who received corticosteroids died compared to 61.8% who did not receive corticosteroids (Wu, C. et al., 2020). A laboratory study on the use of dexamethasone in coronavirus infected pigs revealed the effectiveness of treatment in the acute phase of the disease, and rapid viral replication was observed with prolonged use of dexamethasone (Russell, B. et al., 2020). On the contrary, there have been reports of corticosteroids associated with immunity suppression and increased viral load, and delayed clearance from the body. Chu et al. (2004) indicated an increase in the number of viruses after treatment with corticosteroids in SARS-CoV-2 patients (Chu, C. et al., 2004). Despite the numerous published data on the use of steroids in COVID-19, there is no clear evidence of corticosteroid treatment effectiveness in SARS. Some researchers emphasize the need for cautious use of corticosteroids, but randomized controlled trials are required to approve the positive effects and predict the optimal dosage regimen. However, the possible useful effects should be calculated in comparison to the risks, including secondary infections and delayed viral clearance (Yu, W.C. et al., 2004). On September 2, 2020, WHO issued a guideline on the use of dexamethasone and other corticosteroids (hydrocortisone or prednisone) for the treatment of Covid-19.in the guideline, corticosteroids use was recommended in severe and critical patients (Who, 2020).
4. Risks
The WHO declared corticosteroids an effective medication hailed dexamethasone trial results in treating COVID-19 patients (News, 2020b). Although dexamethasone proved an effective remedy against COVID-19, some severe side effects are associated with CS use. Hormonal imbalance, fluid retention, weight gain, anxiety, and disturbed sleep pattern considered as the most commonly linked risks of dexamethasone. While hemorrhage, blurred vision, and eye disorders rarely occur as a risk of dexamethasone. Conversely, a low dose of dexamethasone is needed for coronavirus patients that limited ARDS chances (Mahase, E., 2020b). Most of the risks associated with CS use for an extended period may change the regular hormonal balance (Curtis, J.R. et al., 2007). However, if CS therapy is halted or diminished too quickly, withdrawal symptoms, including headache, vomiting, arthralgia, myalgia, bone pain, and weight reduction, possibly will also arise (Fields, T., 2009). In a recent research publication, Marinella explained that the immunosuppression could be augmented by dexamethasone in cancerous patients. Dexamethasone is generally recommended as an antiemetic for anticancerous induced severe nausea and vomiting. Dexamethasone induces lymphopenia among those patients by the reduction of B and T cells. As lymphocytes play an essential role in the resistance against COVID-19, for that reason, it is recommended that oncologists should reevaluate the monotonous use of dexamethasone to prevent stimulating lymphopenia (Marinella, M.A., 2020). CS therapy has a direct inhibitory action on β cells and causes lipotoxicity. Potential sequelae of CS treatment are the worsening of latent diabetes, increase insulin resistance, and increased hepatic glucose level by directly interfering with the signaling cascade of the GLUT-4 receptors. Ultimately leads to postprandial hyperglycemia, as well as hepatic glycogenesis (Perez, A. et al., 2014; Tamez-Pérez, H.E. et al., 2015; Lukins, M.B. and Manninen, P.H., 2005). Lastly, work from Youssef et al. (2016) (Youssef, J. et al., 2016) submitted the risk of CS among patients with rheumatic diseases, including severe bacterial infections and opportunistic infections, for example, TB, herpes zoster, and Pneumocystis jiroveci pneumonia. Some well-established risks related to dexamethasone practice summarized in (Table 1 ).
Table 1.
Risks associated with dexamethasone use.
| Dexamethasone | Risks | References |
|---|---|---|
| Short term use (≤1 month) | Prolonged viremia, bacterial super infections, autoimmune and cardiovascular events, resistance to neuromuscular blocking agents | (Manson, S.C. et al., 2009, Walsh, L. et al., 2001, Broadbent, C. et al., 2018, Mattos-Silva, P. et al., 2020) |
| Long term use (≥1–3 months) | Hyperglycemia, glaucoma, cataracts, fluid retention, hypertension, psychological effects including mood swings, confusion and behavior changes, Osteoporosis, Menstrual disorder, Abnormal hair growth | (Hersh, E., 2019, Mayoclinic, 2019; David Zelman, M., 2020, Alessi, J. et al., 2020) |
4.1. Weigh the risks/benefits of corticosteroids
Questions related to the actual practice of steroidal therapy for COVID-19 are gaining considerable debate at the moment among physicians worldwide, Obstetrics Units lacking sufficient data to recommend one steroid regimen over the other. Disparity remains common concerning the dose and period of steroidal therapy to substantially increase the risk (Berton, A. et al., 2020). In this context, the risk-benefit ratio must account for long-term steroidal therapy (≥1–3 months). To achieve the potential benefits of corticosteroids with reduced risk, healthcare providers follow some guidelines (Little, C.P. et al., 2020): Penn Medicine Center clarify about the risks and benefits of CS in a Rapid Guidance Summary for Evidence-based Practice on June 8, 2020 (Matthew D. Mitchell, P.C. and Emilia J. Flores, P., RN (CEP), 2020). Numbers of side effects are associated with the use of CS. However, if CS utilizes cautions, it alleviates ache, inflammation, and distress caused by many different syndromes and conditions.
The potential risks benefits ratio of steroids varies with:
-
•
The severity of the disease
-
•
Nature of the current infection causing disease
-
•
Availability of other treatment substitutes
-
•
Incidence of other significant medical problems
5. Protection
According to the treatment guidelines established by Russell and colleagues (Shang, L. et al., 2020), patients should be treated based on the following principles when administering corticosteroids: (1) risk to benefit ratio should be calculated before use of corticosteroids; (News, A.) corticosteroids use should be limited to critically ill COVID-19 patients; (News, A.) patients already using corticosteroids for comorbidities should use with caution; (4) low to moderate dosage should be administered for a short period.
Following protections can be followed to catch the maximum advantages of CS therapy with the minimum side effects (Staff, M.C., 2020):
-
•
Use intermittent dosing: Using the smallest dose to control the disease risks can be overruled.
-
•
Use a medical alert bracelet: Different corticosteroids are available with varying strengths and intervals of action. So always consult a health care professional (doctor, pharmacist, nurse), especially when using corticosteroids for an extended period. Monitor the patient carefully to detect early signs of severe side effects, and It is advised to wear a medical bracelet or tag.
-
•
Switch to non-oral forms of corticosteroids: Oral corticosteroids cause significant side effects by affecting the whole body rather than a particular area; therefore, replace it with a safer route. For example, In the case of inhaled CS, the drug directly reaches the lungs' surfaces, decreasing other body parts' exposure, ultimately reducing side effects.
-
•
Proper follow-up: When the patients are on long-term CS therapy (≥1–3 months), routinely monitor blood pressure and blood sugar often and treat accordingly.
-
•
Monitor bone density: Corticosteroids carry a risk of osteoporosis (bone thinning), consider monitoring and protect the bones through calcium and vitamin D supplements.
-
•
Choose a healthy diet plan: During the long term CS therapy, strict to a balanced diet plan and physical activities strengthen body muscles and boost immunity.
-
•
Watch withdrawal step: Long term CS therapy alter the average production of adrenal glands. On sudden withdrawal, the patient experiences symptoms, including fatigue, body aches, and lightheadedness as adrenal glands lacking sufficient time to recover. Reduce the dose gradually, giving adrenal glands enough time to restore their activities.
6. Future projections
With the imposition of current outbreaks, the rising rates of COVID-19 infections could quickly worsen the situation in the coming years, so now we need to understand these projections. As we have more powerful means of fighting against coronavirus, the mortality rate will be below the estimates. This review offers a comprehensive evaluation of the dexamethasone outbreak and features a complete assessment by gathering key points from already reported data and trials. In this scenario, dexamethasone's current treatment growth rate (cured cases of COVID-19 from DEXA) started to rise above one threshold. If the current trend continues, the digit of cured patients expected to reach a peak at the beginning of August 2020. Based on these assumptions, experts use different techniques to project the future of game-changer dexamethasone among seriously ill COVID-19 patients.
Risk factors associated with the use of corticosteroids are anticipated to be a key restraint for this forecast period. Risks tend to outweigh the benefits associated with the use of dexamethasone if used for a prolonged period. Further, Long-term use of dexamethasone is projected to act as an impediment to the global corticosteroids market's growth shortly. Researchers are reporting promising results with the role of steroid dexamethasone and establishing data on a case by case basis. Ultimately, the clinical utilization of dexamethasone and its role in COVID-19 management require additional clarity.
7. Conclusion
In this study, research has been reviewed on dexamethasone's fight against coronavirus, emphasizing its mechanism to provide the most up-to-date knowledge into the control and mediation of COVID-19 as possible. Among steroidal drugs, dexamethasone was identified to have inhibitory activities against COVID-19 protease. Our review suggests that drug repurposing screening of dexamethasone is very efficient and makes a substantial contribution to the treatment of novel COVID-19.
Contribution
Sobia Noreen: Conception and design of data, Acquisition of data, acquisition of interpretation of data, revising the manuscript critically for important intellectual content.
Irsah Maqbool: Figures preparation, Data analysis, drafting the manuscript, Performed the analytic calculations and performed the numerical simulations.
Asadullah madni: Approval of the version of the manuscript to be published and supervised the findings of this work, Approval of the version of the manuscript to be published.
References
- Alessi J., de Oliveira G.B., Schaan B.D., Telo G.H. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol. Metab. Syndrome. 2020;12(1) doi: 10.1186/s13098-020-00583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljazeera Dexamethasone reduces death risk in severe COVID-19 cases: Trial. Aljazeera. 2020 [Google Scholar]
- Armitage L.C., Brettell R. Inhaled corticosteroids: a rapid review of the evidence for treatment or prevention of COVID-19. 2020. https://www.cebm.net/covid-19/inhaled-corticosteroids-a-rapid-review-of-the-evidence-for-treatment-or-prevention-of-covid-19/
- Azimi S., Sahebnasagh A., Sharifnia H., Najmeddin F. Corticosteroids administration following COVID-19-induced acute respiratory distress syndrome. Is it harmful or life-saving? Adv. J. Emerg. Med. 2020;4:e43. [Google Scholar]
- Berton A., Prencipe N., Giordano R., Ghigo E., Grottoli S. Systemic steroids in patients with COVID-19: pros and contras, an endocrinological point of view. J. Endocrinol. Invest. 2020;1 doi: 10.1007/s40618-020-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethesda Corticosteroids. 2012. https://www.ncbi.nlm.nih.gov/books/NBK548400/ 2020
- Bhatia M., Zemans R.L., Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am. J. Resp. Cell Mol. 2012;46:566–572. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent C., Pfeffer P., Steed L., Walker S. Patient-reported side effects of oral corticosteroids. Eur. Respir. Soc. 2018 [Google Scholar]
- Broccoli M.C., Pigoga J.L., Nyirenda M., Wallis L., Hynes E.C. Essential medicines for emergency care in Africa. Afr. J. Emerg. Med. 2018;8:110–117. doi: 10.1016/j.afjem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Springer; 2017. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin Immunopathol; pp. 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect. Dis. 2020;20:515–516. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Zhang M., Zhao Wu P., Jia-Wen Li M. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor antagonist Tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K., Kao R., Poon L., Wong C., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral L., Bahamonde A., Delas Revillas F.A., Gomez-Barquero J., Abadia-Otero J., Garcia-Ibarbia C., Mora V., Hernandez J.L., Lopez-Muniz G., Hernandez-Blanco F. GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. MedRxiv. 2020 doi: 10.1007/s00508-020-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J.R., Patkar N., Xie A., Martin C., Allison J.J., Saag M., Shatin D., Saag K.G. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor α antagonists. Arthritis Rheum. 2007;56:1125–1133. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- David Z. Steroid injections. WebMD Med. Ref. 2020 [Google Scholar]
- Fields T. Steroid side effects: how to reduce corticosteroid side effects. Hosp. Spec. Surg. J. 2009 [Google Scholar]
- Group R.C. Dexamethasone in hospitalized patients with covid-19—preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick R.M., Sobieszczyk M.E., Landry D.W., Hollenberg A.N. Prioritizing clinical research studies during the COVID-19 pandemic: lessons from New York City. J. Clin. Invest. 2020;130(9) doi: 10.1172/JCI142151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.S., Capstick T., Ahmed R., Kow C.S., Mazhar F., Merchant H.A., Zaidi S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expet Rev. Respir. Med. 2020;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, N.I.O. 2017. LiverTox: clinical and research information on drug-induced liver injury.https://livertox.nih.gov Nih. gov. [PubMed] [Google Scholar]
- Hersh E. Everything you need to know about steroid injections. Healthline. 2019 [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. MedRxiv. 2020 [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A., Arnaldi G., Boscaro M., Falorni A., Giordano C., Giordano R., Pivonello R., Pofi R., Hasenmajer V., Venneri M. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J. Endocrinol. Invest. 2020;1 doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A.M., Arnaldi G., Boscaro M., Falorni A., Giordano C., Giordano R., Pivonello R., Pofi R., Hasenmajer V., Venneri M.A., Sbardella E., Simeoli C., Scaroni C., Lenzi A. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J. Endocrinol. Invest. 2020 doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K., Weaver J.D., Li Y., Chen X., Liang J., Stabler C.L. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials. 2017;114:71–81. doi: 10.1016/j.biomaterials.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Khan M.M., Noor A., Madni* A., Shafiq M. Emergence of novel coronavirus and progress toward treatment and vaccine. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2116. e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C.P., Birks M.E., Horwitz M.D., Ng C.Y., Warwick D. COVID-19: a rethink of corticosteroid injection? Bone Joint Lett. J. 2020;1:253–256. doi: 10.1302/2633-1462.16.BJO-2020-0050.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang B., Jin Z., Yang H., Rao Z. Protein DataBank; 2020. The Crystal Structure of COVID-19 Main Protease in Complex with an Inhibitor N3. [Google Scholar]
- Lukins M.B., Manninen P.H. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth. Analg. 2005;100:1129–1133. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]
- Mahase E. British Medical Journal Publishing Group; 2020. Covid-19: Demand for Dexamethasone Surges as RECOVERY Trial Publishes Preprint. [DOI] [PubMed] [Google Scholar]
- Mahase E. British Medical Journal Publishing Group; 2020. Covid-19: Low Dose Steroid Cuts Death in Ventilated Patients by One Third, Trial Finds. [DOI] [PubMed] [Google Scholar]
- Manson S.C., Brown R.E., Cerulli A., Vidaurre C.F. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir. Med. 2009;103(7):975–994. doi: 10.1016/j.rmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Marinella M.A. Routine antiemetic prophylaxis with dexamethasone during COVID-19: should oncologists reconsider? J. Oncol. Pharm. Pract. 2020;1078155220931921 doi: 10.1177/1078155220931921. [DOI] [PubMed] [Google Scholar]
- Matthew D., Mitchell P.C., Emilia J., Flores P., Rn (Cep) Center for Evidence-based Practice; 2020. COVID-19: CORTICOSTEROIDS for HOSPITALIZED PATIENTS(as of 8 June 2020) [Google Scholar]
- Mattos S., Paula F., Nathane S.S., Pedro Leme Robba L.R., Chiara B., Denise Pelosi, Paolo R., Patricia Rieken M.C., Fernanda F. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir. Physiol. Neurobiol. 2020;280 doi: 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoclinic . Mayo Foundation for Medical Education and Research; 2019. Cortisone Shots. [Google Scholar]
- Mccreary E.K., Pogue J.M. Open Forum Infectious Diseases. Oxford University Press US; ofaa105: 2020. Coronavirus disease 2019 treatment: a review of early and emerging options. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijvis S.C., Hardeman H., Remmelts H.H., Heijligenberg R., Rijkers G.T., Van Velzen-Blad H., Voorn G.P., Van De Garde E.M., Endeman H., Grutters J.C. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:2023–2030. doi: 10.1016/S0140-6736(11)60607-7. [DOI] [PubMed] [Google Scholar]
- Narrandes N. Oxford study unveils dexamethasone as breakthrough drug in COVID-19 fight. 2020. https://www.capetownetc.com/news/oxford-study-unveils-dexamethasone-as-breakthrough-drug-in-covid-19-fight/
- News A. Potential coronavirus vaccine. 2020. http://www.abc23.com/News/NewsDetails.asp?NewsID=30346
- News J.H. COVID-19 story tip: steroid drug hailed as effective COVID-19 treatment but questions linger about its use for black patients. Johns Hopkins Med. 2020:23. [Google Scholar]
- News T. 2020. WHO Hails Use of Dexamethasone in Treating Covid-19 Patients. [Google Scholar]
- Oxford U.O. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. 2020. https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19
- Patel S.K., Saikumar G., Rana J., Dhama J., Yatoo M.I., Tiwari R., Rodríguez-Morales A.J., Dhama K. Dexamethasone: a boon for critically ill COVID-19 patients? Trav. Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A., Jansen‐Chaparro S., Saigi I., Bernal‐Lopez M.R., Miñambres I., Gomez‐Huelgas R. Glucocorticoid‐induced hyperglycemia. J. Diabetes. 2014;6:9–20. doi: 10.1111/1753-0407.12090. [DOI] [PubMed] [Google Scholar]
- Popov D. Treatment of Covid-19 Infection. A rationale for current and future pharmacological approach. EC Pulmonol. Respir. Med. 2020;9:38–58. [Google Scholar]
- Rees V. Dexamethasone could reduce COVID-19 patient death risk by one-third, study shows. Eur. Pharm. Rev. 2020 [Google Scholar]
- Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin A.R., Erdogan A., Agaoglu P.M., Dineri Y., Cakirci A.Y., Senel M.E., Okyay R.A., Tasdogan A.M. 2019 novel coronavirus (COVID-19) outbreak: a review of the current literature. EJMO. 2020;4:1–7. [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Majumdar S., Singh R., Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a Clinician's perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff M.C. 2020. Prednisone and Other corticosteroids:Weigh the Benefits and Risks of Corticosteroids, Such as Prednisone, when Choosing a Medication. [Google Scholar]
- Tamez-Pérez H.E., Quintanilla-Flores D.L., Rodríguez-Gutiérrez R., González-González J.G., Tamez-Peña A.L. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J. Diabetes. 2015;6:1073. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet. Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L.J., Wong C.A., Oborne J., Cooper S., Lewis S.A., Pringle M., Hubbard R., Tattersfield A.E. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax. 2001;56(4):279–284. doi: 10.1136/thorax.56.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020;60(6):3277–3286. doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who . 2020. Coronavirus Disease (COVID-19): Dexamethasone. [Google Scholar]
- Wong C., Lam C., Wu A., Ip W., Lee N., Chan I., Lit L., Hui D., Chan M., Chung S. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J.A., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.-W., Yuan S., Yuen K.-S., Fung S.-Y., Chan C.-P., Jin D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S.J. Biology of dexamethasone: the first lifesaving drug for covid-19. 2020. https://medium.com/@shinjieyong/biology-of-dexamethasone-the-first-lifesaving-drug-for-covid-19-357ed9daaf7a
- Yong S.J. Biology of dexamethasone: the first lifesaving drug for covid-19. 2020. https://medium.com/@shinjieyong/biology-of-dexamethasone-the-first-lifesaving-drug-for-covid-19-357ed9daaf7a
- Youssef J., Novosad S.A., Winthrop K.L. Infection risk and safety of corticosteroid use. Rheum. Dis. Clin. 2016;42:157–176. doi: 10.1016/j.rdc.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.C., Hui D.S.C., Chan-Yeung M. Antiviral agents and corticosteroids in the treatment of severe acute respiratory syndrome (SARS) Thorax. 2004;59:643–645. doi: 10.1136/thx.2003.017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha L., Li S., Pan L., Tefsen B., Li Y., French N., Chen L., Yang G., Villanueva E.V. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID‐19) Med. J. Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. 108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J., Hu M., Fang M., Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Tar. 2020;5:1–3. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]