Abstract
Corona Virus Disease 2019 (COVID-19) is a new illness caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). With the increasing number of confirmed cases and the accumulating clinical data, a broad spectrum of neurological complications has been reported in the literature, including encephalopathy, stroke, Guillain-Barré syndrome, meningo-encephalitis, acute necrotizing hemorrhagic encephalopathy, and inflammatory central nervous system syndromes. Here, we describe the case of a 38-year-old woman presenting with longitudinally extensive transverse myelitis, revealed by bilateral lower limb weakness, decreased sensation below the Th4 level and urinary retention, and occuring 15 days after she had been diagnosed with COVID-19.
Keywords: Myelitis, LETM, SARS-CoV-2, COVID-19
1. Introduction
As the Severe Acute Respiratory Syndrome Coronavirus 2 (SARs-CoV-2) infection continues to spread around the world, there is increasing evidence that the nervous system is frequently involved in patients with coronavirus disease 2019 (COVID-19) infection. A broad spectrum of neurological complications such as encephalopathy, stroke, Guillain-Barré syndrome, meningo-encephalitis, acute necrotizing hemorrhagic encephalopathy, and inflammatory central nervous system syndromes, has been reported in the literature (Paterson et al., 2020). Here, we describe a case of longitudinally extensive transverse myelitis (LETM) following acute COVID-19 infection.
2. Case presentation
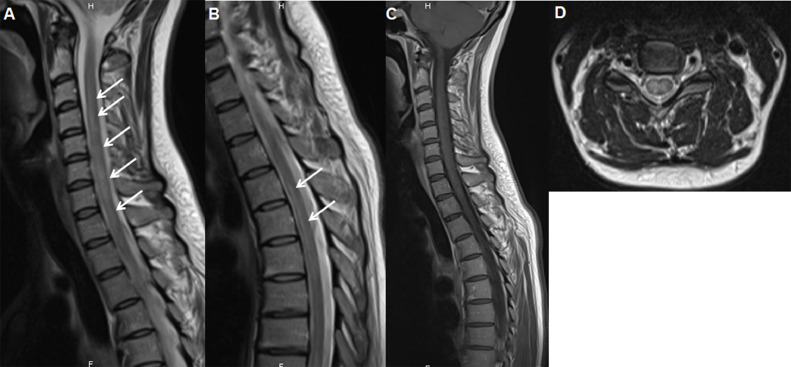
A previously healthy 38-year-old woman was referred to the emergency room of our hospital on November 3, 2020. She had no prior history of neurological disease. Two weeks before admission, she suffered from a dry cough, myalgia, shortness of breath, and a general weakness. In the context of the COVID-19 pandemic, SARS-CoV-2 polymerase chain reaction (PCR) nasopharyngeal swab was performed on October 22, 2020 and tested positive. She did not receive any specific treatment (antiviral or immune modulatory) for COVID-19, and hospitalization was not required. She gradually and completely recovered within 10 days. On October 31, she developed progressive weakness of the lower limbs, hypoesthesia and bladder dysfunction. She was admitted to our emergency department 3 days after the occurrence of these neurological symptoms. Neurological examination revealed decreased sensation below the Th4 level, brisk reflexes, and a moderate paraparesis (Medical Research Council grade 4). Babinski's sign was absent. She was unable to micturate. Whole spinal cord magnetic resonance imaging (MRI) showed a T2 extensive hypersignal involving predominantly the grey matter of the cervical and dorsal regions of the spinal cord, suggestive of acute transverse myelitis (Fig. 1 ). There was no gadolinium enhancement (Fig. 1C). Brain MRI showed no inflammatory lesions (Fig. 1E). Cerebrospinal fluid (CSF) analysis showed lymphocytic pleocytosis (337/µl; N < 5), elevated protein level (0,78 g/l), and absence of/positive CSF-restricted IgG oligoclonal bands. CSF SARS-CoV-2 PCR was negative. PCR screening tests performed on CSF for herpes simplex virus and varicella-zoster virus were all negative. Basic laboratory studies were not significant. There were no copper, vitamin B12 or folate deficiencies. Serological investigations for infectious (syphilis, Lyme disease, brucellosis, HTLV-1, West Nile, CMV, EBV, and HIV) and autoimmune (lupus and vasculitis) diseases were negative. Testing for aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies was negative. Treatment with intravenous methylprednisolone (1g daily for 8 consecutive days) resulted in significant clinical improvement. Follow-up CSF on day 10 after admission showed regressing pleocytosis (62/µl) and normalization of protein levels. Our patient was then transferred to a rehabilitation facility.
Fig. 1.
Spinal cord MRI
(A and B): Sagittal T2 images showing hyperintense longitudinal signal involving a long segment of the spinal cord starting at the level of C3-C4. (C): Sagittal post-contrast T1 weighted-images showing no gadolinium enhancement. (D): Axial T2 images of the cervical spine showing central hyperintense signal of the cervical spinal cord.
3. Discussion
This case highlights a potential neurological complication of COVID-19 infection, in the form of LETM. Here, no other cause of myelitis was identified after extensive workup. The MRI and CSF findings, such as absence of lesions suggestive of demyelination on brain MRI, the presence of pleocytosis over 50 cells/mm³ and the lack of CSF-restricted IgG oligoclonal bands make multiple sclerosis (MS) or a metabolic disorder unlikely, and should imply consideration of other diagnosis. A first attack of double seronegative neuromyelitis optica spectrum disorder (NMOSD) cannot be formally ruled out, but seems however unlikely. Considering the temporal association, a post-infectious etiology was therefore assumed. The interval of 15 days between time of COVID-19 symptoms and time of neurological symptoms onset is similar to the few published cases, and supports a post-infectious neurological complication (AlKetbi et al., 2020; Baghbanian et al., 2020; Chow et al., 2020; Maideniuc and Memon, 2020; Zoghi et al., 2020).
From a pathophysiological point of view, albeit the neuropathogenesis of COVID-19 is yet to be clarified, two underlying mechanisms could be involved, either individually or in combination. The first is based on neuroinvasive and neurotropic properties of SARS-CoV-2, which may facilitate a direct viral dissemination to the central nervous system (CNS) via blood circulation, nasal epithelium or vagus nerve through the angiotensin converting enzyme 2 (ACE2), and then precipitating demyelination (Wu et al., 2020; Desforges et al., 2020; Guadarrama-Ortiz et al., 2020). Interestingly, the ACE2 receptors are also expressed on the membrane of spinal cord neurons (Nemoto et al., 2020). The second hypothesized mechanism is post-viral immunological reaction to COVID-19 resulting in a hyper-inflammatory state characterized by hyperactivity of innate immunity, with activation of inflammatory cells, overproduction of inflammatory cytokines and chemokines such as interleukins (IL) 2, 6, 7, and 10, and tumor necrotizing α factor, causing damage to myelin (Iadecola et al., 2020; Mehta et al., 2020).
A potential important role of IL-6 in the SARS-CoV-2-related neurological manifestations has also been proposed, based on the demonstration of high serum levels of IL-6 in patients with COVID-19 as part of the cytokine storm (Costela-Ruiz et al., 2020), and increased CSF levels of inflammatory cytokines such as IL-6 and IL-1β in patients with neurological manifestations, supporting an exuberant immune response (Bodro et al., 2020). Also, it has been reported that IL-6 levels are directly related to disease severity in patients with COVID-19 (Costela-Ruiz et al., 2020).
Here, SARS-CoV-2 was not detected in the CSF sample, supporting an immune-mediated para-infectious mechanism rather than direct invasion of SARS-CoV-2 into the CNS, although we cannot exclude with certainty that the CSF viral load was insufficient for its detection by PCR assay. In contrast to the previously published cases of LETM or short myelitis following COVID-19, the CSF analysis in our patient yields marked lymphocytic pleocytosis (> 300/µl). This finding has some implications: a) CSF leukocytes count > 50/µl is very uncommon in MS and NMOSD and could therefore be a strong determinant in the differential diagnosis; b) given that the exact pathophysiological mechanism underlying CNS injury in patients with COVID-19 is not fully understood yet, the demonstration of a so marked pleocytosis adds evidence for a host-immune response to SARS-CoV-2 infection. The secondary neurological involvement (no prior neurological initial symptoms), the dramatic clinical improvement and regressing CSF pleocytosis observed after corticosteroids infusions also point towards a post-infectious immune mechanism.
In conclusion, this report adds evidence to post-COVID-19-triggered CNS autoimmunity. COVID-19 infection should be considered in the etiological causes of myelitis. Immunomodulatory treatment such as steroids can result in neurologic improvement as the patient reported here.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Funding
None
Declaration of Competing Interest
The authors declare no conflict of interest regarding this case report.
References
- AlKetbi R., AlNuaimi D., AlMulla M., AlTalai N., Samir M., Kumar N., AlBastaki U. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol. Case Rep. 2020;15:1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbanian S.M., Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)–a case report. Acta Neurol. Belg. Sep. 2020;18:1–2. doi: 10.1007/s13760-020-01497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodro M., Compta Y., Llansó L., Esteller D., Doncel-Moriano A., Mesa A., Rodríguez A., Sarto J., Martínez-Hernandez E., Vlagea A., Egri N., Filella X., Morales-Ruiz M., Yagüe J., Álex Soriano Á., Graus F., García F. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5):e821. doi: 10.1212/NXI.0000000000000821. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.C.N., Magnussen J., Ip J., Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-236720. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costela-Ruiz V., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadarrama-Ortiz P., Choreño-Parra J., Sánchez-Martínez C.M., Pacheco-Sánchez F.P., Rodríguez-Nava A.I., García-Quintero G. Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front. Neurol. 2020;11:1039. doi: 10.3389/fneur.2020.01039. Sept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16–27. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maideniuc C., Memon A.B. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-10145-6. 2020 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto W., Yamagata R., Nakagawasai K., Nakagawa K., Hung W.Y., Fujita M., Tadano T., Tan-No K. Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur. J. Pharmacol. 2020;872 doi: 10.1016/j.ejphar.2020.172950. Apr. [DOI] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi A., Ramenezani M., Roozbeh M., Darazam I.A., Sahraian M.A. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]