Vaccines to protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have risen up the agenda of most policy makers and individuals as the second wave of COVID-19 in northern hemisphere countries grows and there is increasing pressure on health-care systems. For any licensed vaccine, efficacy and duration of protection are key issues. Vaccine efficacies to protect against infection above 80% are desirable,1 but duration of protection will remain uncertain for a number of years post licensure of COVID-19 vaccines. Preliminary evidence suggests waning antibody titres in those who have recovered from SARS-CoV-2 infection,2 but antibodies are only one part of the human immune response and acquired immunity to reinfection or the prevention of disease when reinfected.3, 4, 5 Data on immunity to other coronaviruses suggest that immunity to SARS-CoV-2 might be short lived, perhaps 12–18 months in duration.6 Whether past infection will prevent severe COVID-19 on re-exposure to SARS-CoV-2 is not known at present.
Presently 45 candidate COVID-19 vaccines are in clinical trials in humans and ten of these vaccines are in phase 3 trials,7, 8 with expectations that some results might be announced before the end of 2020. If the results of the phase 3 trials are satisfactory, wide-scale deployment of COVID-19 vaccines is not expected until mid to late 2021.7 Developing the structure of a within-country immunisation programme will be crucial, including defining priorities for receiving vaccination, solving distribution challenges, and encouraging public acceptance of vaccination. Addressing vaccine hesitancy will require good communication strategies on the value of being protected as an individual and the benefits for the community in reducing viral transmission.9
Many governments have plans for the priorities for vaccine distribution once supplies of COVID-19 vaccines become available.10, 11, 12 Priority groups for vaccination typically start with front-line health-care staff, those working in essential services, those with health conditions that predispose to severe morbidity from infection, and then moving down the age groups from old to young in accordance with case fatality rates. A focus on immunisation in care homes for older people is planned in many countries, given the high number of COVID-19 deaths in these facilities during the first wave.
There is less clarity about the main priority of mass vaccination in the shorter term. Is it to minimise net mortality per year, or is it to maximise the average number of years of life gained by an individual receiving the vaccine? To maximise the average years of life gained, calculations need be made using demographic and epidemiological data. For example, with the recorded case fatality rates in the UK during the first COVID-19 wave and with the UK demography, we estimate that vaccinating people older than 70 years in the UK saves more lives than focusing on those aged 50–70 years (appendix). The reason for this is the steep rise in the case fatality rates in the very oldest age groups (appendix). We suggest that governments should therefore minimise mortality in the short term, unless vaccine supplies are short of what is required to protect the entire population for 1 year or more. Such calculations should be expanded to include other statistics, such as years of disability-adjusted life-years gained and impacts related to minimising symptoms of long COVID-19,13 influenced by vaccinating different age groups.
An additional complexity in evaluating these options is the extra burden imposed by COVID-19 on health provision for patients who need urgent treatment for other conditions such as cancer, which have implications for net mortality. COVID-19 has had a negative impact on survival rates for cancer due to the reduced provision of services for patients during the pandemic. Modelling estimates suggest there could be 6270 excess deaths in cancer patients over 1 year of the COVID-19 epidemic in the UK and 33 890 such deaths in the USA.14 More encouragingly, analysis of more than 21 000 hospital admissions of patients with COVID-19 showed that, between March and June, 2020, in the UK, death rates in intensive care had halved from 41% to 21%.15
How much vaccine is required by any given country year by year to create herd immunity to block SARS-CoV-2 transmission, and how long this will take requires calculations with clearly defined assumptions. Vaccine delivery will probably scale up only gradually as manufacturing capabilities develop over 12–24 months post licensure of a COVID-19 vaccine. As such, the impact of vaccination on the transmission of SARS-CoV-2 will start slowly and build up over a few years to reach target coverage levels. The amount of vaccine required for a defined population will depend on evidence from phase 3 COVID-19 vaccine trials on efficacy and what can be assumed about the average duration of vaccine protection—it will be an assumption until the findings of phase 4 trials on duration of both protection against infection and severe disease are reported. For a vaccine with 100% efficacy that gives life-long protection, the level of herd immunity as a proportion of the population, pc, required to block transmission is [1 – 1 / R0], where R0 is the basic reproduction number.16 Given an R0 value before lockdowns in most countries of between 2·5 to 3·5, we estimate the herd immunity required is about 60–72%. If the proportional vaccine efficacy, ε, is considered, the simple expression for pc becomes [1 – 1 / R0] / ε. If we assume ε is 0·8 (80%), then the herd immunity required becomes 75–90% for the defined range of R0 values. For lower efficacies, the entire population would have to be immunised. These overall estimates ignore heterogeneities that can make these figures lower or higher in specific locations.17, 18
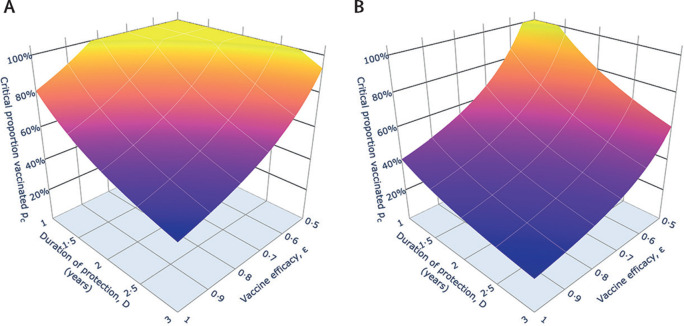
These calculations become more complicated if we assume immunity is short lived.19 Calculations of the proportion of the population that will need to be immunised year by year with a COVID-19 vaccine of defined properties can be derived from transmission models of SARS-CoV-2 (appendix). The simple equation for coverage pcbecomes a more complicated expression that involves the rate at which people are immunised, ε, the magnitude of R0, and the average duration of protection provided by the vaccine (figure ). The surface plotted in the figure shows the percentage of the population in year 1 that must be vaccinated and a similar plot of the percentage that must be vaccinated once the system equilibrates after a few years. A rough idea of this time is given by numerical evaluations of the model and gives equilibration by the end of year 2 (appendix). The percentage of the population that must be vaccinated in year 1 is much larger than the percentage that must be vaccinated once the system has stabilised after a few years, since most of the population will be susceptible as mass immunisation starts, but after a few years, hopefully, a high proportion will be immunised such that effective herd immunity is created. What is clear from our estimates based on the assumptions that efficacy is satisfactory (>80%) but duration of protection is short (1–2 years), is that a large proportion of the total population would need to be vaccinated if there is to be any chance of getting herd immunity to block the continued transmission of SARS-CoV-2. If the vaccine is protective over a longer duration than natural infection, then our estimates will be too pessimistic.
Figure.
Impact of vaccine efficacy and duration of protection on what percentage of the population must be vaccinated in the first year (A) and when the system approaches equilibrium in 2–3 years under continued vaccination (B)
(A) The percentage of the population who must be vaccinated, pc(1) × 100, for the first year of vaccination as a function of vaccine efficacy, ε, and protection duration (D=1/γ2), for Ro=2·5. Here the fraction who must be vaccinated within 1 year, pc(1), is defined in the appendix. If the value is greater than 100%, vaccination must take place more frequently than annually. (B) The percentage requiring vaccination in the population once the system has reached a new equilibrium after a few years of mass vaccination, as a function of vaccine efficacy and protection duration, for Ro=2·5. Ro=basic reproduction number.
What the duration of immunity is for a given COVID-19 vaccine will only be resolved once community-wide vaccination programmes progress. Phase 3 trials will tell us about efficacy and safety, but well designed phase 4 trials are essential based on representative and large numbers of those vaccinated and follow up over time. These studies will record any serious adverse events and identify whether repeatedly exposed individuals acquire coronavirus infections, particularly SARS-CoV-2, and if they do, what is the severity of disease. These cohort-based longitudinal studies will need careful planning and sustained funding, probably from governments with industry contributing. These studies should be targeted at those vaccinated in high-risk groups, such as the individuals older than 70 years and those with comorbidities that predispose to severe disease. Since repeated vaccination of individuals as they age is likely to be required for SARS-CoV-2 control, the pharmaceutical industry should focus on improving the efficacy of the initially licensed COVID-19 vaccines over the coming years.
What happens if countries do not reach high vaccine coverage levels? First, SARS-CoV-2 will become endemic but at a low level, the precise level depending on the degree of vaccine uptake, with peaks in winter and troughs in summer in the northern hemisphere.20 Second, policy makers will have to consider whether to mandate vaccination and to create a certificate to record immunisation for school, college, or university, and the workplace. Given vaccine hesitancy, the creation of herd immunity by vaccination is likely to be challenging in many countries. A further problematic issue for policy makers and vaccine producers is to carefully track the molecular evolution of SARS-CoV-2. Vaccine efficacy will depend on a stable virus target, unless we move to a situation such as that for influenza A vaccination where vaccine composition varies depending on which strains are predicted to be dominant in any given year. Research shows continued viral evolution of SARS-CoV-221 and this aspect needs to be tracked carefully. Taking novel vaccines successfully through phase 1 to phase 3 trials within a year has been an outstanding achievement, but equally challenging over the coming year will be persuading governments and populations to use COVID-19 vaccines effectively to create herd immunity to protect all.
Acknowledgments
RMA was a Non-Executive Director of GlaxoSmithKline for 10 years up to June, 2018. CV, JT, and BSC declare no competing interests. No funding sources were required for this Comment.
Supplementary Material
References
- 1.Bloom BR, Lambert PH. 2nd edn. Academic Press/Elsevier; London: 2016. The vaccine book. [Google Scholar]
- 2.Ward H, Cooke G, Atchinson C. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. 2020 doi: 10.1101/2020.10.26.20219725. published online Oct 27. (preprint) [DOI] [Google Scholar]
- 3.Wajnberg A, Amanat F, Firpo A. Robust neutralizing antibodies to SARS-CovV-2 infection persist for months. Science. 2020 doi: 10.1126/science.abd772.8. published online Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripperger TJ, Uhrlaub JL, Watanabe M. Orthogonal SARS-CoV-2 serological assays enable surveillance of low prevalence communities and reveal durable immunity. Immunity. 2020 doi: 10.1016/j.immuni.2020.10.004. published online Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addetia A, Crawford KHD, Dingens A. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiyuka PK, Agoti CN, Munywoki PK. Human coronavirus NL63 molecular epidemiology and evolutionary patterns in rural coastal Kenya. J Infect Dis. 2018;217:1718–1739. doi: 10.1093/infdis/jiy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–517. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 8.WHO Draft landscape of COVID-19 candidate vaccines. 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 9.WHO Improving vaccination demand and addressing hesitancy. 2020. https://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/
- 10.Bingham K. The UK Government's vaccine taskforce: strategy for protecting the UK and the World. Lancet. 2020 doi: 10.1016/S0140-6736(20)32175-9. published online Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Centers for Disease Control and Prevention Vaccines. 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- 12.Hogan AB, Winskill P, Watson OJ. Imperial College London; London: 2020. Imperial College COVID-19 response team. Report 33: modelling the allocation and impact of a COVID-19 vaccine.https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-33-vaccine/ [Google Scholar]
- 13.Yein D, Wirtheim E, Vetter P. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai AG, Pasea L, Banerjee A. Estimating excess mortality in people with cancer and multimorbidity in the Covid-19 emergency. medRxiv. 2020 doi: 10.1101/2020.05.27.20083287. published online June 1. (preprint) [DOI] [Google Scholar]
- 15.Office for National Statistics Deaths registered weekly in England and Wales including deaths involving coronavirus (Covid-19), by age sex and region. Oct 20, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/weekending9october2020
- 16.Anderson RM, May RM. Oxford University Press; Oxford: 1991. Infectious diseases of humans: dynamics and control. [Google Scholar]
- 17.Britton T, Ball F, Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science. 2020;369:846–849. doi: 10.1126/science.abc6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May RM, Anderson RM. Spatial heterogeneity and the design of immunization programmes. Math Biosci. 1984;72:83–111. doi: 10.1093/imammb/1.3.233. [DOI] [PubMed] [Google Scholar]
- 19.Heffernan JM, Keeling MJ. Implications of vaccination and waning immunity. Proc R Soc Ser B. 2009;276:2071–2080. doi: 10.1098/rspb.2009.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission of SARS-CoV-2 through the post pandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodcroft EB, Zuber M, Nadeau S. Emergence and spread of a SARS-CoV-2 variant through Europe in the summer of 2020. medRxiv. 2020 doi: 10.1101/2020.10.25.20219063. published online Oct 28. (preprint) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.