Abstract
Transcatheter aortic valve implantation (TAVI) outcomes during the coronavirus disease 2019 (COVID-19) pandemic have not been fully evaluated and some structural programs in the world have been suspended during this period. We sought to evaluate and compare clinical outcomes in patients undergoing TAVI in pandemic versus nonpandemic era. In a single center, we compared 198 TAVI patients performed during 2019 to 59 patients performed during the COVID-19 pandemic period (March 1st to June 30th, 2020). Primary outcome was procedural success according to VARC criteria and 30-day mortality rates. VARC-defined procedural success was high in both groups (93.3% vs 96.6%; p = 0.53). There were no differences in any vascular complications (26% vs 19%; p = 0.3), permanent pacemaker implantation (11.8% vs 15.3%; p = 0.63), and length of hospital stay (5.2 vs 4.2 days; p = 0.29). Thirty-day mortality was similar (3% vs 3.4%; p = 1.0). We had no documented COVID-19 disease in our patients during follow up. In conclusion, TAVI procedures can be performed effectively and safely during the COVID-9 pandemic, using a minimalist approach, early discharge, and by maintaining proper use of personal protective equipment.
The current COVID-19 outbreak presents an unprecedented challenge to health services worldwide. With the primary goal of reducing the risk of spread of COVID-19, and preserving access to necessary/emergency care, the ACC/SCAI issued specialty guidance for the management of cardiology patients during this time.1 All hospital trusts were advised early to defer nonurgent cardiovascular diagnostics and interventions. Cardiac complications of COVID-19 include myo-pericarditis, malignant arrhythmias, and biventricular heart failure2 and the fatality rate is significantly higher in those with pre-existing cardiovascular diseases (10.5% vs 2.3%).3 On the other hand, aortic stenosis (AS) is common and affects patient groups particularly vulnerable to a poor outcome with COVID-19 infection, with an overall prevalence of clinically significant AS in those greater than 70 years approximately 1% to 3%.4 Severe symptomatic AS has a uniformly poor prognosis, with an estimated 1-year mortality of up to 40%.5 The mortality rate in patients with severe AS on the waiting list for valve replacement is estimated to be 11.6% in 6 months.6 In our tertiary care hospital, which the main treating hospital of COVID-19 patients in Israel, we have decided to maintain our TAVI program despite many technical and administrative restrictions. The purpose of this current study was described this experience and evaluate whether TAVI program during the COVID19 pandemic is effective and safe.
Methods
We retrospectively analyzed the database of patients undergoing TAVI in 2019 (control group) and in the period between of March 1st to June 30th, 2020 (COVID-19 group), performed in a single, tertiary care hospital (Sheba Medical Center, Tel Hashomer, Israel).
Inclusion criteria were all patients with severe symptomatic AS diagnosed clinically and confirmed by Doppler echocardiography referred to TAVI due to increased surgical risk as assessed by an institutional heart team. All patients included in the study underwent a complete Doppler echocardiography and computed tomographic angiography (CTA) preprocedure. Valve implantation was performed using a self-expandable valve (Evolute Pro or Accurate neo) or a balloon-expandable valve (Sapien-3), according to the operator discretion. Procedural access site was transfemoral, transaxillary, or transcaval.
All procedures were performed using minimalist strategy including avoidance of general anesthesia, and no use of intra-procedural transesophageal echo in order to focus on rapid reconditioning, early mobilization, and accelerated return to baseline function and activities of daily living driven by a nursing protocol.
Patients undergoing TAVI during COVID-19 pandemic were screened for symptoms according to CDC guidelines, and tested for PCR SARS-CoV-2 if needed. WHO's recommendations for the rational use of personal protective equipment (PPE) in health care (patients and operators) were applied.7
TAVI device success and postprocedural complications were recorded according to the Valve Academic Research Consortium-2 (VARC-2).8 Thirty-day mortality rates were ascertained with the Israeli ministry of interior mortality database.
Continuous variables that have normal distributed were expressed as median and standard deviation values, and difference between groups was assessed using the student's t test. For continuous variables not normally distributed were reported as median and interquartile range (IQR, 25th-75th percentiles) values, and significance was assessed using the Mann-Whitney U or Kruskal-Wallis tests. Categorical variables were presented in frequencies and percentages and significance was assessed using the chi-square test or Fisher's exact test. Kaplan-Meier estimates of survival analysis were calculated. Differences among the groups were compared with the log-rank test. Statistical significance was accepted for a 2-sided p <0.05. All data were initially entered into statistical program SPSS (version 25.0.0 IBM, Armonk, NY, USA) and R version 4.0.0 software (The R foundation).
Results
Of the 257 patients included in the present analysis, 59 (23%) underwent TAVI during the COVID 19 pandemic. This group was compared with the TAVI patients performed in 2019 (n = 198, 77%).
As shown in Table 1 , baseline characteristics including age, gender, co-morbidities, and STS score were similar in both study groups. Patients in COVID-19 pandemic were using significantly more anticoagulant therapy at baseline.
Table 1.
Baseline characteristics
| Variable | All patients N = 257 |
Pre-COVID N = 198 |
COVID period N = 59 |
p Value |
|---|---|---|---|---|
| Age (years ± SD) | 80.3 ± 9 | 80.2 ± 9.5 | 80.5 ± 6.9 | 0.82 |
| Men | 127 (50%) | 97 (49%) | 30 (51%) | 0.95 |
| Body mass index (Kg/m2 ± SD) | 28.1±5.3 | 28.4±5.7 | 27.3±3.7 | 0.16 |
| NYHA class III-IV | 121 (47%) | 89 (45%) | 32 (54%) | 0.28 |
| STS score, (mean ± SD) | 3.1± 2.4 | 3.2± 2.4 | 2.1± 0.6 | 0.13 |
| Hypertension | 197 (77%) | 151 (77%) | 46 (78%) | 0.97 |
| Diabetes mellitus | 97 (38%) | 75 (38%) | 22 (37%) | 1 |
| Chronic obstructive pulmonary disease | 17 (7%) | 12 (6%) | 5 (10%) | 0.56 |
| Malignancy | 48 (19%) | 33 (17%) | 15 (25%) | 0.19 |
| Cerebrovascular disease | 26 (10%) | 21 (11%) | 5 (9%) | 0.77 |
| Atrial fibrillation | 66 (26%) | 46 (24%) | 20 (34%) | 0.15 |
| Chronic renal failure | 83 (32%) | 65 (33%) | 18 (31%) | 0.86 |
| Liver disease | 4 (2%) | 3 (2%) | 1 (2%) | 1 |
| Peripheral artery disease | 12 (7%) | 6 (5%) | 6 (10%) | 0.28 |
| Previous myocardial infarction | 32 (21%) | 20 (22%) | 12 (20%) | 0.84 |
| Previous revascularization (PCI or CABG) | 96 (37%) | 79 (40%) | 16 (27%) | 0.09 |
| Anticoagulant therapy | 71 (28%) | 47 (24%) | 24 (41%) | 0.01 |
| Baseline albumin (mg/dL ± SD) | 3.97±1.78 | 3.98±2.03 | 3.97±0.32 | 0.98 |
CABG = Coronary artery bypass graft; PCI = Percutaneous Coronary Intervention; STS = Society of Thoracic Surgeons Score.
Echocardiographic and tomographic characteristics previous TAVI of the participants are shown in Table 2 with no significant differences between the 2 groups. The vast majority of cases were done via femoral access. There were no differences in the type of access, type of valve, valve in valve procedures, and other technical issues during the procedure, such as balloon pre- and postdilation (Table 3 ).
Table 2.
Echocardiographic and tomographic characteristics
| Variable | All patients N = 257 | Pre-Covid N = 198 |
Covid period N = 59 | p Value |
|---|---|---|---|---|
| Echocardiography | ||||
| Left ventricular ejection fraction (%, [IQR]) | 55% [50,65] | 55% [50,60.7] | 56% [50,65] | 0.65 |
| Aortic valve area cm2 ± SD | 0.74 ± 0.17 | 0.75 ± 0.17 | 0.71 ± 0.16 | 0.11 |
| Moderate or severe regurgitation | ||||
| Aortic | 24 (14%) | 18 (15%) | 6 (10%) | 0.48 |
| Mitral | 53 (21%) | 40 (29%) | 13 (22%) | 0.38 |
| Tricuspid | 50 (25%) | 35 (25%) | 15 (25%) | 1 |
| Computed tomography | ||||
| Calcium score aortic valve (Agatstone Units ± SD) | 2842 ± 1496 | 2865± 1456 | 2785 ± 1603 | 0.73 |
| Systolic annular perimeter, (mm ± SD) | 73.8 ± 7.8 | 74 ± 7.9 | 73.1 ± 7.8 | 0.48 |
| Systolic annular area (mm2 ± SD) | 408 ± 126 | 439 ± 94 | 430 ± 91 | 0.55 |
IQR: Inter-quartile range.
Table 3.
Procedural details
| Variable | All patients N= 257 |
Pre-Covid19 N = 198 |
Covid19 period N = 59 |
p Value |
|---|---|---|---|---|
| Femoral vascular access | 246 (96%) | 186 (94%) | 58 (98%) | 0.30 |
| Balloon expandable valve | 92 (36%) | 67 (34%) | 24 (41%) | 0.35 |
| Valve size (mean) 23 26 29 34 |
26 [25,29] 48 (19%) 80 (31%) 72 (28%) 28 (11%) |
26 [25,29] 38 (19%) 56 (28%) 58 (29%) 25 (12%) |
26 [25.5, 29] 10 (17%) 24 (41%) 14 (24%) 3 (5%) |
0.16 |
| Pre dilatation | 117 (46%) | 91 (47%) | 25 (42%) | 0.65 |
| Post dilatation | 68 (27%) | 54 (28%) | 14 (24%) | 0.61 |
| Valve in valve | 16 (6%) | 14 (7%) | 2 (3%) | 0.37 |
| Moderate and severe paravalvular leak per angiography | 14 (5%) | 12 (6%) | 2 (3%) | 0.74 |
| Numbers of staff TAVI operators | 5 | 5 | 5 | 1 |
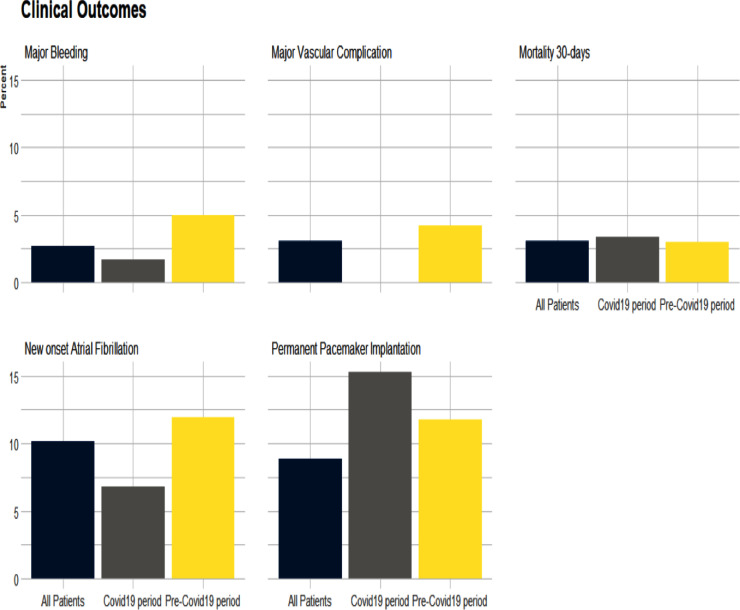
Outcomes are presented in Table 4 . In both groups there was a high TAVI device success. There were no differences in rates of any vascular complications, major or minor bleeding and permanent pacemaker implantation. Thirty-day mortality was similar (3% vs 3.4%; p = 0.26) (Figure 1 )
Table 4.
Outcomes
| Variable | All patients (N= 257) |
Pre-COVID 19 (N = 198) |
COVID 19 period (N = 59) |
p Value |
|---|---|---|---|---|
| TAVI device success VARC-2 | 239 (93%) | 182 (93%) | 56 (97%) | 0.53 |
| Vascular complications (VARC-2) | 61 (24%) | 50 (26%) | 11 (19%) | 0.3 |
| Minor | 53 (21%) | 42 (22%) | 11 (19%) | 0.71 |
| Major | 8 (3%) | 8 (4%) | 0 | 0.20 |
| Permanent pacemaker implantation | 23 (9%) | 14 (12%) | 9 (15%) | 0.63 |
| Procedural bleeding (BARC) | 25 (10%) | 20 (17%) | 5 (9%) | 0.17 |
| Minor | 18 (7%) | 14 (12%) | 4 (7%) | 0.43 |
| Major | 7 (3%) | 6 (5%) | 1 (2%) | 0.43 |
| New atrial fibrillation | 18 (10%) | 14 (12%) | 4 (7%) | 0.42 |
| Hospitalization days ± SD | 5.04 ± 6.9 | 5.2 ± 7.7 | 4.2 ± 2.9 | 0.29 |
| 30-day mortality | 8 (3%) | 6 (3%) | 2 (3%) | 1 |
VARC-2 = Valve Academic Research Consortium-2; BARC = Bleeding Academic Research Consortium.
Figure 1.
Clinical outcomes.
In the TAVI group performed during the COVID-19 pandemic, there was a tendency for a shorter length of hospital stay after the procedure (4.2 vs 5.2 days; p = 0.29). None of the patients in the COVID-19 era were infected with the virus from the procedure to the latest follow-up day.
Discussion
This study shows that TAVI program could have been maintained despite the limitations and restrictions applied by the COVID-19 outbreak with excellent and comparable outcomes to a nonpandemic era.
The ACC/SCAI group proposed criteria for not delaying intervention in hospitalized patients with severe AS despite the outbreak including associated reduction in ejection fraction, presence of class III-IV congestive heart failure symptoms, or syncope secondary to AS. The document states that It would be reasonable to schedule TAVI for elective patients with severe to critical aortic stenosis and class III-IV CHF symptoms. In patients with mild symptoms decision should be made according to quantitative measures of valve severity that indicate a critically tight valve. For truly asymptomatic patients, it is reasonable to postpone TAVI for 3 months or until after hospital operations resume elective procedures. Close outpatient monitoring, possibly via telehealth, should continue for all patients with severe AS.
On February 21st, 2020, Israel confirmed the first case of COVID-19. In the following months, we saw an exponential increase in cases, reaching a peak during April 2020. Until the end of June, 25,244 confirmed cases were registered with 354 deaths.9 During this time, dynamic quarantines and total lockdown were carried out in the country. With widespread community transmission of COVID-19, the overarching goal was to minimize the risk of COVID-19 exposure and to preserve limited resources such as anesthesia care, ventilators, intensive care unit (ICU) beds, and PPE, making TAVI implants more difficult. In our experience, we continued our regular TAVI program without a selection of priority cases. We have maintained our program focusing on early discharge and WHO's recommendations for the rational use of PPE in health care. Close monitoring of symptoms after discharge was achieved via telehealth.
Patients with severe symptomatic AS have poor overall survival without definitive treatment.10 Outcome data from the PARTNER-1 cohort B trial showed 1-year all-cause mortality of 50.7% in the medical arm group. Even for asymptomatic patients, long-term survival is poor, with a mortality of 21.1% at 3 years from diagnosis,11 , 12 suggesting that all severe AS patients do poorly in the long term in the absence of intervention.
Sudden cardiac death can occur with asymptomatic severe AS (without intervention) at a rate of approximately 1 in a 100,13 and may occur without any prodromal symptoms. Should symptoms develop, clinical deterioration may progress rapidly, and the risk of sudden death can escalate if AS is managed conservatively (4% at 1 month, 12% at 6 months).14 In a recent study of AS patients with a high probability of LV decompensation, more than 50% were either dead or hospitalized with heart cardiac failure within 2 years.15
Timing of intervention is also crucial, as perioperative morbidity is markedly increased if advanced left ventricular systolic dysfunction occurs due to a delay in intervention.16 , 17 Registry data unsurprisingly revealed greater mortality during the preoperative period for patients with established heart failure and advanced myocardial scarring.18 There is an increasing awareness that aortic valve intervention is often performed too late, and several studies are examining the effects of earlier intervention in asymptomatic patients.19
It is important to focus on the concept of minimalist TAVI using local anesthesia with minimal sedation and supported by patient coaching and avoidance of invasive lines; and early recovery monitoring protocol with accelerated reconditioning and continuity/consistency of medical care. Accelerated mobilization and reconditioning are pivotal to avoiding a cascade of in-hospital adverse events in older patients, including loss of motor function and increased risk of falls.20 Similarly, the avoidance of hospitalization-related modifiable risk factors such as the use of general anesthesia and "deep" monitored anesthesia care, administration of opioids, urinary tract and other infections, immobility, deconditioning, and long length of stay may reduce the incidence of procedure-related delirium to its near elimination in the era of contemporary TAVI. We believe that this strategy is useful in these times of pandemic, where the patient's exposure should be as low as possible, being the strategy that we use during the pandemic.
This is a retrospective, nonrandomized, nonblinded observational study, and therefore it is subjected to limitations inherent in this design. We hope that early experience from our center may prove useful for others adapting their practice in preparation for local COVID-19 surges.
In conclusion, experience from out single center study shows that TAVI procedures can be performed effectively and safely during the COVID-19 pandemic with a minimalist implant strategy, early discharge and WHO's recommendations for the rational use of personal protective equipment. However, the decision to intervene in a patient needing a valve procedure will be determined largely by hospital resources and the burden of COVID-19 admissions at any given time and hospital.
Author Contribution Statement
Martín Valdebenito, MD: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project Administration; Software; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing.
Eias Massalha, MD: Data curation; Formal analysis; Investigation; Methodology; Software.
Israel M. Barbash, MD: Conceptualization; Investigation; Supervision; Validation; Writing - review & editing.
Elad Maor MD, PhD: Conceptualization; Investigation; Supervision; Validation.
Paul Fefer, MD: Conceptualization; Investigation; Supervision; Validation.
Victor Guetta, MD: Conceptualization; Investigation; Supervision; Validation; Writing - review & editing.
Amit Segev, MD: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project Administration; Software; Supervision; Validation; Visualization; Writing - review & editing.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Shah PB, Welt FP, Mahmud E, Phillips A, Kleiman NS, Young MN, Sherwood M, Batchelor W, Wang DD, Davidson L, Wyman J, Kadavath S, Szerlip M, Hermiller J, Fullerton D, Anwaruddin S. Triage considerations for patients referred for structural heart disease intervention during the coronavirus disease 2019 (COVID-19) pandemic: an ACC /SCAI consensus statement. JACC Cardiovasc Interv. 2020;13:1484–1488. doi: 10.1016/j.jcin.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman J, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Sengelov M, Cheng S, Biering-Sorensen T, Matsushita K, Konety S, Solomon SD, Folsom AR, Shah AM. Ideal cardiovascular health and the prevalence and severity of aortic stenosis in elderly patients. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 6.Malaisrie SC, McDonald E, Kruse J, Russell H, McCarthy P, Andrei AC. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98:1564–1571. doi: 10.1016/j.athoracsur.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 7.WHO | Personal protective equipment for COVID-19. https://www.who.int/medical_devices/priority/COVID_19_PPE/en. Last uptdate September 19th 2020.
- 8.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. Eur J Cardio-Thorac Surg. 2012;42:S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 9.Worldometer COVID-19 Data. https://www.worldometers.info/coronavirus. Last uptdate September 19th 2020.
- 10.Marquis-Gravel G, Redfors B, Leon MB, Généreux P. Medical treatment of aortic stenosis. Circulation. 2016;134:1766–1784. doi: 10.1161/CIRCULATIONAHA.116.023997. [DOI] [PubMed] [Google Scholar]
- 11.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Smith CR, Mack M, Miller D, Moses JW, Svensson LG, Tuzcu EM, Webb J, Fontana G, Makkar R, Brown D, Block P, Guyton R, Pichard A, Bavaria JE, Herrmann H, Douglas P, Petersen JL, Akin JJ, Anderson W, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 13.Campo J, Tsoris A, Kruse J, Karim A, Andrei A, Liu M, Bonow RO, McCarthy P, Malaisrie SC. Prognosis of severe asymptomatic aortic stenosis with and without surgery. Ann Thorac Surg. 2019;108:74–79. doi: 10.1016/j.athoracsur.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Greve AM, Gerdts E, Boman K, Gohlke-Baerwolf C, Rossebo AB, Devereux RB, Kober L, Ray S, Willenheimer R, Wachtell K. Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis: the SEAS (simvastatin and ezetimibe in aortic stenosis) study. J Am Coll Cardiol. 2012;59:1142–1149. doi: 10.1016/j.jacc.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawasw Y, Izumi C, Kato T, Ishii K, Nagao K, Nakagawa Y, Toyofuku M, Saito N, Minatoya K, Kimura T. Sudden death in patients with severe aortic stenosis: observations from the CURRENT AS registry. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores-Marín A, Gómez-Doblas JJ, Caballero-Borrego J, Cabrera-Bueno F, Rodríguez-Bailón I, Melero J, Porras C, Sánchez-Espín G, Such M, Olalla E, de Teresa E. Long- Term predictors of mortality and functional recovery after aortic valve replacement for severe aortic stenosis with left ventricular dysfunction. Rev Esp Cardiol. 2010;63:36–45. doi: 10.1016/s1885-5857(10)70007-4. [DOI] [PubMed] [Google Scholar]
- 17.Gillam LD, Marcoff L, Shames S. Timing of surgery in valvular heart disease: prophylactic surgery vs watchful waiting in the asymptomatic patient. Can J Cardiol. 2014;30:1035–1045. doi: 10.1016/j.cjca.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Everett RJ, Clavel MA, Pibarot P, Dweck MR. Timing of intervention in aortic stenosis: a review of current and future strategies. Heart. 2018 Dec;104:2067–2076. doi: 10.1136/heartjnl-2017-312304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banva W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Penell DJ, Newby DE, Mohjaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 20.Bing R, Everett RJ, Tuck C, Semple S, Lewis S, Harkess R, Mills N, Treibel T, Prasad S, Greenwood J, McCann G, Newby D, Dweck M. Rationale and design of the randomized, controlled early valve replacement guided by biomarkers of left ventricular decompensation in asymptomatic patients with severe aortic stenosis (evolved) trial. Am Heart J. 2019;212:91–100. doi: 10.1016/j.ahj.2019.02.018. [DOI] [PubMed] [Google Scholar]