Abstract
The present outburst of coronavirus-associated (SARS-CoV-2) acute respiratory disease coronavirus disease 19 (COVID-19) in December 2019 in Wuhan, China is the third recognised spill over due to the zoonotic transmission. SARS-CoVs are about 29.7 kb positive, single stranded (ss) RNA viruses that are considered as zoonotic pathogens, bat being their natural reservoirs and also shows transmission within humans. The rapidly increasing COVID-19 cases and need of best and efficient drug/vaccine/strategy to counteract the virus entry and its pathogenesis has made it a Herculean challenge for scientists. Synthetic drugs associated complications has attracted scientific attention for natural product-based drugs. Chemo-diversity of algae and cyanobacteria offers a novel approach and can be recognized as a relevant source for developing a future natural “antiviral drug”. The aim of this review is to highlight important features of SARS-CoV-2/COVID-19 and the antiviral compounds recognized in algae and cyanobacteria, with their mechanisms of actions. Algae possess both immunity improving capacity and suppresses many viruses. Thus, they can be recommended as a preventive and curative remedy against SARS-CoV-2.
Keywords: Coronavirus, 2019-nCoV, Algal compounds
Coronavirus (CoV) has caused two large-scale pandemics in the last two decades i.e. Severe Acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [1]. An increasing number of patients with pneumonia occurred in Hubei province of Wuhan, China since December 2019, which has globally attracted attention [2]. World Health Organization (WHO) has named the novel pneumonia as Corona Virus Disease 19 (COVID-19), where ‘CO’ stands for corona, ‘VI’ for virus, ‘D’ for disease and the epithet 19 symbolises the year of outbreak i.e. 2019 and declared it as the sixth “Public Health Emergency of International Concern” (PHEIC) on 30th January 2020 and a “Global Pandemic” on 11th March 2020 [3]. Scientists have successfully isolated a novel coronavirus from human airway epithelial cells [Fig. 1] [4]. The high virulence of these viruses and the absence of effective therapies has posed an ongoing threat to the public health. The conventional one-bug-one-drug hypothesis is inadequate to discourse the challenge of emerging and re-emerging viral pathogens, and only few drugs are available at present to control the viral diseases [5]. Moreover, identification of targets is equally necessary to find highly specific drugs. Recently, Angiotensin-converting enzyme 2 (ACE2) expressing cells along with spike protein (S-protein) and non-structural proteins (nsp) have been identified as the target cells for neutralizing antibody and antiviral peptides that can prove to be the potential therapeutic target against SARS-CoV-2 [6]. Thus, the development of a broad-spectrum class of natural antiviral agents that bind to these specific targets is urgent in view of the global pandemic.
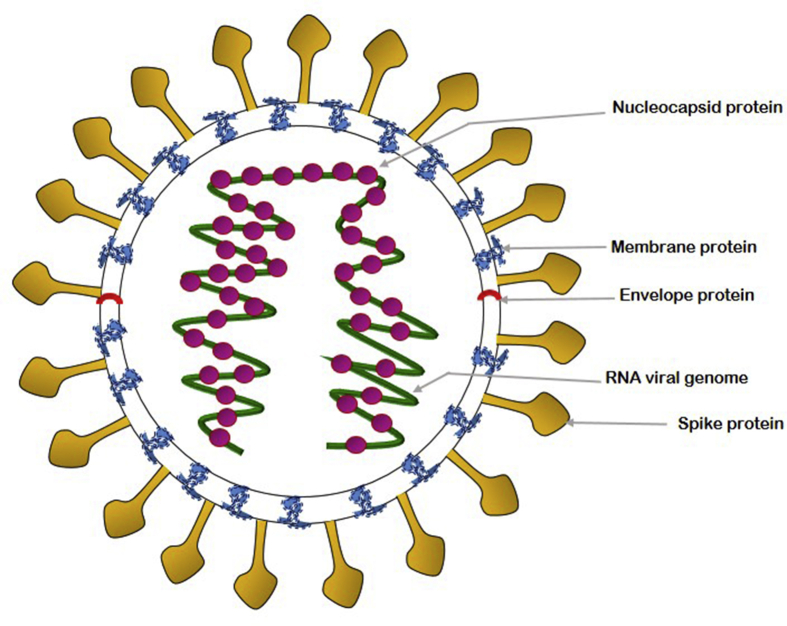
Fig. 1.
Structure of SARS-CoV-2 virus.
Algae and cyanobacteria are one of the richest sources of bioactive compounds that exhibit antiviral properties and are pharmacologically active [7]. Metabolites like flavanones, flavonols, and alkaloids are known to inhibit proteins like 3CLpro, Transmembrane Serine Protease 2 (TMPRSS2) and ACE2 involved in replication of COVID-19. Consequently, the antiviral compounds present in algae and cyanobacteria need to be explored to find the effective therapy for SARS-CoV-2. The present review discusses about the therapeutic potential of such compounds in details but to begin with, the classification and virology of SARS-CoV-2, its pathogenesis and associated symptoms of COVID-19 have been also briefly explained.
SARS CoV-2: virus behind the pandemic
Classification and nomenclature
The Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses has placed the virus within the Coronaviridae and provisionally named it as 2019-nCoV [8] [Table 1]. The external subdomain of the 2019-nCoV receptor-binding domain (RBD) share similarity with SARS and is closer to bat-SLCoVZXC21 and bat-SL-CoVZC45 at the whole-genome level. Since 2019-nCoV forms a sister clade to the prototype human and bat SARS-CoVs therefore it has been renamed as SARS-CoV-2 [9].
Table 1.
Classification of SARS-CoV-2.
| Realm | Riboviria |
| Order | Nidovirales |
| Sub-order | Cornidovirineae |
| Family | Coronaviridae |
| Sub-family | Coronavirinae |
| Genus | Betacoronavirus |
| Sub-genus | Sarbecovirus |
| Species | Severe acute respiratory syndrome-related coronavirus |
| Lineage | Lineage B |
Virology and pathogenesis of SARS-CoV-2
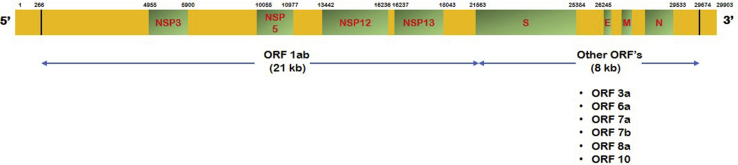
SARS-CoV-2 is highly stable in the environment and can survive for at least 2–4 days in stool and on dry surfaces at room temperature [10]. It is an enveloped positive sense ss-RNA virus with genome size of approximately 30 kb that encodes structural, non-structural and accessory proteins [Table 2] [11].
Table 2.
Details about SARS-CoV-2 genome organization [13].
| Region | Nucleotide length | Protein Formed |
|---|---|---|
| 5′ UTR | 265 | Non-coding region |
| ORF 1 ab gene | 21290 | ORF 1 ab poly-protein |
| S gene | 3822 | Spike glycoprotein |
| ORF 3a gene | 828 | ORF 3a protein |
| E gene | 228 | Envelope protein |
| M gene | 669 | Membrane protein |
| ORF 6a gene | 186 | ORF 6a protein |
| ORF 7a gene | 366 | ORF 7a protein |
| ORF 7b gene | 132 | ORF 7b protein |
| ORF 8a gene | 193 | ORF 8a protein |
| N gene | 908 | Nucleocapsid protein |
| ORF 10 gene | 117 | ORF 10 protein |
| 3′ UTR | 229 | Non-coding region |
Structural proteins include spike (S), envelope (E), membrane (M) and nucleocapsid (N) protein [Fig. 2]. The surface S-glycoprotein assures appropriate interactions between the virus and the host receptor during viral entry. The recombinant receptor binding domain (RBD) of S-protein specifically binds to ACE2 protein, mediates host cell invasion and initiates pathogenesis [12]. The binding efficiency is about 10–20 times higher in SARS-CoV-2 that results in its higher transmissibility and contagiousness. The other three structural proteins help in viral assembly.
Fig. 2.
Gene organization of SARS-CoV-2 virus.
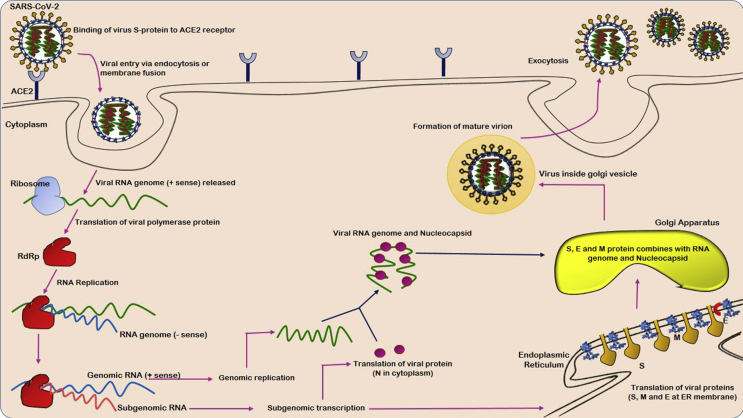
Non-structural proteins include 3-chymotrypsin-like protease (3CLpro), papain-like protease (PLpro), helicase, and RNA-dependent RNA polymerase (RdRp) that play an important role in the viral life cycle [Table 3]. The virus releases its genome as ss-positive RNA that subsequently gets translated into polyproteins by the host cell translation machinery [13] [Fig. 3].
Table 3.
Proteins coded by SARS-CoV-2.
| Region | Type of Protein | Protein | Functions |
|---|---|---|---|
| NSP 3 | Papain like protease (PLPro) |
|
|
| NSP 5 | 3CL-protease (3CLPro) |
|
|
| NSP 12 | Non-structural Proteins | RNA dependent Polymerase (RdRp) |
|
| NSP 13 | Helicase |
|
|
| S | Spike protein |
|
|
| E | Structural Proteins | Envelope protein |
|
| M | Membrane protein | ||
| N | Nucleocapsid |
Fig. 3.
Pathogenesis mechanism of SARS-CoV-2.
The pathogenesis starts with evading the innate antiviral response, then, embracing the host metabolic apparatus, replicating proficiently inside host cell and consequently inducing cytolysis [14]. Acute respiratory distress syndrome (ARDS) is the common immuno-pathological event for the coronavirus infection and the principal cause of COVID-19 deaths. The main mechanism involved in ARDS is the cytokine storm syndrome (CSS) which is the deadly hysterical systemic inflammatory response generated in response to the release of massive pro-inflammatory cytokines [TNF-α, TGFβ, IFN-(α, γ), IL-(1β, 6, 8, 9 etc.)] and chemokines [CCL-(2, 3 and 5), CXCL-(8, 9 etc.)] by immune effector cells [15]. The immune system consequently triggers a vicious attack on the body by causing acute lung injury (ALI), cardiac injury, RNAaemia, sepsis and multiple organ failure [16].
Prominent symptoms of COVID-19
SARS-CoV-2 attacks the respiratory system, gastrointestinal system, central nervous system, kidney, heart and liver leading to multiple organ failure [4]. The general COVID-19 symptoms include mostly the upper respiratory infection, onset of fever, dry cough, myalgia, fatigue, dyspnea, abnormal leukocyte counts, increased amount of lactate dehydrogenase (LDH) and C-reactive protein (CRP) [10]. Additionally, some patients might also suffer from diarrhoea, vomiting, nausea, headache, dizziness and abdominal pain. The disease when severely progresses causes sepsis, sudden cardiac arrest, pneumonia with ARDS or ALI [11].
Therapeutical approach against COVID-19 till date
The strategies being used in the drug development focusses on two aspects i.e. modulating the host defense system and targeting infectivity of virus. The former method involves blocking the signal transduction pathways in human cells that aids viral replication. The latter targets SARS-CoV-2 itself by inhibiting its RNA synthesis, replication (through acting on critical viral enzymes and blocking the virus binding to human cell receptors) or inhibiting it's self-assembly. The most reliable therapy being used till date is remdesivir which has shown potent in vitro activity against SARS-CoV-2, but it is not US Food and Drug Administration (US FDA) approved. Chloroquine has also shown good activity in vitro but the cardiovascular toxicity concerns limit its use. Existing clinical proofs support the administration of “Angiotensin Receptor Blockers (ARB)” or ACE inhibitors in patients with COVID-19. However, the fear of development of the drug-resistant form creates a void in between the disease and its therapy. Moreover, reports from randomized clinical trials also hints that the available therapy fails to improve the condition of suspected or confirmed COVID-19 patients.
Thus, exploration of natural sources in perspective of producing new pharmaceutical tools and development of a broad-spectrum class of effective antiviral agent(s) against SARS-CoV-2 is an urgent need [17]. Algal-derived polysaccharides and lectins serve as potential antiviral agents. Herein, bioactivity of some important polysaccharides and lectins including carrageenan, galactans, nostoflan, cyanovirin, microvirin have been explained.
Antiviral activity of algal-derived polysaccharides
Algal polysaccharides are natural polymers that are nontoxic, cheap, biodegradable and biocompatible. They have been tested for their antiviral efficacy against many viruses including human immunodeficiency virus (HIV), dengue virus (DENV) etc. Thus, they have acquired importance in biomedical and pharmaceutical industries that can be further explored to develop drug molecules targeting SARS-CoV-2 [18].
Carrageenan
A sulphated polymer obtained from red algae such as Chondrus, Gigartina, Hypnea and Eucheuma that obstructs the entry of viruses by inhibiting their binding or incorporation into the host cells [[19], [20], [21]]. It hinders the replication of dengue virus in mosquito and mammalian cells. They are effective against a range of sexually transmitted human papillomavirus (HPV) that leads to cervical cancer and genital warts. In vivo studies have revealed that low molecular weight carrageenans (3, 5 and 10 kDa) exhibit considerable inhibitory effects against influenza virus [20,22]. The administration of a carrageenan nasal spray (Iota-carrageenan) also known as “super-shedders” increased viral clearance, reduced the duration of common cold disease and relapses and has proved to be an effective treatment of the common cold [23,24]. Kwon et al. [25] have reported that sulfated polysaccharides bind tightly to the S-protein of SARS-CoV-2 which suggests that they can act as decoys to interfere with S-protein binding to the heparan sulfate co-receptor in host tissues inhibiting viral infection. Sulfated polysaccharides from Porphyridium have been used as a coating material on the sanitary items and for the production of antiviral drugs [26]. Exopolysaccharides from Porphyridium along with carrageenan and sulfated polysaccharides inhibits the internalization or binding of virus on the host cells. Therefore, they reduce COVID-19 proliferation and can prove to be a promising antiviral agent against respiratory viruses belonging to the coronavirus's family [27]. Recently, it has also been confirmed that iota-carrageenan is capable of inhibiting SARS-CoV-2 infection in Vero cell cultures (isolated from kidney epithelial cells extracted from African green monkey) [28].
Alginates
The natural polymers that contain linear copolymers of β- (1–4) linked d-mannuronic acid and β-(1–4) linked l-guluronic acid units, derived from brown algae like Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [29]. Marine polysaccharide drug 911 derived from alginate significantly inhibit the acute infection of MT4 cells and the chronic infection of H9 cells with HIV-1 [30]. The drug inhibits the viral replication of HIV via significantly decrementing the activity of reverse transcriptase (RTase), discontinuing the virus adsorption, and improving the defense mechanisms of the host cells and inhibiting the virus replication by suppressing the activity of DNA polymerase activity [[31], [32], [33]]. The sulfated form of alginate i.e. sulphated polymannuroguluronate (SPMG) inhibits HIV-1 infection through attachment of virus glycoprotein, gp120 with CD4 molecules on the surface of T-cells. It further blocks the virus replication and the syncytium formation between uninfected and infected cells [34].
Galactans
Red algae Agardhiella tenera produces extracellular polysaccharides with linear chains of galactoses that exhibit antiviral potency against enveloped viruses including herpes simplex virus-1 and -2 (HSV-1 and HSV-2), DENV, HIV-1 and HIV-2, and hepatitis A virus (Hep A) virus. Like alginates, they also block the replication of the virus and the syncytium formation between uninfected and infected cells [35]. Three galactans polysaccharide fractions isolated from marine alga Callophyllis variegate have shown activity against HSV-1, HSV-2 and DENV-2 with considerable inhibitory effects along with low cytotoxicity [36]. The sulphated galactan isolated from Schizymenia binderi have been found to active against HSV-1 and HSV-2 with lowest cytotoxicity [37]. It has been reported that D, L-galactan hybrid C2S-3, extracted from the Brazilian marine alga Cryptonemia crenulata inhibits the multiplication of DENV-2 in Vero cell line [38]. Furthermore, it blocks the replication of HIV-1 and the syncytium formation between uninfected and infected cells as well [39].
Fucans
They are strong anionic high molecular weight polysaccharides found in brown algae. They have been classified into three major groups: glycuronogalacto fucans, fucoidans and xylofuco glycuronans. Sulphated fucans of brown seaweed species Dictyota mertensii, Lobophora variegata, Fucus vesiculosus, and Spatoglossum schroederi have been found to prevent HIV infection by blocking the activity of reverse transcriptase [40]. The fucan polysaccharide isolated from Cladosiphon okamuranus inhibits DENV-2 infection in baby hamster kidney cell (BHK-21) cell line [41]. The anti-influenza virus compound named MC26 (a new type of fucose polysaccharides), isolated from marine brown algae, Sargassum piluliferum, exhibited a stronger anti-influenza virus activity with low cytotoxicity in vivo and in vitro as compared to the known active compounds [42]. Fucoidans isolated from several algal species such as Adenocytis utricularis, Undaria pinnatifida, Stoechospermum marginatum and Cystoseira indica, possesses both in vivo as well as in vitro antiviral potential against many RNA and DNA viruses like HSV-1 and HSV-2, dengue virus, and cytomegalovirus [43]. They block interaction of virus with the cells and inhibit syncytium formation [44].
Nostoflan
It is the acidic polysaccharide isolated from blue-green algae Nostoc flagelliforme [45] and possess antiviral activity against viruses having carbohydrates as cellular receptors. It exhibits potent antiviral activity against HSV-1, HSV-2, human cytomegalovirus and influenza A virus. It inhibits the initial stage of virus infection including the virus binding and internalization processes [46].
Calcium spirulan (Ca-SP)
It has been isolated from the hot water extract of Spirulina platensis that exhibits promising antiviral activity against HSV-1, HIV-1 and HSP-1. It inhibits the virus entry into the host cells and syncytium formation even at low concentrations [47].
Naviculan
A sulphated polysaccharide isolated from Navicula directa that is composed of galactose, xylose, rhamnose, fucose, mannose and sulphate [48]. It has shown novel antiviral activities against HSV-1, HSV-2 and influenza A virus. It inhibits fusion between the cells that express CD4 receptor and HIV gp160-expressing HeLa cell line [49].
A1 and A2 polysaccharide
The extracellular sulphated polysaccharides isolated from marine microalga Cochlodinium polykrikoides that inhibit influenza type-A and type-B virus in MDCK cells, respiratory virus types-A and B in Hep-2 cells, immunodeficiency virus type-1 in MT-4 cells. Inhibition of viral activity is suggested by its potential to reduce blood coagulation [50].
Laminarin
Brown seaweeds like Laminaria japonica, Ecklonia kurome, Eisenia bicyclis produce two types of laminarin i.e. one made of glucose residues while the other terminated by D-mannitol residues [51]. Both possess great antiviral activity and are bio-compatible. It prevents adsorption of HIV reverse transcriptase [52].
p-KG03
The sulphated exo-polysaccharide p-KG03, produced by marine microalga Gyrodinium impudicum, exhibits unique antiviral activity against encephalomyocarditis virus (EMCV) without showing any toxic effects on HeLa cells. In addition, p-KG03 also inhibit influenza A virus replication by targeting mainly the viral adsorption and incorporation steps [53,54].
Sea algae extract (SAE)
A member of carrageenan isolated from Schizymenia pacifica (red algae) that impedes the function and replication of reverse transcriptase in avian retrovirus (avian myeloblastosis virus) and mammalian retrovirus (Rauscher murine leukemia virus) [55,56].
Antiviral activity of algal-derived lectins
Lectins are the class of proteins that bind reversibly to viral receptors in non-covalent and highly specific manner. They counteract several viruses including HIV which makes them possible drug for drug development [57].
Cyanovirin
Lectin isolated from Nostoc ellipsosporum that consist of 101 amino acids and has a molecular weight of 11 kDa. It efficiently binds to the envelope glycoprotein (gp120) and inhibit many viruses including HIV-1, HIV-2, simian immunodeficiency virus (SIV) and feline immunodeficiency virus [58]. Cyanovirin acts once the virus-cell attachment is complete or after CD-4 binding step in the entry process [59,60].
Microvirin
Microcystis aeruginosa produces microvirin which is composed of 108 amino acids and is more than 50-fold less toxin than cyanovirin. It does not increase the level of activation markers such as CD25, CD69 and HLA-DR in CD4+ T lymphocytes [61]. It inhibits syncytium formation between HIV-1 infected T cells and uninfected CD4+ T cells.
Griffithsin
The lectin isolated from marine red algae Griffithsia sp. and is considered to be the most considerable HIV inhibitor till date [62]. It is made up of 120 amino acids and shows anti-HIV activity with IC50 in the picomolar (pm) range. Griffithsin binds to the HIV envelope protein gp120 and inhibits viral infection [63,64]. It is also known to inhibit hepatitis C Virus (HCV) infection of mice having human primary hepatocytes in the liver and prevents in vitro HCV infection of Huh-7 hepatoma cells [65]. It binds to the HCV envelope glycoproteins (E1 and E2) and block entry of virus into human hepatocytes [66,67]. Moreover, it has been further observed that griffithsin protects mice infected with genital HSV-2 as well and prevent cell-to-cell spread with no significant adverse effects [68].
Griffithsin is also known to prevent SARS-CoV infection through specific binding to the S-protein. These inhibitory effects gets accompanied with a specific inhibition of deleterious host immune reactions in response to SARS [69]. MERS-CoV gets inhibited at the entry level by griffithsin to prevent infection in vitro [70].
Scytovirin
Scytonema varium produces a 95-amino-acid lectin called scytovirin that is active against multiple viruses, including HIV, Zaire ebolavirus, Marburg virus, and SARS-CoV [71,72]. Subcutaneous administration of scytovirin (30 mg/kg/day) for every 6 h to the ebola virus infected mice resulted in survival of 9 out of 10 animals [73]. Scytovirin binds with high affinity to mannose-rich oligosaccharides on the envelope glycoprotein, blocking entry into target cells.
Other lectins (KAA-2, BCA)
Red algae Kappaphycus alvarezii and green alga Boodlea coacta synthesizes high mannose-specific lectin, KAA and agglutinin, BCA respectively that inhibit infection of multiple influenza strains like the pandemic H1N1-2009. They interfere with the viral entry into host cells upon direct binding of hemagglutinin (HA) on the viral envelope [74,75].
Allophycocyanin
Blue green algae S. platensis allophycocyanin neutralizes enterovirus 71-induced cytopathic effect in human rhabdomyosarcoma cells and African green monkey kidney cells. It delay viral RNA synthesis and subside the apoptotic process along-with DNA fragmentation, decrease in membrane damage and declining cell sub-G1 phase [76].
Pheophorbide like compounds
Ethanolic extract of the marine green algae Dunaliella primolecta contain pheophorbide like compounds that inhibit cytopathic effect of HSV-1 during its adsorption and invasion into the host cells [77].
Phlorotannins (6,6′-bieckol)
Ecklonia cava produces phlorotannins that inhibit syncytia formation, lytic effects and viral p24 antigen production both in vitro and in vivo [78]. It has shown potent inhibition of HIV-1 reverse transcriptase enzyme [79].
Conclusions
Novel infectious diseases resulting from RNA viruses will continue to be a serious global health threat. Despite two former major outbreaks of coronavirus infections i.e. the SARS and MERS, the world is still underprepared to effectively manage the current COVID-19 pandemic outbreak. A rigorous effort to develop effective drugs and vaccines against existing and potential future coronavirus infections and other highly pathogenic virus outbreaks is essential to reduce devastating impacts on human life and global healthcare systems. Clinical drug development is too costly and a strenuous process, so there is a need to develop relatively broad-spectrum natural antiviral drugs. Algae and cyanobacteria are the fruitful reservoir of many metabolites like sulfated polysaccharides, lectins, etc. that possess strong antiviral activities and immunity boosting effects. Therefore, these natural resources should be screened thoroughly as there is enormous probability of getting novel compounds that can inhibit SARS-CoV-2.
Success stories
Till date, there are two scientific groups actively involved in developing algae-based edible vaccines for SARS-CoV-2. The first group belong to the Laboratory of Photosynthesis and Bioenergy of the Department of Biotechnology at the University of Verona, Italy. They have adopted two approaches i.e. nuclear transgenesis and chloroplast transformation to introduce a DNA sequence corresponding to the receptor-binding domain of SARS-CoV-2 S-protein in single-celled alga Chlamydomonas reinhardtii that resulted in production of antibodies. The algae has been lyophilized and encapsulated to develop an oral vaccine against SARS-CoV-2 [80].
Similar work has been done by the Biotech Company, TransAlgae. They also genetically modified algae, C. reinhardtii to produce oral vaccine for SARS-CoV-2. If contamination is prevented then it is possible to accumulate up to 1 mg of the recombinant antigen for each gram of biomass of dried algae. Subsequently, the dehydrated/lyophilized algae can be encapsulated to generate an “oral vaccine.” The cell wall from the dry algae protects the antigens from the harsh acidic and protease-rich gastric environment, enabling the bioactive molecule to reach the intestinal immune system where it can stimulate cellular and humoral responses, hopefully, leading to effective immunization [81].
Future scope
Prevention is better than cure hence the fight against novel coronavirus needs immediate and well-planned strategies. The governments can supply raw algae powders/capsules to improve immunity of individuals that will prevent the viral infection. It is worth mentioning that the recombinant antigen obtained from genetically modified algae can prove to be a boon as it can be dried out and used directly, saving the cost incurred on extraction and purification. Further, the algal cell wall protects the antigen for longer periods without any loss in its efficacy. This will certainly help developing countries that face the problem of storage and transportation of vaccines. Genetic modification of algae will make the vaccine development easy but further research is needed to develop strategies that can inhibit the recurrence of these viral diseases.
Funding
R. Ahmad sincerely thanks University Grants Commission (F.No. 61–1/2019 (SA-III)) for Maulana Azad National Fellowship (MANF-JRF).
Author contributions
All authors have contributed to the manuscript. All authors have revised, edited and approved the final manuscript.
Conflicts of interest
The authors declare no conflict of interest. No conflicts, informed consent, or human or animal rights are applicable to this study.
Acknowledgements
R. Ahmad sincerely thanks University Grants Commission (F.No. 61-1/2019 (SA-III)) for Maulana Azad National Fellowship (MANF-JRF).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang B., Bragazzi N.L., Li Q., Tang S., Xiao Y., Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infectious Disease Modelling. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.canaryhealthtech.com/news/naming-the-coronavirusdisease-covid-2019-and-the-virus-that-causes-it [Google Scholar]
- 4.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carossino M., Thiry E., de la Grandière A., Barrandeguy M.E. Novel vaccination approaches against equine alphavirus encephalitides. Vaccine. 2014;3:311–319. doi: 10.1016/j.vaccine.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 6.Lu R.P., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deig E.F., Ehresmann D.W., Hatch M.T., Riedlinger D.J. Inhibition of herpesvirus replication by marine algae extracts. Antimicrob Agents Chemother. 1974;6:524–525. doi: 10.1128/aac.6.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbalenya A.E., Baker S.C., Baric R.S., de Groo R.J., Drosten C., Gulyaeva A.A. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19:100682. doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Weber H.K., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J.H., Skinner G.W., Harcum W.W., Barnum P.E. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharmaceut Sci Technol Today. 1998;1:254–261. [Google Scholar]
- 19.Lahaye M. Developments on gelling algal galactans, their structure and physico-chemistry. J Appl Phycol. 2001;13:173–184. [Google Scholar]
- 20.Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J. 2008;5:107. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilliou L., Larotonda F.D., Abreu P., Ramos A.M., Sereno A.M., Gonçalves M.P. Effect of extraction parameters on the chemical structure and gel properties of κ/ι-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol Eng. 2006;23:201–208. doi: 10.1016/j.bioeng.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Zeitlin L., Whaley K.J., Hegarty T.A., Moench T.R., Cone R.A. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–335. doi: 10.1016/s0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]
- 23.Eccles R., Meier C., Jawad M., Weinmullner R., Grassauer A., Prieschl-Grassauer E. Efficacy and safety of an antiviral iota-carrageenan nasal spray: a randomized, double-blind, placebo controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res. 2010;16:1–11. doi: 10.1186/1465-9921-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenighofer M., Lion T., Bodenteich A., Prieschl-Grassauer E., Grassauer A., Unger H. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Respir Med. 2014;9:1–12. doi: 10.1186/2049-6958-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon P.S., Oh H., Kwon S.J., Jin W., Zhang F., Fraser K. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramus J. Cell surface polysaccharides of the red alga Porphyridium. In: Loewus F., editor. Biogenesis of plant cell wall polysaccharides. Academic Press; New York: 1973. pp. 333–359. [Google Scholar]
- 27.Nagle V., Gaikwad M., Pawar Y., Dasgupta S. Marine red alga Porphyridium sp. as a source of sulfated polysaccharides (SPs) for combating against COVID-19. https://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjeu7ia-dnuAhUUyYsBHbe7D3QQFjADegQIARAC&url=https%3A%2F%2Fwww.preprints.org%2Fmanuscript%2F202004.0168%2Fv1%2Fdownload&usg=AOvVaw0mip2oHJATrTtfNZ7hKuJ2 2020. [Preprints] 2020040168. Available from:
- 28.Bansal S., Jonsson C.B., Taylor S.L., Figueroa J.M., Dugour A.V., Palacios C. 2020. Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture. bioRxiv 2020.08.19.225854 [Preprint]. Available from: doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabeau S., Kloareg B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F.serratus, Bifurcaria bifurcata and Laminaria digitate. J Exp Bot. 1987;38:1573–1580. [Google Scholar]
- 30.Xianliang X., Hua D., Meiyu G., Pingfang L., Yingxia L., Huashi G. Studies of the anti-AIDS effects of marine polysaccharide drug 911 and its related mechanisms of action. Chin J Mar Drugs. 2000;19:4–8. [Google Scholar]
- 31.Xin X., Geng M., Guan H., Li Z. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitro. Chin J Mar Drugs. 1999;19:15–18. [Google Scholar]
- 32.Xianliang X., Meiyu G., Huashi G., Zelin L. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitro. Chin J Mar Drugs. 2000;19:15–22. [Google Scholar]
- 33.Jiang B.F., Xu X.F., Li L., Yuan W. Study on ‘911’ anti-HBV effect in HepG2 2115 cells culture. Mod Prev Med. 2003;30:517–518. [Google Scholar]
- 34.Wang W., Wang S.X., Guan H.S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witvrouw M., Este J.A., Mateu M.Q., Reymen D., Andrei G., Snoeck R. Activity of a sulfated polysaccharide extracted from the red seaweed Aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antiviral Chem Chemother. 1994;5:297–303. [Google Scholar]
- 36.Rodríguez M.C., Merino E.R., Pujol C.A., Damonte E.B., Cerezo A.S., Matulewicz M.C. Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales) Carbohydr Res. 2005;340:2742–2751. doi: 10.1016/j.carres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Matsuhiro B., Conte A.F., Damonte E.B., Kolender A.A., Matulewicz M.C., Mejías E.G. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta) Carbohydr Res. 2005;340:2392–2402. doi: 10.1016/j.carres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Talarico L.B., Duarte M.E., Zibetti R.G., Noseda M.D., Damonte E.B. An algal-derived DL-galactan hybrid is an efficient preventing agent for in vitro dengue virus infection. Planta Med. 2007;73:1464–1468. doi: 10.1055/s-2007-990241. [DOI] [PubMed] [Google Scholar]
- 39.Bouhlal R., Haslin C., Chermann J.C., Colliec-Jouault S., Sinquin C., Simon G. Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (rhodophytha, gigartinales) and Boergeseniella thuyoides (rhodophyta, ceramiales) Mar Drugs. 2011;9:1187–1209. doi: 10.3390/md9071187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Queiroz K.C., Medeiros V.P., Queiroz L.S., Abreu L.R., Rocha H.A., Ferreira C.V. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed Pharmacother. 2008;62:303–307. doi: 10.1016/j.biopha.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 41.McCandless E.L., Craigie J.S. Sulfated polysaccharides in red and brown algae. Annu Rev Plant Physiol. 1979;30:41–53. [Google Scholar]
- 42.Akamatsu E., Shimanaga M., Kamei Y. Isolation of an anti-influenza virus substance, MC26 from a marine brown alga, Sargassum piluliferum and its antiviral activity against influenza virus. Coast Bioenviron. 2003;1:29–34. [in Japanese] [Google Scholar]
- 43.Hidari K.I., Takahashi N., Arihara M., Nagaoka M., Morita K., Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun. 2008;376:91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 44.Hemmingson J.A., Falshaw R., Furneaux R.H., Thompson K. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta) J Appl Phycol. 2006;18:185–193. [Google Scholar]
- 45.Whitton B.A., Potts M. 1st ed. Springer; Netherlands: 2007. The ecology of cyanobacteria: their diversity in time and space. [Google Scholar]
- 46.Kanekiyo K., Hayashi K., Takenaka H., Lee J.B., Hayashi T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol Pharm Bull. 2007;30:1573–1575. doi: 10.1248/bpb.30.1573. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T., Hayashi K., Maeda M., Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J Nat Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 48.Kubo Y., Shozen K., Seto Y. Hyaluronidase inhibitory effect in diatom extracts isolated from deep sea water. Deep Ocean Water Res. 2002;3:71–76. [Google Scholar]
- 49.Lee J.B., Hayashi K., Hirata M., Kuroda E., Suzuki E., Kubo Y. Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol Pharm Bull. 2006;29:2135–2139. doi: 10.1248/bpb.29.2135. [DOI] [PubMed] [Google Scholar]
- 50.Hasui M., Matsuda M., Okutani K., Shigeta S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int J Biol Macromol. 1995;17:293–297. doi: 10.1016/0141-8130(95)98157-t. [DOI] [PubMed] [Google Scholar]
- 51.Nelson T.E., Lewis B.A. Separation and characterization of the soluble and insoluble components of insoluble laminaran. Carbohydr Res. 1974;33:63–74. doi: 10.1016/s0008-6215(00)82940-7. [DOI] [PubMed] [Google Scholar]
- 52.Muto S, Niimura K, Oohara M, Oguchi Y, Matsunaga K, Hirose K, et al. Polysaccharides and antiviral drugs containing the same as active ingredient. U.S. Patent No. 5,089,481. Washington, DC: U.S. Patent and Trademark Office. United States 1992;5:481.
- 53.Yim J.H., Kim S.J., Ahn S.H., Lee C.K., Rhie K.T., Lee H.K. Antiviral effects of sulfated exopolysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar Biotechnol. 2004;6:17–25. doi: 10.1007/s10126-003-0002-z. [DOI] [PubMed] [Google Scholar]
- 54.Kim M., Yim J.H., Kim S.Y., Kim H.S., Lee W.G., Kim S.J. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir Res. 2012;93:253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Nakashima H., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Antiretroviral activity in a marine red alga: reverse transcriptase inhibition by an aqueous extract of Schizymenia pacifica. J Canc Res Clin Oncol. 1987;113:413–416. doi: 10.1007/BF00390034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakashima H.Y., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob Agents Chemother. 1987;31:1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashita K., Hara-Kuge S., Ohkura T. Intracellular lectins associated with N-linked glycoprotein traffic. Biochim Biophys Acta Gen Subj. 1999;1473:147–160. doi: 10.1016/s0304-4165(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 58.Mori T., Boyd M.R. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. Antimicrob Agents Chemother. 2001;45:664–672. doi: 10.1128/AAC.45.3.664-672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dey B., Lerner D.L., Lusso P., Boyd M.R., Elder J.H., Berger E.A. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J Virol. 2000;74:4562–4569. doi: 10.1128/jvi.74.10.4562-4569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiwari V., Shukla S.Y., Shukla D. A sugar binding protein cyanovirin-N blocks herpes simplex virus type-1 entry and cell fusion. Antivir Res. 2009;84:67–75. doi: 10.1016/j.antiviral.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahzad-ul-Hussan S., Gustchina E., Ghirlando R., Clore G.M., Bewley C.A. Solution structure of the monovalent lectin microvirin in complex with Manα (1–2) Man provides a basis for anti-HIV activity with low toxicity. J Biol Chem. 2011;286:20788–20796. doi: 10.1074/jbc.M111.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori T., O'Keefe B.R., Sowder R.C. 2nd, Bringans S., Gardella R., Berg S. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 63.Barton C., Kouokam J.C., Lasnik A.B., Foreman O., Cambon A., Brock G. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue J., Hoorelbeke B., Kagiampakis I., Demeler B., Balzarini J., Li Wang P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob Agents Chemother. 2013;57:3976–3989. doi: 10.1128/AAC.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meuleman P., Albecka A., Belouzard S., Vercauteren K., Verhoye L., Wychowski C. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takebe Y., Saucedo C.J., Lund G., Uenishi R., Hase S., Tsuchiura T. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PloS One. 2013;8 doi: 10.1371/journal.pone.0064449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kachko A., Loesgen S., Shahzad-Ul-Hussan S., Tan W., Zubkova I., Takeda K. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin: mechanistic differences between the high mannose specific lectins MVL, CV-N, and GNA. Mol Pharm. 2013;10:4590–4602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nixon B., Stefanidou M., Mesquita P.M., Fakioglu E., Segarra T., Rohan L. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J Virol. 2013;87:6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millet J.K., Séron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R. MiddleEast respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bokesch H.R., O'Keefe B.R., McKee T.C., Pannell L.K., Patterson G.M., Gardella R.S. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42:2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Zhang X., Chen G., Wei D., Chen F. Algal lectins for potential prevention of HIV transmission. Curr Med Chem. 2008;15:1096–1104. doi: 10.2174/092986708784221421. [DOI] [PubMed] [Google Scholar]
- 73.Garrison A.R., Giomarelli B.G., Lear-Rooney C.M., Saucedo C.J., Yellayi S., Krumpe L.R. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivir Res. 2014;112:1–7. doi: 10.1016/j.antiviral.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato Y., Hirayama M., Morimoto K., Yamamoto N., Okuyama S., Hori K. High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pickett E., Brown J., van Schalkwyk M., Hunter A., Edwards K., Edwards S. Access to influenza immunisation services by HIV-positive patients in the UK. Influenza Other Respir Viruses. 2018;12:544–546. doi: 10.1111/irv.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shih S.R., Tsai K.N., Li Y.S., Chueh C.C., Chan E.C. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J Med Virol. 2003;70:119–125. doi: 10.1002/jmv.10363. [DOI] [PubMed] [Google Scholar]
- 77.Ohta S., Ono F., Shiomi Y., Nakao T., Aozasa O., Nagate T. Anti-herpes simplex virus substances produced by the marine green alga, Dunaliella primolecta. J Appl Phycol. 1998;10:349–356. [Google Scholar]
- 78.Artan M., Li Y., Karadeniz F., Lee S.H., Kim M.M., Kim S.K. Anti-HIV-1 activity of phloroglucinol derivative, 6, 6′-bieckol, from Ecklonia cava. Bioorg Med Chem. 2008;16:7921–7926. doi: 10.1016/j.bmc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 79.Wijesekara I., Yoon N.Y., Kim S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): biological activities and potential health benefits. Biofactors. 2010;36:408–414. doi: 10.1002/biof.114. [DOI] [PubMed] [Google Scholar]
- 80.Specht E.A., Mayfield S.P. Algae-based oral recombinant vaccines. Front Microbiol. 2014;5:60. doi: 10.3389/fmicb.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gunasekaran B., Gothandam K.M. A review on edible vaccines and their prospects. Braz J Med Biol Res. 2020;53:e8749. doi: 10.1590/1414-431X20198749. [DOI] [PMC free article] [PubMed] [Google Scholar]