Abstract
High-throughput screening of cell-biomaterial interactions combined with machine learning algorithms leads the way toward the future of medical device manufacturing: in silico modeling of cell and tissue response. Bio-compatible medical implants will have a huge clinical impact.
High-throughput screening of cell-biomaterial interactions combined with machine learning algorithms leads the way toward the future of medical device manufacturing: in silico modeling of cell and tissue response. Bio-compatible medical implants will have a huge clinical impact.
Main Text
Here and Now
In times of COVID-19, it is not difficult to advocate the importance of large volumes of high-quality data and powerful predictive models. Epidemiologists are the new heroes. Data help us to understand and design an informed strategy to tackle complex medical problems—and not just COVID-19.
While the coronavirus is being combated with unified forces, a slower, silent revolution is taking place that will affect the health of even more people; the article by Rostam and co-workers in this issue of Matter is a feat of that.1 Smart medical implants play an increasingly important role in human health care, and many unmet clinical challenges can be solved using biomaterials. But the proper interaction of the inorganic material surface with the biological system is of utmost importance for the success of the implant. The relation between surface properties and biological response is complex, and that’s where data-driven research kicks in.
Medical implants have saved and/or improved the lives of millions of people and include pacemakers (I wear one myself!), hip implants, catheters, and stents to name a few out of many hundreds of implants. With all the benefit they bring the patient, many unwanted side effects occur. Patients wearing a stent must take blood thinners for a lifetime to prevent coagulation at the stent surface, and implant infection occurs in a large percentage of recipients. Another major problem is that many implants fail because of encapsulation and fibrosis, as seen in pelvic floor mat adhesion, brain implant encapsulation, and pathological encapsulation of breast implants. It is a typical problem of biological incompatibility, in which macrophages play a central role. These white blood cells belong to the innate immune system, which is the front line of defense against everything that invades the body. Macrophages recognize the foreign implant and unleash an inflammatory reaction that ultimately leads to the implant being encapsulated with scar tissue by resident fibroblasts. At least, that happens to most materials—but not all! It turns out that the physical and chemical properties of the material influence the inflammatory reaction, but the mechanism behind it, and the relationship between material properties and macrophage reaction has not been properly mapped.2 , 3 If only we could predict how material properties tune the immune system…But for predictions we need models and for models we need data. In short, clinical challenges must be boiled down to hard biological and material data. Now, how do we do that?
Peeking at the Neighbors
The biomaterial field can learn a lot from two adjacent disciplines: molecular cell biology and materials science. Molecular biologists are not afraid of measurements: the whole genome of humans (Human Genome project), the gene expression patterns of all cell types in the human body (Human Cell Atlas), or 1.2 million gene expression profiles of cells that have been genetically or pharmacologically treated (Connectivity Map).4 DNA sequencing technology is so powerful that this is everyday practice, and bioinformatics has been developed in its wake. Standards and nomenclature have been developed for creating and analyzing data; user-friendly software is available that allows many cell biologists to use the techniques. And thus, biological complexity is unraveled step by step.
Steps are also being taken on the materials science side. Quantitative structure activity relationships have long been a used to describe cause and effect. Here, too, complexity is a challenge. Even though we only have 94 naturally occurring elements on Earth, the number of materials we can make from them is (virtually) infinite. Therefore, materials science has embraced high-throughput screening and machine learning to structure chaos, and the field is organizing itself through initiatives such as the Material Genome Initiative to build a structural approach and tackle real world challenges, such as building efficient solar cells or converting CO2 in useful hydrocarbon compounds. The future in material engineering may lie in closed loop systems, where algorithms set up hypotheses based on literature and databases and control automated material manufacturing and analysis units to generate and analyze data (Figure 1 ).5, 6, 7
Figure 1.
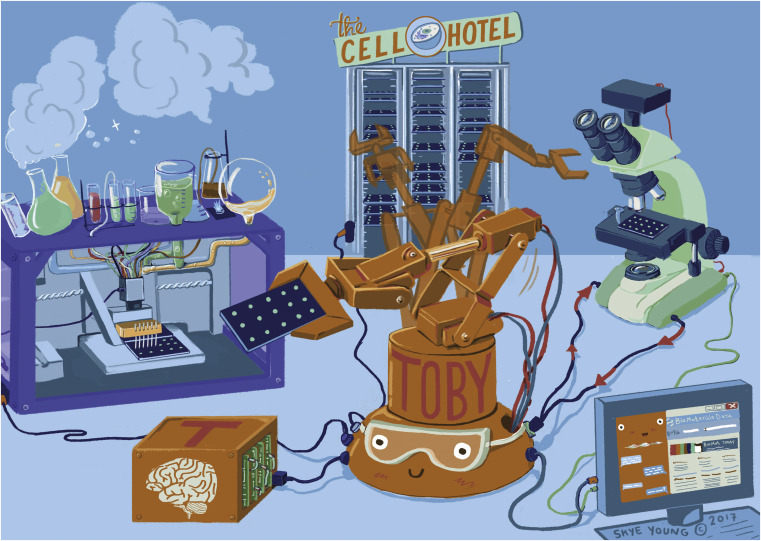
A Closed Loop for Biomaterials Discovery
The complexity of both biology and materials science requires an integrated approach to materials discovery and synthesis exploiting big data via parallel experimental and computational approaches guided by machine learning (from Vasilevich and de Boer,7 used with permission, ©2018 Elsevier B.V.).
A New Science
We are not yet ready for this in the biomaterial field, but we are heading in the right direction, kick started by the 2004 work of Dan Anderson and colleagues, who made polymer libraries for culture of embryonic stem cells.8 A huge challenge specific for biomaterial engineering is that we need to bridge the complexity of both biology and materials science and want to have predictive models of it that hold not only at the cell but also at the tissue and organism level. For such a challenge, you need a well-stocked expedition and the expedition headed by Rostam and co-workers,1 described in this issue of Matter was impressive. It was able to take all steps from material engineering, macrophage screening, data modeling, and testing the materials in an animal model. Libraries of (met) acrylates were printed, high content imaging has been used to fish out materials that triggered a mild inflammatory reaction in macrophages using automated image analysis, the correlation between material properties and inflammation has been modeled with machine learning algorithms, the adhesion of serum proteins was mapped by mass spectrometry, and the hit surfaces were found to influence the immune system and the encapsulation reaction in an animal model. Mission accomplished.
Looking Ahead
But there is a hidden gem in this paper. The study generated much more data than has been reported. Rostam and co-workers have taken photos of an estimated 150,000 macrophages.1 The expression of M1 and M2 markers is known for each macrophage but also the shape of the cells, the shape of the nucleus, the intensity of DNA staining, how well they adhere to 540 different materials, and the expression of 150 proteins for 8 surfaces. Think of all the hypotheses you can test with this dataset! Come to think of it, wouldn’t it be great if we had our own Biomaterial Connectivity Map, if we could link the data from Rostam’s work to other published high-throughput screens? And what if the Human Cell Atlas had a sister book called the Human Cell-Biomaterial Atlas? And then, when we can swim in data from hundreds of thousands of experiments, then science will really change. As David Winkler, co-author on the article and data scientist, and I agreed during a hike along the Trent River: in the future, the first step in any material discovery project will be in silico because most of the experiments will be done already. We can then predict cell and tissue response to biomaterials using large volumes of high-quality data and powerful predictive models. Data will help us to understand and design an informed strategy to tackle complex medical problems. We are not that far yet, but Rostam et al.1 show us that we are on the right track.
Acknowledgments
Declaration of Interests
J.d.B. is co-founder and shareholder of Materiomics b.v., which commercializes the TopoChip platform technology in the area of improved culture ware and medical device surface optimization.
References
- 1.Rostam H.M., Fisher L.E., Hook A.L., Burroughs L., Luckett J.C., Figueredo G.P., Mbadugha C., Teo A.C.K., Latif A., Kämmerling L. Immune-Instructive Polymers Control Macrophage Phenotype and Modulate the Foreign Body Response In Vivo. Matter. 2020;2:1564–1581. this issue. [Google Scholar]
- 2.Vegas A.J., Veiseh O., Doloff J.C., Ma M., Tam H.H., Bratlie K., Li J., Bader A.R., Langan E., Olejnik K. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 2016;34:345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown B.N., Barnes C.A., Kasick R.T., Michel R., Gilbert T.W., Beer-Stolz D., Castner D.G., Ratner B.D., Badylak S.F. Surface characterization of extracellular matrix scaffolds. Biomaterials. 2010;31:428–437. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian A., Narayan R., Corsello S.M., Peck D.D., Natoli T.E., Lu X., Gould J., Davis J.F., Tubelli A.A., Asiedu J.K. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017;171:1437–1452.e17. doi: 10.1016/j.cell.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King R.D., Whelan K.E., Jones F.M., Reiser P.G., Bryant C.H., Muggleton S.H., Kell D.B., Oliver S.G. Functional genomic hypothesis generation and experimentation by a robot scientist. Nature. 2004;427:247–252. doi: 10.1038/nature02236. [DOI] [PubMed] [Google Scholar]
- 6.Steiner S., Wolf J., Glatzel S., Andreou A., Granda J.M., Keenan G., Hinkley T., Aragon-Camarasa G., Kitson P.J., Angelone D. Organic synthesis in a modular robotic system driven by a chemical programming language. Science. 2019;363:eaav2211. doi: 10.1126/science.aav2211. [DOI] [PubMed] [Google Scholar]
- 7.Vasilevich A., de Boer J. Robot-scientists will lead tomorrow’s biomaterials discovery. Curr. Opin. Biomed. Eng. 2018;6:74–80. [Google Scholar]
- 8.Anderson D.G., Levenberg S., Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]


