Abstract
Chalcones and its derivatives are reported to exhibit anti-cancer effects in several cancer cell lines, including colon cancer cells. However, the in vivo anticancer effects and associated mechanisms of chalcones against intestinal tumorigenesis currently remain unclear. The aim of the present study was to investigate the chemopreventive effect of a chalcone derivative, 4′-hydroxychalcone (4-HC), in a transgenic adenomatous polyposis coli multiple intestinal neoplasia mouse model (ApcMin) of spontaneous intestinal adenomas. ApcMin mice were fed 4-HC (10 mg/kg/day) or the vehicle control by oral gavage starting at 8 weeks of age, and were sacrificed at 20 weeks. The administration of 4-HC significantly decreased the number of colon adenomas by 45% and the size of colon adenomas by 35% compared with the respective controls. Similarly, the number of adenomas in the distal small intestine (DSI) and proximal small intestine also decreased by 35 and 33%, respectively, in 4-HC-treated mice, and adenoma size in the DSI decreased by 39% compared with the respective controls. Treatment with 4-HC strongly decreased proliferation in colon and DSI adenomas, as detected by immunofluorescence staining with the proliferation marker protein Ki-67, and promoted apoptosis in colon adenomas, as detected by TUNEL immunofluorescence staining. In addition, decreased mRNA expression of β-catenin target genes, including c-Myc, Axin2 and CD44, in colon adenomas of 4-HC-treated animals demonstrated the involvement of the Wnt/β-catenin signaling pathway in the initiation and progression of colon neoplasms. Treatment with 4-HC also decreased the protein levels of β-catenin in colon adenomas, as demonstrated by immunofluorescence staining. The results suggested that 4-HC may be a promising candidate for the chemoprevention of intestinal tumorigenesis, and further investigations are required to evaluate its clinical utility.
Keywords: colorectal cancer, chalcone derivatives, chemoprevention, adenomatous polyposis coli, β-catenin
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-associated mortality in the USA, with an estimated 145,600 new cases and 51,020 deaths in both men and women in 2019 (1). Although the death rate is decreasing as a result of increased use of colonoscopy in early screening, the decline in incidence has tapered in recent years (2). The pathogenesis of CRC is a multistep process that originates from premalignant neoplastic lesions, known as adenomas, and progresses to invasion and further metastasis (3). Early adenoma formation is accompanied by the mutation of the tumor suppressor gene adenomatous polyposis coli (APC), which is present in ~80% of sporadic CRCs and all familial adenomatous polyposis cases (4). Most somatic APC mutations result in a truncated Apc protein and contribute in CRC development (5). Mechanistically, dysregulation of the Wnt signaling pathway as a result of the APC mutation leads to dysfunction of the multiprotein ‘destruction complex’ and translocation of β-catenin into the nucleus to activate transcription factors that belong to the T-cell factor (TCF)/lymphoid enhancer factor family (6,7). This gives rise to increased expression of genes that regulate cell proliferation and apoptosis, such as c-Myc and Axin2. Mutated APC multiple intestinal neoplasia mouse model (ApcMin) possess a nonsense mutation at codon 850 of APC, increasing its predisposition to intestinal adenoma formation, which is similar to the human somatic mutation that is associated with the progression of human CRC (8). Therefore, ApcMin mice have been widely used as a model for the study of intestinal tumorigenesis. This model is particularly advantageous for assessing the effect of anticancer agents in the early stages of cancer formation, since ApcMin mice spontaneously grow detectable intestinal adenomas within several months (9).
It has been well established that diets high in fruit and vegetables decrease the risk of developing various types of cancer, including CRC (10–12). A class of natural products abundant in these types of foods are flavonoids, which have been widely reported to have preventive effects against CRC, as demonstrated in animal studies (13,14) and population-based studies (15–17). The chemistry of chalcones attracts much attention owing to its simple structure, easy synthesis, variable construct derivates and promising biological functions, including its activity against inflammation, angiogenesis, bacterial infection, diabetes and cancer, as well as its role in immunomodulation (18–21). Chalcones are reported to possess chemopreventive activities in a variety of solid tumors, such as prostate cancer, melanoma and colon cancer (22,23). Chalcones serve as precursors for flavonoid synthesis and are considered promising candidates for various disease treatments, including dietary cancer prevention (24,25). Previous in vitro studies have indicated that chalcones and their derivatives selectively induce apoptosis and restrain proliferation in human cancer cell lines (26), such as Caco-2 cells, particularly when the hydroxyl groups are present in chalcone molecules (27), and these are thought to be potent modulators of angiogenesis (19). However, the in vivo anticancer effects of these chalcone derivatives remain to be fully elucidated. In the present study, the chemopreventive effects of 4′-hydroxychalcone (4-HC; an α,β-unsaturated ketone with the chalcone backbone and one hydroxyl-substituent at the 4′ position of the A ring; Fig. 1A), was evaluated for the first time on the spontaneous intestinal tumorigenesis in ApcMin mice. The results of the present study provide scientific evidence that supports 4-HC as a potential chemopreventive regimen for intestinal neoplasms originating from APC mutations.
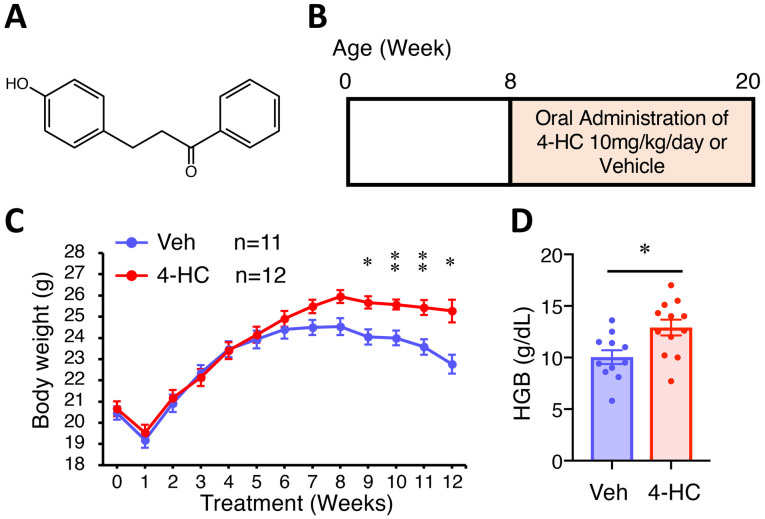
Figure 1.
Experimental design and general in vivo observations. (A) The chemical structure of 4-HC. (B) Experimental design for the evaluation of the chemopreventive effect of 4-HC in male ApcMin mice. Mice were randomly allocated into groups for oral administration with 10 mg/kg/day 4-HC (n=12) or Veh control (n=11) from 8 to 20 weeks of age. Changes in (C) body weight over time and (D) HBG levels at 20 weeks for ApcMin mice orally treated with 4-HC (n=12) or Veh (n=11). Data are presented as the mean ± SEM. *P<0.05, **P<0.01 vs. Veh. 4-HC, 4′-hydroxychalcone; ApcMin, adenomatous polyposis coli multiple intestinal neoplasia mouse model; HGB, hemoglobin; Veh, vehicle.
Materials and methods
Animals and chemicals
A total of 4 male ApcMin (8 weeks old, average starting weight, 22 g) and 8 female C57BL/6 mice (8 weeks old, average starting weight, 19 g) were obtained Beijing Vital River Laboratory Animal Technology Co., Ltd. Animals were housed under optimal conditions (21°C, 60% relative humidity, 12-h light/dark cycle, free access to food and water) in the barrier facility of the Laboratory Animal Center, Sichuan University. The animal experiments were reviewed and approved by the Animal Investigation Committee of the West China Second University Hospital, Sichuan University (Chengdu, China). The 4-HC was purchased from Selleck Chemicals and was dissolved in DMSO to a final concentration of 10 mg/ml for storage, and further dissolved in corn oil prior to administration.
Adenoma burden assessment
Male ApcMin mice were bred with C57BL/6 mice and the offspring were genotyped by polymerase chain reaction (PCR) using the following primers according to the manufacturer's instructions (Sigma-Aldrich: Merck KGaA): IMR0033, 5′-GCCATCCCTTCACGTTAG-3′; IMR0034, 5′-TTCCACTTTGGCATAAGGC-3′; and IMR0758, 5′-TTCTGAGAAAGACAGAAGTTA-3′. Male 8-week-old ApcMin mice were treated with vehicle (DMSO in corn oil; n=11) or 10 mg/kg/day 4-HC (n=12) by oral gavage every day for 12 weeks. The dose of 4-HC used was considered based on a previous study (28) and the final concentration was tested across 3 different concentrations (4-week-old mice were treated with 5, 10 or 20 mg/kg of 4-HC for 8 weeks; Fig. S1). At the end of the experimental period, 20-week-old mice were euthanized by CO2 asphyxiation, with a flow rate of 30% displacement of the cage volume per min, followed by cervical dislocation. Small intestines and colons were removed and opened longitudinally along the mesenchymal side. A stereoscopic dissection microscope (Stemi 2000-c; Carl Zeiss AG) was utilized to assess the tumor burden by counting the number of adenomas and determining their dimensions (AxioVision Application, version 4.6, Cal Zeiss Microscopy). Cardiac puncture was performed as soon as mice were euthanized and mouse blood was collected. Complete blood cell counts were performed by the clinical laboratory at West China Second University Hospital.
Tissue processing, immunofluorescence staining and TUNEL
Adenoma-containing mouse intestines were prepared using the Swiss-roll technique and fixed in 4% paraformaldehyde overnight at 4°C. Tissues were then embedded in paraffin and subsequently cut into 5-µm-thick sections. Paraffin-embedded slides were deparaffinized in room temperature with xylene and rehydrated with a graded ethanol series (100, 100, 95, 95 and 50%). Histological analysis was performed using hematoxylin and eosin staining (5 min each in room temperature). For tissue immunofluorescence staining, antigen retrieval was performed in citrate buffer (Sigma-Aldrich; Merck KGaA) with heating for 13.5 min in a microwave oven at 95°C. The samples were then incubated in blocking buffer consisting of PBS supplemented with 3.5% normal goat serum (Thermo Fisher Scientific, Inc.) at room temperature for 30 min. Samples were then incubated in a humidified chamber overnight at 4°C with primary antibodies diluted in blocking buffer. The primary antibodies included: β-catenin (1:200; cat. no. ab32572) and proliferation marker protein Ki-67 (1:500; cat. no. ab15580) (both from Abcam). Following three washes in PBS and one wash in blocking buffer, tissue samples were incubated with goat anti-rabbit Alexa Fluor 488-conjugated secondary antibody in room temperature for 20–30 min (1:400; cat. no. A11008; Thermo Fisher Scientific, Inc.) and then washed three times in PBS, followed by the addition of Hoechst 33342 nuclear stain (15 min at room temperature) and coverslip mounting with SlowFade Gold Antifade reagent (Thermo Fisher Scientific, Inc.).
To determine if 4-HC affects apoptosis of intestinal adenomas, TUNEL staining was performed using the DeadEnd™ Fluorometric TUNEL System (Promega Corporation) according to manufacturer's instructions. A total of 12 polyps from 4 mice in each treatment group were selected for TUNEL staining, and five fields per slide were examined to quantify the TUNEL-positive cells.
Image acquisition and quantification
Bright-field microscopy images were acquired with an Optronics MicroFire charge-coupled device camera on a Leica DM2000 Upright Compound Microscope (Leica Microsystems, Inc.). Fluorescence microscopy images were acquired using a Nikon A1R confocal microscope (×20 magnification used; p to ×60/1.4 oil immersion objective lens; Nikon Corporation). Images were analyzed using ImageJ software (v2.0.0-rc-69/1.52p; National Institutes of Health) (29). Quantification of Ki-67-, TUNEL- and β-catenin-positive cells was performed by measuring the area of target fluorescence and normalizing it to the area of nuclear fluorescence, and was expressed as fold change over the vehicle treated group.
Tissue mRNA expression analysis by reverse transcription-quantitative PCR (RT-qPCR)
Adenoma tissue samples were collected and stored in RNAlater (Qiagen GmbH) overnight, and total RNA was extracted using the RNeasy Mini kit (Qiagen GmbH) according to the manufacturer's instructions. For cDNA synthesis, 2–5 µg of the total RNA was reverse transcribed using a SuperScript III Reverse Transcriptase kit according to the manufacturer's instructions (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was performed using SYBR Green-based detection (cat. no. 4364346, Invitrogen; Thermo Fisher Scientific, Inc.) and a Mastercycler (Eppendorf) with the following thermocycling conditions: 2 min of initial denaturation at 94°C, followed by 25–30 cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and elongation at 72°C for 60 sec, and then 10 min of final extension at 94°C. The gene targets and corresponding primers used in this experiment included: Mouse c-Myc, forward, 5′-ATGCCCCTCAACGTGAACTTC-3′, and reverse, 5′-CGCAACATAGGATGGAGAGCA-3′; mouse Axin2, forward, 5′-TGACTCTCCTTCCAGATCCCA-3′, and reverse, 5′-TGCCCACACTAGGCTGACA-3′); and mouse CD44, forward, 5′-TCGATTTGAATGTAACCTGCCG-3′, and reverse, 5′-CAGTCCGGGAGATACTGTAGC-3′. Technical triplicates were used, and data were normalized to the housekeeping gene GAPDH (forward, 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse, 5′-TGTAGACCATGTAGTTGAGGTCA-3′), and the relative abundance of transcripts was calculated by the comparative 2−ΔΔCq method (30).
Statistical analysis
All data are presented as the mean ± SEM analyzed using GraphPad Prism v7 (GraphPad Software, Inc.). Data for continuous variables involving two groups were analyzed by unpaired Student's t-test. For multiple-time-point comparisons (Figs. 1C and S1A), two-way ANOVA followed by Sidak post hoc test was performed using built-in functions in GraphPad Prism v7 (GraphPad Software, Inc.). For multigroup comparisons (Fig. S1B), one-way ANOVA followed by Tukey's post hoc test was performed. P<0.05 was considered to indicate a statistically significant difference.
Results
General in vivo observations
To determine the dose of 4-HC used in the present study, a toxicity test was performed. The 4-week-old C57BL/6 mice were orally treated with vehicle or gradient doses of 4-HC (5, 10 or 20 mg/kg/day) for 8 weeks. The body weight change was similar among mice treated with vehicle, 5 mg/kg/day 4-HC and 10 mg/kg/day, but the body weight of mice treated with 20 mg/kg/day 4-HC was significantly lower than that of mice treated with vehicle over time (Fig. S1A and B). Therefore, 10 mg/kg/day of 4-HC administration was considered to exert no marked toxicity and was selected for further treatment for the tumor burden assessment of ApcMin animals.
A total of 24 male ApcMin animals were randomly allocated for treatment with vehicle or 10 mg/kg/day of 4-HC by oral administration from 8 to 20 weeks (Fig. 1B). ApcMin mice treated with 4-HC exhibited significantly less body weight loss than those treated with vehicle (Fig. 1C). Mice treated with 4-HC weighed 4% more at 7 weeks of treatment (P=0.049) and 11% more at 12 weeks (P<0.01). Body weight loss in both groups occurred at the first week of treatment due to inadaptation to oral gavage handling, and also at a late timepoint (8–12 weeks of treatment) owing to the increased intestinal adenoma burden and subsequent anemia. Similarly, the endpoint hemoglobin level in 4-HC-treated mice was significantly higher compared with that in control mice (P=0.01; Fig. 1D).
Oral administration of 4-HC prevents spontaneous intestinal polyposis in ApcMin mice
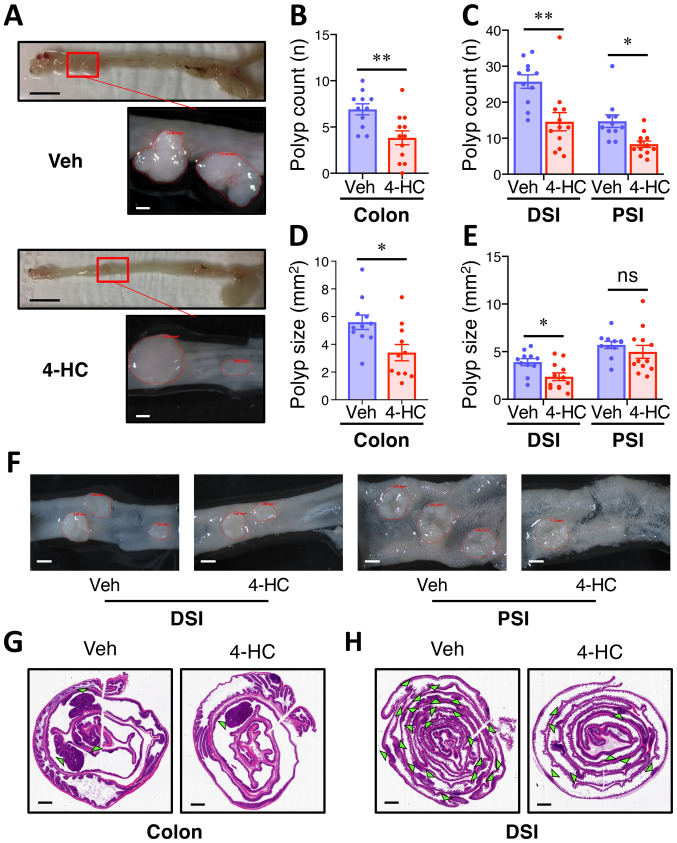
Most adenomas in ApcMin mice developed in the small intestine, with fewer in the colon, and were identified histologically as adenomatous polyps using H-E stained slides. At the age of 20 weeks, mice in the control group developed an average of 6.9, 25.7 and 12.5 polyps in the colon, distal small intestine (DSI) and proximal small intestine (PSI), respectively; whereas 4-HC treatment led to a significant reduction (45%) in the number of colon adenomas (P<0.01) (Fig. 2A and B), and also a decrease in colon adenoma size (35% reduction) compared with the control treatment (P<0.05; Fig. 2D). Similarly, 4-HC strongly decreased the number of polyps by 35% (P<0.01) and 33% (P=0.03) in the DSI and PSI (Fig. 2C), respectively. A prominent decrease in polyp size was also observed in the DSI, with a 39% reduction in adenoma surface area (P=0.01; Fig. 2E). Hematoxylin and eosin staining of Swiss-rolled intestines exhibited similar phenotypes, and histological analysis showed comparable dysplasia and invasion in the polyps in both groups (Fig. 2F and G).
Figure 2.
Oral administration of 4-HC prevents spontaneous intestinal polyposis in ApcMin mice. (A) Representative images of colon polyps in ApcMin mice treated with 4-HC or Veh for 12 weeks. Images were depicted macroscopically (scale bar, 10 mm; top images) and under a stereoscopic dissection microscope (scale bar, 1 mm; bottom images). ApcMin mice treated with 10 mg/kg/day 4-HC (n=12) and Veh (n=11) for 12 weeks and (B) colon polyp number, (C) DSI/PSI polyp number, (D) colon polyp size, and (E) DSI/PSI polyp size were analyzed. Polyp size is the tumor surface area observed under the microscope. (F) Representative images of polyps in the small intestine of ApcMin mice under a stereoscopic dissection microscope. Scale bar, 1 mm. Representative images of adenoma burden in the (G) colon and (H) DSI from mice treated with Veh or 4-HC. Green triangles indicate microscopic intestinal adenomas. Scale bar, 2 mm. Data are presented as the mean ± SEM. *P<0.05, **P<0.01. 4-HC, 4′-hydroxychalcone; ApcMin, adenomatous polyposis coli multiple intestinal neoplasia mouse model; DSI, distal small intestine; ns, not significant; PSI, proximal small intestine; Veh, vehicle.
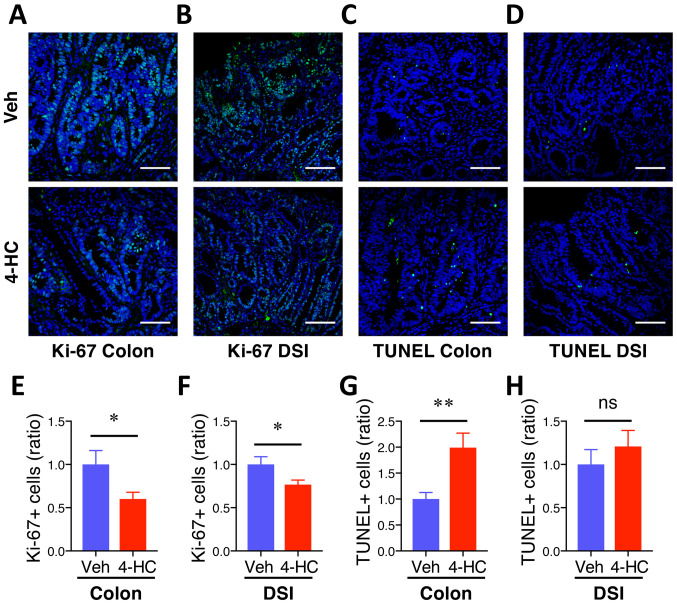
4-HC treatment prevents proliferation and induces apoptosis during intestinal adenoma formation
To assess whether 4-HC efficacy is associated with antiproliferative and proapoptotic properties, Ki-67 and TUNEL immunofluorescence staining were performed on colon and DSI adenomas. Qualitative microscopic examination of Ki-67-stained sections showed a decrease in Ki-67-positive cells in both colon and DSI adenomas from mice treated with 4-HC (Fig. 3A and B) compared with the vehicle control. The quantification of Ki-67 immunofluorescence staining showed 40% (P=0.03) and 23% (P=0.03) decreases in Ki-67 positive cells from polyps from the colon and DSI, respectively, compared with the vehicle control (Fig. 3E and F). Fig. 3C, D, G and H summarizes the effects of 4-HC on adenoma cell apoptosis. Qualitative microscopic examination of TUNEL-stained sections showed an increase in TUNEL-positive cells selectively in colon adenomas from mice treated with 4-HC compared with the vehicle. The quantification of TUNEL staining showed a 99% increase in TUNEL-positive cells in colon polyps with 4-HC treatment compared with those that received control treatment (P<0.01; Fig. 3G). No significant difference in the percentage of TUNEL-positive cells was identified in DSI polyps between the treatment groups (P>0.05; Fig. 3H).
Figure 3.
4-HC prevents proliferation and induces apoptosis during intestinal adenoma formation. DSI and colon adenomas of 20-week-old ApcMin mice were sectioned for immunofluorescent (IF) staining and corresponding analysis. 12 polyps from 4 individual mice in each treatment group were included in the analysis. (A) Representative images of Ki-67 IF staining of colon adenomas. (B) Representative images of Ki-67 IF staining of DSI adenomas. (C) Representative images of TUNEL IF staining of colon adenomas. (D) Representative images of TUNEL IF staining of DSI adenomas. (E) Relative quantification of Ki-67+ cells in colon adenomas. (F) Relative quantification of Ki-67+ cells in DSI adenomas. (G) Relative quantification of TUNEL+ cells in colon adenomas. (H) Relative quantification of TUNEL+ cells in DSI adenomas. Data are presented as the mean ± SEM. Scale bar, 100 µm. ns, not significant; *P<0.05, **P<0.01. 4-HC, 4′-hydroxychalcone; DSI, distal small intestine; ApcMin, adenomatous polyposis coli multiple intestinal neoplasia mouse model; ns, not significant; Veh, vehicle.
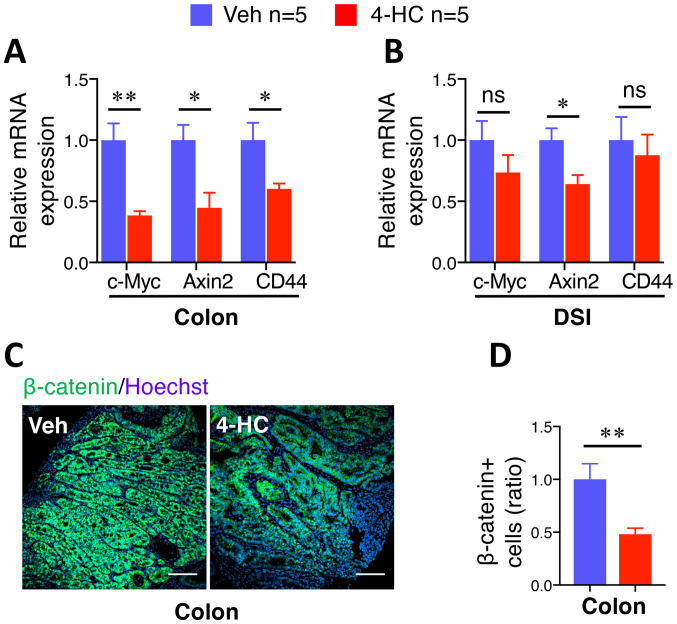
β-catenin and related gene expression levels are selectively suppressed by 4-HC in colon adenomas
The aforementioned data demonstrated the antiproliferative and apoptosis-promoting role of 4-HC in spontaneous intestinal tumorigenesis, which is associated with the properties of the β-catenin signaling pathway in human CRC progression (31). Furthermore, chalcone and a number of its analogues have been described to possess the ability to inhibit β-catenin signaling pathways (25,32–35). To examine this hypothesis, β-catenin target gene expression levels were analyzed in adenomas by RT-qPCR. A marked suppression of c-Myc, Axin2 and CD44 gene expression was observed in colon adenomas treated with 10 mg/kg/day 4-HC compared with those treated with the vehicle (Fig. 4A). Notably, the suppressive effect of 4-HC was demonstrated to occur selectively in adenomas from the colon, but not in adenomas from the DSI (Fig. 4B). This effect was confirmed by immunofluorescence staining of β-catenin. Microscopic examination of β-catenin staining images depicted a decrease in the accumulation of cellular β-catenin in the colon adenomas of 4-HC-treated mice (Fig. 4C). The quantification of β-catenin staining showed a significant 52% reduction in colon adenomas (P<0.01; Fig. 4D), whereas no significant difference was observed in the DSI adenomas (data not shown).
Figure 4.
β-catenin and related gene expression level are selectively suppressed by 4-HC in colon adenomas. mRNA expression levels of β-catenin-related genes c-Myc, Axin2 and CD44, were determined using reverse transcription-quantitative PCR analysis in (A) colon and (B) DSI adenomas from ApcMin mice treated with 4-HC and Veh (n=5 mice/group). (C) Representative images of β-catenin immunofluorescence staining in colon polyps from ApcMin mice following oral administration of 4-HC or Veh. (D) Relative quantitative analysis of β-catenin staining from (C) (n=5 per group). Data are presented as the mean ± SEM. *P<0.05, **P<0.01. 4-HC, 4′-hydroxychalcone; ApcMin, adenomatous polyposis coli multiple intestinal neoplasia mouse model; DSI, distal small intestine; ns, not significant; Veh, vehicle.
Discussion
To the best of our knowledge, the present study demonstrated the chemopreventive effects of 4-HC on spontaneous intestinal adenoma formation in ApcMin mice for the first time, which is a model that mimics numerous gene regulatory changes present in sporadic human CRC (8). Examination of intestinal adenoma number and size under a dissection microscope revealed that 10 mg/kg/day 4-HC led to a significant suppression of both colon and small intestinal adenoma formation. Treatment with 4-HC decreased both the number and size of adenomas, particularly those from the colon, suggesting its ability to affect tumor initiation and also tumor progression. Although different biological changes are involved in these two events, inhibition of tumor initiation and progression could both be beneficial for the treatment of CRC. Generally, intestinal polyps are often found in middle-aged patients during colonoscopy examinations, and their progression to cancerous lesions occurs within 10–15 years (36). Therefore, agents with the ability to inhibit tumor initiation and progression have a wider window of time to intervene with cancer development and could be used effectively even years after cancer initiation (37).
Although the association between flavonoid intake and CRC risk remains to be fully elucidated, mechanistic studies have revealed the anti-CRC properties of various flavonoids, such as anthocyanidins, apigenin and quercetin. Anthocyanidins have been reported to decrease CRC risk (38,39), largely due to their ability to negatively regulate inflammatory signaling pathways, including NF-κB, MAPK, JNK and STAT. Apigenin can induce G2/M cell cycle arrest in multiple colon cancer cell lines with decreased expression of cyclin B1 proteins and the cyclin-dependent kinase p34 (40), and possesses proapoptotic features that are associated with its pro-oxidative effect, leading to the increased production of reactive oxygen species and oxidative stress (41). A chalcone derivative, L2H17, was previously reported to have cytotoxic effects on colon cancer cell lines through various biological processes, including induction of G0/G1 cell cycle arrest and apoptosis, attenuation of cell migration and invasion, and inactivation of the NF-κB signaling pathway (42). L2H17 also possesses in vivo antitumor activity, as determined using a xenograft mouse model of colon cancer (42).
Wnt/β-catenin signaling pathways are abnormally activated in the early stages of CRC (43). A crucial and heavily studied Wnt pathway is canonical Wnt signaling, which functions by regulating the transcriptional coactivator β-catenin and promotes the expression of key developmental genes (44). For example, c-Myc, which is a key component of the Wnt signaling pathway and an important transcription factor, is often constitutively expressed in CRC and leads to increased expression of numerous genes that are involved in cell proliferation, differentiation and apoptosis, including c-MYC, CDKN1A, LGR5, CD44, AXIN2 and CCND1 (45–47). Several studies have demonstrated the chemopreventive effect of flavonoids that act as inhibitors of components in the Wnt/β-catenin signaling pathways. For example, apigenin can inhibit Wnt signaling in CRC cells in vitro, potentially through lysosomal degradation of β-catenin and downregulation of Wnt target genes such as cyclin D1 and c-Myc (48). Quercetin has been reported to disrupt the TCF/β-catenin interaction by suppressing the binding of the Tcf complexes to its specific DNA-binding sites (49). Chalcone lonchocarpin is reported to be a potent inhibitor of the Wnt/β-catenin pathway that acts downstream to stabilize β-catenin expression and impair TCF-mediated transcription (34). Cardamonin, a natural chalcone, was recently described to increase 5-fluorouracil chemosensitivity in gastric cancer cells (BGC-823 cells) by targeting Wnt/β-catenin signaling pathways and blocking β-catenin/TCF4 complex formation (33).
To the best of our knowledge, the present study is the first to identify 4-HC as a negative modulator of the Wnt/β-catenin signaling pathway exclusively in colon adenoma formation in ApcMin mice. Treatment with 4-HC attenuated β-catenin expression at the post-transcriptional level and also affected the expression of downstream genes, including c-Myc, Axin2 and CD44. Further studies are required to ascertain why 4-HC inhibits the Wnt/β-catenin pathway selectively in adenomas from the colon, but not in those from the small intestine. Interactions between dietary flavonoids and intestinal microbiota have been proven to be important in the metabolism of dietary flavonoids, and to influence their efficacy on human disease, which may contribute to the phenotypical difference between small intestine and colon adenoma formation (50,51).
There are a number of limitations to the present study. The experimental design in the present study is not optimal. Using other drugs as positive controls may improve the ability to demonstrate chemopreventive effects of 4-HC. Furthermore, additional studies are needed to investigate the dose-dependent effect of 4-HC in intestinal tumorigenesis. Such studies are currently under investigation in our laboratory, awaiting results. Last, the assessment of toxicity in the present study relies on the change of body weight. However, the result would be biased if there is a significant difference in the food consumption, which is lacking in the present study. We will include this in future studies. Overall, the results of the present study provided compelling evidence that 4-HC may prevent the development of small intestinal and colon adenomas in ApcMin mice by inhibiting cancer cell proliferation (Ki-67), increasing cancer cell apoptosis (TUNEL), and in colon adenomas, by attenuating β-catenin activation and limiting downstream gene expression. 4-HC treatment may therefore be a promising natural preventive agent against intestinal tumorigenesis.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was funded by Shanghai Science and Technology Committee (grant no. 19441905700) and Shanghai Tongji Hospital [grant no. ITJ(ZD)1802].
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
QC, QH and BG designed and supervised the project. QC, JL, JZ and SM performed the experiments. QC, JL, JZ and SM performed the data analysis and figure preparation. QC drafted the manuscript. QH and BG edited the manuscript and oversaw the project and made intellectual efforts in paper revision. All authors have read and approved the final manuscript.
Ethics approval and consent for participate
All animal experiments were approved by and performed in accordance with the recommendations of the Animal Investigation Committee of the West China Second University Hospital, Sichuan University (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, Murphy EA, Sajish M, Sheth A, Buckhaults PJ, Berger FG. Early-onset colorectal cancer: Initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17:352–364. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 5.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 6.Roose J, Clevers H. TCF transcription factors: Molecular switches in carcinogenesis. Biochim Biophys Acta. 1999;1424:M23–M37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 7.Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser AR, Luongo C, Gould KA, McNeley MK, Shoemaker AR, Dove WF. ApcMin: A mouse model for intestinal and mammary tumorigenesis. Eur J Cancer. 1995;31A:1061–1064. doi: 10.1016/0959-8049(95)00181-H. [DOI] [PubMed] [Google Scholar]
- 9.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the mod'el system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 10.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo WP, Fang YJ, Lu MS, Zhong X, Then YM, Zhang CX. High consumption of vegetable and fruit colour groups is inversely associated with the risk of colorectal cancer: A case-control study. Br J Nutr. 2015;113:1129–1138. doi: 10.1017/S0007114515000331. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Lamprecht SA, Liu Y, Shinozaki H, Fan K, Leung D, Newmark H, Steele VE, Kelloff GJ, Lipkin M. Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane-treated mouse colon. Carcinogenesis. 2000;21:1655–1660. doi: 10.1093/carcin/21.9.1655. [DOI] [PubMed] [Google Scholar]
- 14.Du WJ, Yang XL, Song ZJ, Wang JY, Zhang WJ, He X, Zhang RQ, Zhang CF, Li F, Yu CH, et al. Antitumor Activity of total flavonoids from daphne genkwa in colorectal cancer. Phytother Res. 2016;30:323–330. doi: 10.1002/ptr.5540. [DOI] [PubMed] [Google Scholar]
- 15.Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary flavonoids and the risk of colorectal cancer: An updated Meta-analysis of epidemiological studies. Nutrients. 2018;10:950. doi: 10.3390/nu10070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, Kim J. Dietary flavonoids, CYP1A1 genetic variants, and the risk of colorectal cancer in a korean population. Sci Rep. 2017;7:128. doi: 10.1038/s41598-017-00117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamora-Ros R, Not C, Guinó E, Luján-Barroso L, García RM, Biondo S, Salazar R, Moreno V. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case-control study (the Bellvitge Colorectal Cancer Study) Cancer Causes Control. 2013;24:549–557. doi: 10.1007/s10552-012-9992-z. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CT, Hsieh TJ, El-Shazly M, Chuang DW, Tsai YH, Yen CT, Wu SF, Wu YC, Chang F. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg Med Chem Lett. 2012;22:3912–3915. doi: 10.1016/j.bmcl.2012.04.108. [DOI] [PubMed] [Google Scholar]
- 19.Mirossay L, Varinska L, Mojzis J. Antiangiogenic effect of flavonoids and chalcones: An update. Int J Mol Sci. 2017;19:27. doi: 10.3390/ijms19010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes I, Pérez-Gregorio R, Soares S, Mateus N, de Freitas V. Wine flavonoids in health and disease prevention. Molecules. 2017;22:292. doi: 10.3390/molecules22020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kar Mahapatra D, Asati V, Bharti SK. An updated patent review of therapeutic applications of chalcone derivatives (2014-present) Expert Opin Ther Pat. 2019;29:385–406. doi: 10.1080/13543776.2019.1613374. [DOI] [PubMed] [Google Scholar]
- 22.Mahapatra DK, Bharti SK, Asati V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur J Med Chem. 2015;98:69–114. doi: 10.1016/j.ejmech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Karthikeyan C, Moorthy NS, Ramasamy S, Vanam U, Manivannan E, Karunagaran D, Trivedi P. Advances in chalcones with anticancer activities. Recent Pat Anticancer Drug Discov. 2015;10:97–115. doi: 10.2174/1574892809666140819153902. [DOI] [PubMed] [Google Scholar]
- 24.Kim HG, Oh HJ, Ko JH, Song HS, Lee YG, Kang SC, Lee DY, Baek NI. Lanceoleins A-G, hydroxychalcones, from the flowers of Coreopsis lanceolata and their chemopreventive effects against human colon cancer cells. Bioorg Chem. 2019;85:274–281. doi: 10.1016/j.bioorg.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Shin S, Son Y, Liu KH, Kang W, Oh S. Cytotoxic activity of broussochalcone a against colon and liver cancer cells by promoting destruction complex-independent β-catenin degradation. Food Chem Toxicol. 2019;131:110550. doi: 10.1016/j.fct.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 26.Kello M, Drutovic D, Pilatova MB, Tischlerova V, Perjesi P, Mojzis J. Chalcone derivatives cause accumulation of colon cancer cells in the G2/M phase and induce apoptosis. Life Sci. 2016;150:32–38. doi: 10.1016/j.lfs.2016.02.073. [DOI] [PubMed] [Google Scholar]
- 27.Loa J, Chow P, Zhang K. Studies of structure-activity relationship on plant polyphenol-induced suppression of human liver cancer cells. Cancer Chemother Pharmacol. 2009;63:1007–1016. doi: 10.1007/s00280-008-0802-y. [DOI] [PubMed] [Google Scholar]
- 28.Qu Q, Dai B, Yang B, Li X, Liu Y, Zhang F. 4-Hydroxychalcone attenuates hyperaldosteronism, inflammation, and renal injury in cryptochrome-null mice. Biomed Res Int. 2014;2014:603415. doi: 10.1155/2014/603415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Polakis P, Hart M, Rubinfeld B. Defects in the regulation of beta-catenin in colorectal cancer. Adv Exp Med Biol. 1999;470:23–32. doi: 10.1007/978-1-4615-4149-3_3. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca BF, Predes D, Cerqueira DM, Reis AH, Amado NG, Cayres MC, Kuster RM, Oliveira FL, Mendes FA, Abreu JG. Derricin and derricidin inhibit Wnt/β-catenin signaling and suppress colon cancer cell growth in vitro. PLoS One. 2015;10:e0120919. doi: 10.1371/journal.pone.0120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou G, Yuan X, Li Y, Hou G, Liu X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Invest New Drugs. 2019;38:329–339. doi: 10.1007/s10637-019-00781-9. [DOI] [PubMed] [Google Scholar]
- 34.Predes D, Oliveira LFS, Ferreira LSS, Maia LA, Delou JMA, Faletti A, Oliveira I, Amado NG, Reis AH, Fraga CAM, et al. The chalcone lonchocarpin inhibits wnt/beta-catenin signaling and suppresses colorectal cancer proliferation. Cancers (Basel) 2019;11:1968. doi: 10.3390/cancers11121968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin L, Niu C, Liao LX, Dou J, Habasi M, Aisa HA. An isoxazole chalcone derivative enhances melanogenesis in B16 melanoma cells via the Akt/GSK3β/β-catenin signaling pathways. Molecules. 2017;22:2077. doi: 10.3390/molecules22122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 37.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 38.Charepalli V, Reddivari L, Vadde R, Walia S, Radhakrishnan S, Vanamala JK. Eugenia jambolana (Java Plum) fruit extract exhibits anti-cancer activity against early stage human HCT-116 colon cancer cells and colon cancer stem cells. Cancers (Basel) 2016;8:29. doi: 10.3390/cancers8030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazewski C, Liang K, Gonzalez de Mejia E. Comparison of the effect of chemical composition of anthocyanin-rich plant extracts on colon cancer cell proliferation and their potential mechanism of action using in vitro, in silico, and biochemical assays. Food Chem. 2018;242:378–388. doi: 10.1016/j.foodchem.2017.09.086. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. doi: 10.1002/1098-2744(200006)28:2<102::AID-MC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee K, Mandal M. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 2015;5:153–162. doi: 10.1016/j.redox.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S, Chen M, Chen W, Hui J, Ji J, Hu S, Zhou J, Wang Y, Liang G. Chemopreventive effect of chalcone derivative, L2H17, in colon cancer development. BMC Cancer. 2015;15:870. doi: 10.1186/s12885-015-1901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaemmerer E, Jeon MK, Berndt A, Liedtke C, Gassler N. Targeting wnt signaling via notch in intestinal carcinogenesis. Cancers (Basel) 2019;11:555. doi: 10.3390/cancers11040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 47.Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CM, Chen HH, Lin CA, Wu HC, Sheu JJ, Chen HJ. Apigenin-induced lysosomal degradation of β-catenin in Wnt/β-catenin signaling. Sci Rep. 2017;7:372. doi: 10.1038/s41598-017-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 50.Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. 2017;105:10–22. doi: 10.3945/ajcn.116.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murota K, Nakamura Y, Uehara M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci Biotechnol Biochem. 2018;82:600–610. doi: 10.1080/09168451.2018.1444467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.