Abstract
Objectives
During severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, dramatic endothelial cell damage with pulmonary microvascular thrombosis have been was hypothesized to occur. The aim was to assess whether pulmonary vascular thrombosis (PVT) is due to recurrent thromboembolism from peripheral deep vein thrombosis or to local inflammatory endothelial damage, with a superimposed thrombotic late complication.
Design
Observational study.
Setting
Medical and intensive care unit wards of a teaching hospital.
Participants
The authors report a subset of patients included in a prospective institutional study (CovidBiob study) with clinical suspicion of pulmonary vascular thromboembolism.
Interventions
Computed tomography pulmonary angiography and evaluation of laboratory markers and coagulation profile.
Measurements and Main Results
Twenty-eight of 55 (50.9%) patients showed PVT, with a median time interval from symptom onset of 17.5 days. Simultaneous multiple PVTs were identified in 22 patients, with bilateral involvement in 16, mostly affecting segmental/subsegmental pulmonary artery branches (67.8% and 96.4%). Patients with PVT had significantly higher ground glass opacity areas (31.7% [22.9-41] v 17.8% [10.8-22.1], p < 0.001) compared with those without PVT. Remarkably, in all 28 patients, ground glass opacities areas and PVT had an almost perfect spatial overlap. D-dimer level at hospital admission was predictive of PVT.
Conclusions
The findings identified a specific radiologic pattern of coronavirus disease 2019 (COVID-19) pneumonia with a unique spatial distribution of PVT overlapping areas of ground-glass opacities. These findings supported the hypothesis of a pathogenetic relationship between COVID-19 lung inflammation and PVT and challenged the previous definition of pulmonary embolism associated with COVID-19 pneumonia.
Key Words: COVID-19, thrombosis, D-dimer increase, inflammation, critical care, computed tomography
Graphical abstract
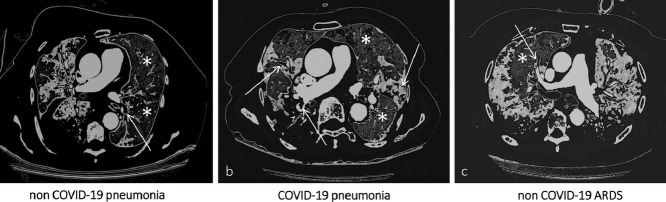
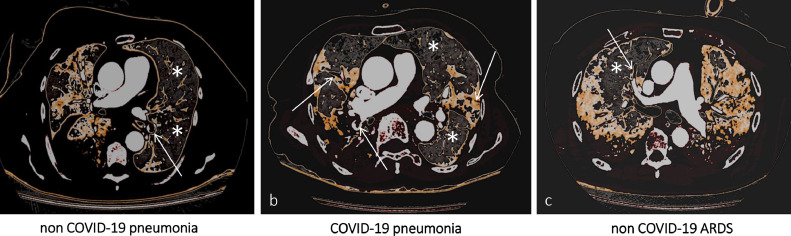
Computed Tomography Pulmonary Angiography (CTPA) imaging demonstrating, in three different clinical scenarios, eventual filling defects in branches of the pulmonary arteries (white arrows) and their spatial distribution according to the corresponding ventilation maps (white asterisks indicate normal lung parenchyma). In (a) [non COVID-19 pneumonia] and (c) [non COVID-19 ARDS] filling defects are electively found in the branches of the pulmonary arteries accountable for the vascularization of healthy lung segments. On the contrary, in (b) [COVID-19 pneumonia] there is an almost perfect topographical overlap between filling defects distribution and pneumonia extent.
CLINICAL MANIFESTATIONS of coronavirus disease 2019 (COVID-19) include a variety of phenotypes, spanning from asymptomatic disease to severe interstitial pneumonia with acute respiratory distress syndrome (ARDS) and death. The clinical evolution of COVID-19 can be described in three major patterns1: mild illness with upper respiratory tract clinical symptoms; non-life-threatening pneumonia; and severe pneumonia with ARDS, which begins with mild symptoms for seven-to-eight days and then rapidly progresses to symptoms requiring advanced life support.
In addition to a possible direct cytopathic effect, the virus may elicit a local cytokine-dependent inflammatory and potentially detrimental immune reaction. The authors previously hypothesized that this host immune response may cause massive vascular endothelial and alveolar epithelial cell damage, with microvascular thrombosis leading to worsening of ventilation/perfusion imbalances and loss of hypoxic vasoconstrictor reflexes.2 Progression of this endothelial thrombo-inflammatory syndrome to the microvascular bed of other vital organs may result in multiple organ failure and, eventually, death. Direct viral infection of the endothelial cells, with diffuse endothelial inflammation, and apoptosis has been reported in kidney, small bowel, and lung tissue specimens from patients with COVID-19.3 On the other hand, pulmonary embolism has been described as part of the clinical manifestation associated with COVID-19 pneumonia,4, 5, 6 as have elevated D-dimers.6, 7, 8 In this peculiar pathogenic scenario, it could be argued whether pulmonary vascular thrombosis is due to recurrent thromboembolism from peripheral deep vein thrombosis (DVT) or rather to local inflammatory endothelial damage with a superimposed thrombotic late complication.
A possible hint to such a pathogenic dilemma could come from imaging. Computed tomography (CT) pulmonary angiography is a noninvasive imaging tool able to identify filling defects in pulmonary artery branches,9 as well as radiologic hallmarks of COVID-19 pneumonia: bilateral ground-glass opacities (GGOs), crazy paving pattern, and/or consolidations predominantly in subpleural locations in the lower lobes.10 , 11 Furthermore, chest CT (irrespective of contrast medium administration) has been used to quantify disease burden.12 , 13
To address the question of whether pulmonary vascular thrombosis reflects recurrent thromboembolism from peripheral DVT or rather local thrombosis secondary to inflammatory endothelial damage, along with inflammatory markers and coagulation profile, spatial distribution of pulmonary vascular thrombosis in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia was evaluated in a consecutive cohort of patients with COVID-19 with clinical suspicion of pulmonary thromboembolism. Specifically, the topographic pattern of distribution of pulmonary vascular thrombosis was investigated using CT pulmonary angiography and then correlated with pneumonia extent.
Methods
This series was a subset of a larger prospective study at the San Raffaele Scientific Institute, a 1,350-bed tertiary care academic hospital in Milan, Italy; the COVID-BioB study is an institutional observational study with the aim to collect and analyze biologic samples and clinical outcomes in patients with COVID-19 (COVID-BioB, ClinicalTrials.gov NCT04318366). The study was approved by the institutional review board (protocol number 34/INT/2020). All procedures were conducted in agreement with the Declaration of Helsinki (1,964 and further amendments); informed consent was collected from all patients according to the institutional review board guidelines.
Between March 29 and April 9, 2020, patients with a positive nasopharyngeal swab result for SARS-CoV-2, who underwent CT pulmonary angiography for clinical suspicion of pulmonary vascular thrombosis, were enrolled in this study. Last follow-up date was set at July 15, 2020. Clinical suspicion of pulmonary vascular thrombosis in patients with SARS-CoV 2 was defined as ARDS nonresponsive to increasing O2 therapy.
Exclusion criteria were defined as (1) severe respiratory and/or motion artifacts that did not allow proper evaluation of lung parenchyma and identification of eventual filling defects in pulmonary arteries branches, and (2) pneumothorax (Fig 1 ).
Fig 1.
Flowchart of the study.
Clinical Data Collection
Data were entered into a dedicated electronic case report form specifically developed on site for the COVID-BioB study. Before analysis, data managers and clinicians verified data for accuracy. Details regarding treatments administered to patients other than anticoagulants previously have been reported extensively by the authors’ group.14 Data regarding lower- and upper-limb compression ultrasonography with Doppler evaluation also were collected to evaluate possible DVT.
CT Protocol
CT pulmonary angiography examinations first were performed in a dedicated CT suite (GE CT Light Speed VCT CardiacPro), easily accessible via assigned elevators and paths from the emergency department, SARS-CoV-2-dedicated intensive care units (ICUs), and COVID-dedicated wards.15 Two additional ICUs specifically dedicated to critically ill COVID-19 patients, with a total of 24 beds, were created with a dedicated novel CT scanner (Philips CT Incisive 128 pro).15
CT pulmonary angiography protocol included an unenhanced, breath-hold axial scan of the thorax (from lung apex to the lowest hemidiaphragm), followed by an additional scan starting a few seconds after intravenous administration of nonionic iodinate contrast medium to specifically enhance pulmonary arteries and their branches; the automatic bolus tracking technique had the region of interest positioned in the right ventricle, with a trigger threshold of 100 Hounsfield units (HU). This protocol ensured evaluation of lung parenchyma and eventual filling defects in the branches of the pulmonary arteries because an enhanced scan alone would not allow proper characterization of pneumonia.16
Image Evaluation
All CT pulmonary angiography images were reviewed independently by two radiologists experienced in thoracic imaging (R.N. and D.P., with 28 and five years of experience, respectively) both blinded to the patient symptoms and outcome; differences in assessment were resolved with consensus. Pulmonary vascular thrombosis was defined when contrast-enhanced CT demonstrated filling defects in the branches of the pulmonary arteries and classified in distribution (number and site of pulmonary lobes affected) and extent (down to subsegmental branches). Irrespective of presence or absence of pulmonary vascular thrombosis, the diameter of perilesional subsegmental vessels always was measured on unenhanced slices; a subsegmental vessel was defined as enlarged when its axial diameter exceeded 3 mm.17 Imaging after processing was carried out using a commercially available software (Intellispace version 8.0, Philips Medical Systems, Chronic Obstructive Pulmonary Disease tool); after automated identification of pulmonary lobes on unenhanced slices, the software differentiated diverse areas of lung parenchyma based on HU thresholds. In the setting of SARS-CoV-2 pneumonia, the following settings were used: to quantify and differentiate between normal and pathologic lung parenchyma, a -740 HU threshold was set: areas with higher-density values were considered pathologic (disease burden). To distinguish between GGO and non-GGO (crazy paving and/or consolidation) areas, the authors applied a 660-HU threshold.
Descriptor definitions are taken from the Fleischner Society: Glossary of Terms for Thoracic Imaging 18: Ground-glass opacity is defined as a hazy increased opacity of lung, with preservation of bronchial and vascular margins; consolidation as an opacity of lung that obscures bronchial and vascular margins; and crazy paving pattern as thickened interlobular septa and intralobular lines superimposed on a background of GGO, resembling irregularly shaped paving stones.
Software outputs refer to both lungs and single pulmonary lobes as percentages of the overall lung volume. Finally, to accurately assess the precise contribution of GGO and non-GGO patterns to the overall disease burden, the authors calculated the so-called GGO ratio, defined as the percentage of pneumonia made up by GGO.
Statistical Analysis
Median values with respective interquartile ranges (IQR) were used to express continuous variables, while frequencies in percentages were used for categorical variables. Patient-related variables and imaging parameters of patients with and without pulmonary vascular thrombosis were compared using the chi-square or Fisher exact test for categorical variables, and the Wilcoxon rank sum or Student t test (according to the assessment of normality) for continuous variables. The ability of GGO ratio in predicting the risk of pulmonary vascular thrombosis was determined by the area under the curve of the receiver operating characteristic curve. The optimal cutoff value predicting pulmonary vascular thrombosis was determined on the highest Youden index value (sensitivity + specificity – 1). To evaluate the cutoff accuracy, sensitivity and specificity were estimated. Comparison of GGO values between patients with or without thrombosis was evaluated using a Wilcoxon/Student's t test. Univariate and multivariate analyses were used to explore potential risk factors associated with pulmonary vascular thrombosis. The final model included D-dimer level at hospital admission and treatment with anticoagulant before CT pulmonary angiography. A p value of less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 25 (SPSS Inc./IBM, Armonk, NY) and R version 3.3.1.
Results
Patient Characteristics
Sixty-seven patients underwent CT pulmonary angiography during the study period. Following exclusions due to severe respiratory and motion artifacts (n = 9) and pneumothorax (n = 3) (Fig 1), 55 patients (39 male [70.9%] with a median age of 62 years [IQR 56-71 years]) were included in this study. Overall, 28 (50.9%) patients showed findings of pulmonary vascular thrombosis at CT pulmonary angiography imaging.
Patient characteristics are summarized in Table 1 ; most patients had histories of comorbidities, with hypertension being reported in 43.1%. The median time interval between COVID-19 symptom onset and CT pulmonary angiography was 17.5 days (range: 1-38). At the time of CT pulmonary angiography, 27 patients were in COVID-19–dedicated medical wards, 19 in the ICU and nine in the emergency department.
Table 1.
Baseline Characteristics of the 55 Patients With COVID-19 Who Had a Computerized Tomography Pulmonary Angiography Scan Performed at the Institute
| Characteristics (%) | Overall, n = 55 | Without Pulmonary Vascular Thrombosis (n=27) | With Pulmonary Vascular Thrombosis (n=28) | p Value* |
|---|---|---|---|---|
| Age, y (IQR) | 62 (56-71) | 62 (56-74) | 62 (54.5-68) | 0.76 |
| Sex, M | 39 (72) | 19 (73) | 20 (71) | 0.89 |
| Ethnicity | ||||
| White | 47 (85) | 20 (74) | 27 (96) | |
| Asian | 8 (15) | 7 (26) | 1 (4) | 0.02 |
| Onset of COVID-19 symptoms, d | 17.5 (10-23) | 19 (10-25) | 14 (9-22) | 0.17 |
| Body temperature °C | 37.6 (36.5-38.3) | 38 (37- 38.7) | 37.4 (36- 38.2) | 0.07 |
| PaO2/FIO2 ratio | 247 (106.7-307) | 252 (91-310) | 247 (121-307) | 0.96 |
| BMI kg/m2 | 26 (25-31) | 25 (24-29) | 28(25-34) | 0.14 |
| Presence of comorbidities | 27 (52.9) | 11 (45.8) | 16 (59.3) | 0.33 |
| Hypertension | 0.84 | |||
| No | 29 (56.9) | 14 (58.3) | 15 (56) | |
| Yes | 22 (43.1) | 10 (41.7) | 12 (44) | |
| Coronary artery disease | 0.69 | |||
| No | 44 (86.3) | 20 (83) | 24 (89) | |
| Yes | 7 (13.7) | 4 (17) | 3 (11) | |
| Diabetes | 0.58 | |||
| No | 40 (78) | 19 (79) | 21 (78) | |
| Yes | 11 (22) | 5 (21) | 6 (22) | |
| COPD | 0.64 | |||
| No | 47 (92) | 22 (91.7) | 25 (92.6) | |
| Yes | 4 (8) | 2 (8.3) | 2 (7.4) | |
| Chronic kidney disease | 0.47 | |||
| No | 50 (98) | 23 (88.2) | 27 (100) | |
| Yes | 1 (2) | 1 (11.8) | 0 | |
| Cancer | 0.52 | |||
| No | 50 (98) | 24 (100) | 26 (96) | |
| Yes | 1 (2) | 0 | 1 (4) | |
| Neurodegenerative disorders | 0.49 | |||
| No | 52 (97.1) | 25 (97.1) | 27 (100) | |
| Yes | 1 (2.9) | 1 (2.9) | 0 | |
| WBC, × 109/L | 9.0 (6.3-12.3) | 9.2 (6.0-12.8) | 8.90 (6.52-12.15) | 0.78 |
| Missing | 0 | 0 | 0 | |
| Lymphocyte count, × 109/L | 0.8 (0.7-1.2) | 0.9 (0.7-1.2) | 0.8 (0.7-1.2) | 0.83 |
| Missing | 3 | 2 | 1 | |
| Neutrophil count, × 109/L | 7.5 (4.9-10.0) | 7.90 (4.65-10.65) | 6.8 (4.9-9.9) | 0.57 |
| Missing | 3 | 2 | 1 | |
| Hemoglobin, g/dL | 13.5 (12.3-14.6) | 13 (11.5-14.2) | 13.6 (13.0-14.8) | 0.08 |
| Missing | 0 | 0 | 0 | |
| Platelet count, × 109/L | 247 (194-372) | 248 (214-430) | 247 (170-328) | 0.27 |
| Missing | 6 | 3 | 3 | |
| Albumin, g/L | 25.4 (23.6-28.2) | 26.0 (22.6-28.2) | 25.3 (23.7-28.3) | 0.9 |
| Missing | 11 | 4 | 7 | |
| ALT, U/L | 50 (31-73) | 49 (27-67) | 50 (32-83) | 0.66 |
| Missing | 6 | 3 | 3 | |
| AST, U/L | 58 (45-78) | 59 (47-79) | 57 (36-87) | 0.52 |
| Missing | 7 | 4 | 3 | |
| Creatinine, mg/dL | 10 (0.84-1.19) | 0.99 (0.88-1.09) | 11 (0.81-1.20) | 0.57 |
| Missing | 0 | 0 | 0 | |
| Glucose, mg/dL | 109 (97-151) | 117 (92-154) | 106 (99-146) | 0.54 |
| Missing | 3 | 1 | 2 | |
| Lactate dehydrogenase, U/L | 490 (354-540) | 471 (387-599) | 530 (342-666) | 0.91 |
| Missing | 1 | 1 | 0 | |
| C-reactive protein, mg/L | 144 (78-218) | 152 (80-218) | 121.3 (58.2-238.2) | 0.78 |
| Missing | 0 | 0 | 0 | |
| Lactate, mmol/L | 1.52 (1.22-1.91) | 1.46 (1.13-1.72) | 1.53 (1.25-2.15) | 0.30 |
| Missing | 3 | 3 | 0 | |
| Prothrombin time | 1.14 (1.05-1.23) | 1.11 (1.05-1.20) | 1.18 (1.06-1.25) | 0.44 |
| Missing | 2 | 1 | 1 | |
| PTT | 0.97 (0.89-1.04) | 0.97 (0.93-1.07) | 0.97 (0.89-1.02) | 0.46 |
| Missing | 2 | 1 | 1 | |
| D-dimer, µg/mL | 3.00 (1.18-18.98) | 1.62 (0.93-6.06) | 10.06 (1.78-21.00) | 0.009 |
| Missing | 7 | 4 | 3 | |
| Fibrinogen, mg/dL | 623 (503-760) | 686 (527-773) | 594 (451-701) | 0.18 |
| Missing | 24 | 13 | 11 | |
| IL6, pg/mL | 74 (38-134) | 41.8 (34.8-71.8) | 93.6 (48.1-216) | 0.02 |
| Missing | 17 | 12 | 5 | |
| Ferritin, ng/mL | 1517 (690-2869) | 1130 (748-2479) | 1990 (675-3365) | 0.22 |
| Missing | 9 | 6 | 3 | |
| Creatine kinase, U/L | 108 (69-212) | 115 (75-745) | 85 (39-165) | 0.09 |
| Missing | 11 | 6 | 5 | |
| Procalcitonin, ng/mL | 0.51 (0.32-1.72) | 0.50 (0.34-1.07) | 0.95 (0.30-2.29) | 0.26 |
| Missing | 14 | 6 | 8 | |
| Pro-BNP, pg/mL | 287 (86-756) | 230 (77-747) | 490 (156-1121) | 0.28 |
| Missing | 12 | 3 | 9 | |
| Cardiac troponin, ng/L | 14.1 (7.07-28.7) | 12.4 (7.0-23.8) | 15.8 (7.6-44.6) | 0.53 |
| Missing | 11 | 4 | 7 |
NOTE. Results reported as median (IQR) or frequency (%).
Abbreviations: ALT, alanine aminotransferase, AST, aspartate transaminase; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; IQR, interquartile range; M, male; PTT, partial thromboplastin time; WBC, white blood cells.
P values are calculated by chi-square or Fisher exact test (categorical variables) or Mann Whitney test (continuous variables).
At hospital admission (Table 1), the median values for white blood cell count, lymphocyte count, lactic dehydrogenase, C-reactive protein, serum ferritin, and D-dimer were 9.9 (IQR 6.3-12.3) × 109/L, 0.8 (IQR 0.7-1.2) × 109/L, 490 (IQR 354-540) U/L, 144 (IQR 78-212) mg/L, 1,517 (IQR 690-2,869) mg/L, and 3 (IQR 1.1-18) µg/mL, respectively. Baseline laboratory findings were not different between patients with or without pulmonary vascular thrombosis, except for D-dimer level (10.06 [1.78-21.00] v 1.62 [0.93-6.06]) and IL6 (93.6 [48.1-216] v 41.8 [34.8-71.8]), which were increased (p = 0.009 and p = 0.02, respectively) in patients with pulmonary vascular thrombosis.
Laboratory findings at the time of CT pulmonary angiography are summarized in Supplemental Table 1. Before CT pulmonary angiography, 28 patients already were receiving low-molecular-weight heparin or direct thrombin inhibitor (12/28 with and 16/27 without pulmonary vascular thrombosis, p = 0.06).
After a median follow-up of 29.5 (IQR 15-55.7) days after hospital admission, 17 patients died (eight of the patients with and nine of those without pulmonary vascular thrombosis, p = 0.7), 36 were discharged after a median time of 30 (IQR 20-56) days, and two patients were still hospitalized (one with indication of bilateral lung transplantation due to severe fibrosis). Univariate and multivariate analyses are reported in Table 2 . An increased level of D-dimer at baseline (odds ratio 1.09, 95% confidence interval 1-1.18, p = 0.02) and absence of anticoagulation before occurrence of pulmonary vascular thrombosis (odds ratio 3.81, 95% confidence interval 1.21-11.9, p = 0.04) were associated independently with the increased risk of pulmonary vascular thrombosis.
Table 2.
Univariate and Multivariate Logistic Regression Models on the Risk of Pulmonary Vascular Thrombosis
| Characteristics | Univariate, Odds Ratio (95% Confidence Interval) | p Value | Multivariable, Odds Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|---|
| Sex (female v male) | 0.95 (0.29-3.04) | 0.93 | ||
| Age (continuous) | 0.99 (0.95-1.04) | 0.91 | ||
| COVID-19 symptoms onset, d (continuous) | 0.94 (0.88-1.01) | 0.10 | ||
| Ethnicity (Asian v white) | 0.10 (0.02-0.93) | 0.04 | ||
| Hypertension (yes v no) | 1.12 (0.36-3.40) | 0.84 | ||
| Absence of anticoagulant prophylaxis before lung thrombosis (yes v no) | 3.81 (1.21-11.9) | 0.02 | 3.85 (1.01-13.9) | 0.04 |
| PO2/FIO2 (continuous) | 0.99 (0.95-1.00) | 0.73 | ||
| Neutro/lympho ratio (continuous) | 0.97 (0.91-1.04) | 0.43 | ||
| Lymphocyte count, × 109/L (continuous) | 1.04 (0.48-2.24) | 0.92 | ||
| C-reactive protein, mg/L (continuous) | 1 (0.99-1.05) | 0.90 | ||
| Lactate dehydrogenase, U/L (continuous) | 1 (0.99-1.03) | 0.70 | ||
| D-dimer, μg/mL (continuous) | 1.09 (1.01-1.17) | 0.02 | 1.09 (1-1.18) | 0.02 |
Abbreviations: COVID-19, coronavirus disease 2019.
Imaging Findings
Evaluation of Pulmonary Vascular Thrombosis
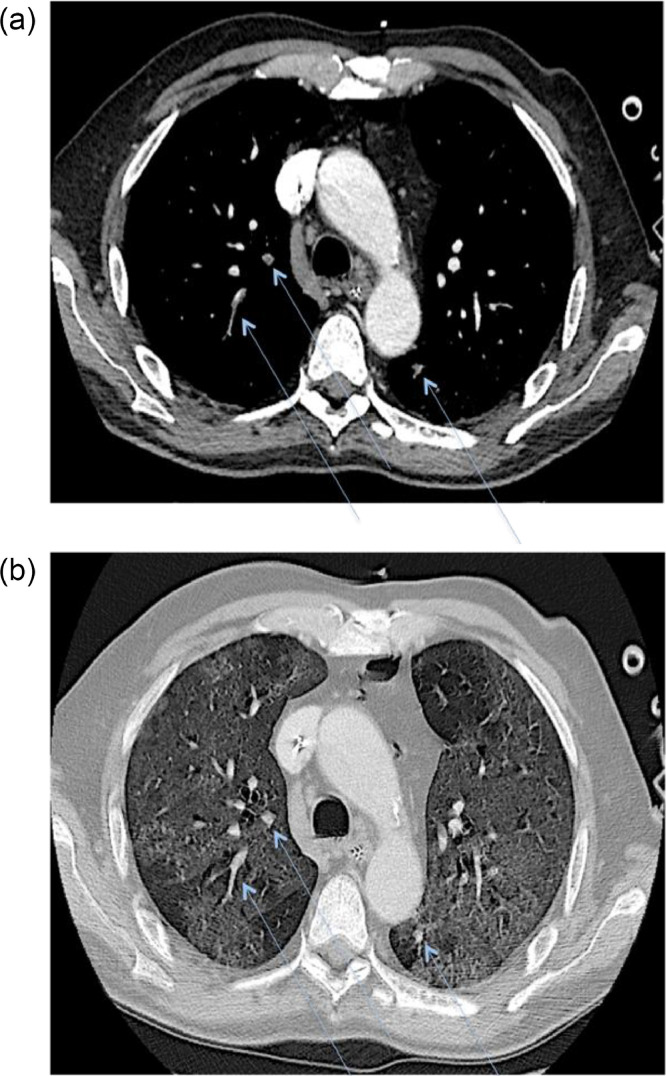
Simultaneous multiple thromboses were identified in 22 patients (78.6%), with bilateral involvement in 16 of 28 patients (57.1%) (Fig 2 ). The right lung was the most affected, with the highest involvement in the right lower lobe (18/28, 64.3%), followed by the upper right lobe (17/28, 60.7%) and middle lobe (8/28, 28.6%). The left lung showed involvement in 17 of 28 (60.7%) patients in the lower left lobe and 13 of 28 in the upper left (46.4%).
Fig 2.
Axial contrast-enhanced CT scan demonstrating simultaneous multiple thrombi with bilateral involvement (A) (blue arrows) affecting subsegmental pulmonary artery branches within extensive ground-glass opacities (B).
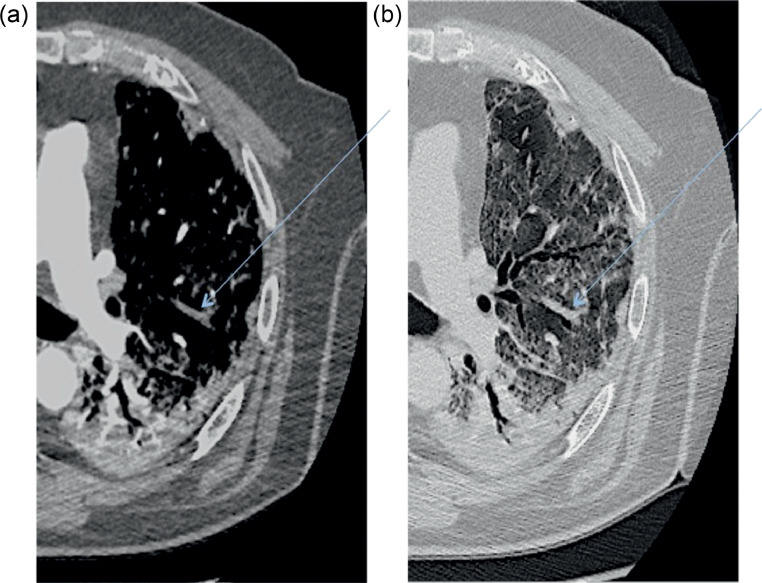
Filling defects typically were found in segmental (19/28, 67.8%) and subsegmental (27/28 96.4%) branches (Fig 3 ); lobar branches were affected in only six patients (21.4%).
Fig 3.
Axial contrast-enhanced CT scan demonstrating a filling defect (A) (blue arrow) in the left upper lobe overlapping the segmental distribution of ground-glass opacities (B). Notably, no filling defects are present in the left lower lobe, where consolidation is the main radiologic pattern of pneumonia.
The presence of an enlarged subsegmental vessel was observed in 31 patients (56.4%, median diameter: 4.2 mm [3-5.7]). Patients with pulmonary vascular thrombosis demonstrated larger subsegmental vessels compared with those without thrombosis (5.25 mm [4.42-6.4] v 2.9 mm [2.55-3.2], p < 0.001).
Lower and upper limb compression ultrasonography with Doppler evaluation was performed in 27 of 55 patients (12 with and 15 without pulmonary vascular thrombosis). Deep vein thrombosis was detected in four patients (14.8%) (three in the pulmonary vascular thrombosis group, with popliteal and tibial vein thrombosis in one and two patients, respectively).
Pneumonia Evaluation
CT features of SARS-CoV-2 pneumonia are summarized in Table 3 . The median disease burden was 74.7% (55.4-83.6); the most affected pulmonary lobes were the lower ones (right: 84.5% [68.35-95.17], left: 88.3% [71.8-95.5]), followed by the upper lobes (right: 67.2% [36.9-85.5], left: 71.9% [35.6-83.9]) and the middle lobe (45.7 [20.6-69.5]). No significant differences were found in disease burden between patients with pulmonary embolism and patients without (73% [33.8-82.4] v 75.4% [57.2-85.9] p = 0.35). No differences were found even when considering separately right and left lung (p = 0.26 and 0.51, respectively) or single pulmonary lobes.
Table 3.
Evaluation of Lung Involvement in 55 COVID-19 Patients With Suspected Pulmonary Vascular Thrombosis
| Disease Burden (%) | GGO (%) | GGO Ratio (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 55) | LT (n = 28) | Non-LT (n = 27) | p Value* | Overall (n = 55) | LT (n = 28) | Non-LT (n=27) | p Value* | Overall (n = 55) | LT (n = 28) | Non-LT (n = 27) | p Value* | |
| Lungs | ||||||||||||
| Both (RL + LL) | 74.75 (55.45-83.57) | 73.00 (33.80-82.40) | 75.40 (57.20-85.90) | 0.35 | 22.20 (11.50-36.02) | 31.70 (22.90-41.00) | 17.80 (10.80-22.10) | <0.001 | 36.88 (23.58-64.79) | 57.77 (42.99-71.90) | 27.26 (14.50-33.91) | <0.001 |
| RL | 72.35 (49.62-81.97) | 62.20 (36.90-78.70) | 77.70 (53.20-83.50) | 0.36 | 21.50 (13.55-30.55) | 30.20 (21.50-41.00) | 16.80 (11.60-22.00) | <0.001 | 39.37 (22.65-65.11) | 64.97 (41.03-72.35) | 27.46 (17.39-34.69) | <0.001 |
| LL | 76.30 (57.67-88.82) | 77.20 (36.60-89.50) | 75.40 (60.80-88.60) | 0.51 | 20.30 (11.27-31.60) | 29.10 (20.10-48.10) | 16.20 (8.40-20.70) | <0.001 | 36.08 (21.76-63.13) | 62.31 (46.46-72.90) | 24.38 (12.64-33.39) | <0.001 |
| Lobes | ||||||||||||
| RUL | 67.20 (36.87-85.55) | 65.50 (28.30-79.00) | 71.50 (46.00-86.10) | 0.35 | 23.65 (11.55-32.72) | 30.10 (15.00-47.10) | 20.30 (10.70-25.60) | <0.001 | 45.83 (30.77-71.62) | 70.75 (50.53-83.75) | 34.82 (13.83-44.67) | <0.001 |
| RML | 45.70 (20.65-69.55) | 31.70 (17.30-59.80) | 50.40 (26.00-73.30) | 019 | 16.95 (11.65-29.55) | 23.10 (11.80-40.80) | 14.20 (11.20-20.10) | <0.001 | 53.77 (32.20-80.67) | 80.21 (72.06-86.15) | 37.30 (27.15-52.54) | <0.001 |
| RLL | 84.55 (68.35-95.17) | 84.20 (59.80-94.40) | 87.50 (74.80-96.30) | 0.41 | 19.95 (11.72-31.87) | 29.00 (23.20-43.20) | 13.80 (8.60-17.80) | <0.001 | 29.22 (14.59-56.94) | 48.10 (31.56-70.47) | 15.41 (11.54-24.64) | <0.001 |
| LUL | 71.90 (35.62-83.95) | 69.60 (26.70-84.40) | 74.80 (42.80-80.70) | 0.71 | 21.15 (12.15-40.62) | 35.70 (16.30-50.70) | 15.50 (10.80-24.60) | <0.001 | 47.51 (28.77-72.06) | 71.88 (53.66-77.80) | 35.83 (15.54-47.07) | <0.001 |
| LLL | 88.30 (71.80-95.47) | 80.70 (53.30-95.30) | 89.60 (77.60-96.00) | 0.34 | 19.85 (9.77-31.2) | 31.00 (21.60-38.60) | 11.70 (5.00 -19.20) | <0.001 | 29.98 (12.81-49.36) | 43.15 (30.11-65.36) | 13.41 (8.33-27.99) | <0.001 |
NOTE. Results reported as frequency (%) and range.
Abbreviations: COVID-19, coronavirus disease 2019; GGO, ground-glass opacities; LL, left lobe; LLL, left lower lobe; LT, lung thrombosis; LUL, left lower lobe; RL, right lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
P values are calculated by chi-square or Fisher exact test (categorical variables) or Mann Whitney test (continuous variables).
GGOs were present in all patients (55/55, 100%), with a median GGO burden of 22.2% (11.5-36); patients with pulmonary vascular thrombosis had significantly higher GGO areas compared with those without pulmonary vascular thrombosis (31.7% [22.9-41] v 17.8% [10.8-22.1] p < 0.001). Differences also were found when considering separately right and left lung (p < 0.001 in both cases) or single pulmonary lobes. Non-GGO pattern (crazy paving and/or consolidation) was present in 49 of 55 patients (89.1%). GGO ratio analysis also highlighted significant differences between the patients with or without pulmonary vascular thrombosis (57.8% [42.9-71.9] v 27.3% [14.5-33.9], p < 0.001). Differences also were found when considering separately right and left lung (p < 0.001 in both cases) or single pulmonary lobes.
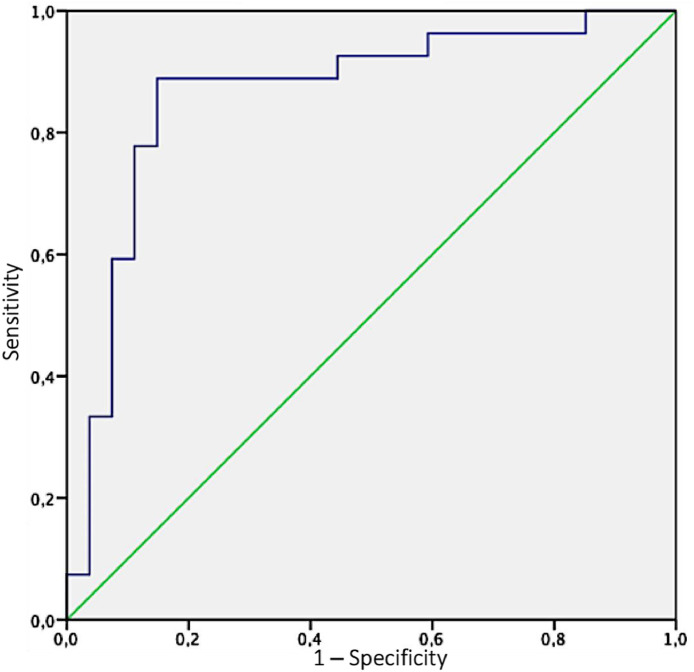
By means of receiver operating characteristic analysis, the authors identified as best threshold for GGO ratio a percentage of 36.4, above which patients demonstrated a higher risk of pulmonary vascular thrombosis (area under the curve: 0.864; sensitivity: 88.9%; specificity: 85.2%) (Fig 4 ).
Fig 4.
The ability of GGOs (ground-glass opacities) ratio in predicting the risk of pulmonary vascular thrombosis was determined by the area under the curve (AUC) of receiver operating characteristics (ROC) curve; the authors identified as best threshold for GGO ratio a percentage of 36.4, above which patients demonstrated a high risk of pulmonary vascular thrombosis (AUC: 0.864; sensitivity: 88.9%; specificity: 85.2%).
Discussion
This study showed a unique pattern of distribution of pulmonary vascular thrombosis overlapping areas of active GGOs in patients with severe COVID-19 ARDS. Most patients with pulmonary vascular thrombosis showed multiple thrombi (78.6%), with frequent bilateral involvement (57.1%) in segmental and subsegmental pulmonary artery branches, in the presence of larger subsegmental vessels within GGO areas. Furthermore, the authors confirmed an association between D-dimer values and the presence of pulmonary vascular thrombosis.
The percentage of pneumonia characterized by pure GGO (GGO ratio) was significantly higher in patients who developed pulmonary vascular thrombosis, with a cutoff, estimated with the receiver operating characteristics analysis, of 36.4%. As a consequence, the results provided the opportunity to define a specific pattern of COVID-19 pneumonia; that is, GGO predominant, significantly associated with occurrence of pulmonary vascular thrombosis (Fig 5 , central image). Ground-glass opacity is believed to represent the initial, typical response to lung injury, and roughly represents the amount of active inflammation. Furthermore, the observed subsegmental vascular enlargement previously described in SARS-CoV-2 pneumonia17 , 19 also has been associated with SARS-CoV-2–triggered hyperemia. The results pointed out a reliable association between subsegmental vascular enlargements within a GGO prevalent pneumonia and the presence of multiple bilateral pulmonary vascular thromboses in the peripheral branches of each lobe artery.
Fig 5.
Central image/visual abstract: Computed tomography pulmonary angiography (CTPA) imaging demonstrating, in three different clinical scenarios, eventual filling defects in branches of the pulmonary arteries (white arrows) and their spatial distribution according to the corresponding ventilation maps (white asterisks indicate normal lung parenchyma). In (A) (non-COVID-19 pneumonia) and (C) (non-COVID-19 ARDS) filling defects are electively found in the branches of the pulmonary arteries, accountable for the vascularization of healthy lung segments. On the contrary, in (B) (COVID-19 pneumonia), there is an almost perfect topographical overlap between filling defects distribution and pneumonia extent. COVID-19, coronavirus disease 2019.
Poissy et al.20 recently reported a 20.6% pulmonary embolism incidence among the first 107 consecutive patients with COVID-19 admitted to their ICU in France and receiving prophylactic antithrombotic treatment. Grillet et al.21 also reported that the proportion of patients with acute pulmonary embolism was 23% in the subgroup of 100 patients with COVID-19 infection and severe clinical features (among the 280 hospitalized patients in the study period).
Overall, the current findings confirmed the ability of CT scan to provide a quantitative assessment of the disease severity and confirm the extension of active inflammation as the major determinant of pulmonary vascular thrombosis.22 Multiple and bilateral pulmonary thromboembolic events already have been reported in patients with COVID-19.23 However, a systematic topographic analysis of this pattern was lacking and, instead, an embolism concept was proposed; the authors showed stringent pulmonary vascular thrombosis topographic distribution in overlap with GGO areas. Overall, these features supported the concept of an atypical COVID-19-associated ARDS, because the pattern of distribution of pulmonary thromboembolic events in typical ARDS involves areas spared by inflammatory changes.24 The presence of a high percentage of lung involvement on chest CT has been shown to range between six and 13 days from symptom onset10 , 25; the median time in pulmonary vascular thrombosis presentation (17.5 days) fits with the hypothesis of an intermediate stage disease,26 in which lung inflammation is at its highest, resulting in possible progressive endothelial damage. Subsequent activation of the coagulation cascade in the microvascular compartment could lead to a critical functional deterioration of O2 exchange, ultimately unresponsive to mechanical ventilation.
There is increasing evidence supporting the important role of endothelial cells in the initiation of inflammation and in the development of extensive pulmonary intravascular coagulopathy, which is common in COVID-19 patients with ARDS.27, 28, 29 However, it long has been known that not only diffuse alveolar damage but also pulmonary vascular injury are central pathologic features of ARDS.30 , 31 Disregulated inflammation and endothelial cell direct injury promote the expression of coagulation-initiating factors, like tissue factor on cell surfaces, thereby causing downstream activation of coagulation. At the same time, natural anticoagulant mechanisms, including antithrombin, tissue factor pathway inhibitor, and the protein C system, are suppressed and this further propagates an uncontrolled cycle of coagulation.32 , 33 Other authors suggest that the disruption of pulmonary circulation causes platelet and fibrinogen depletion, suggesting local thrombosis formation.34
Histologic postmortem studies revealed diffuse extensive fibrin thrombosis of small and large pulmonary arteries and lung necrosis distal to vascular obstruction in ARDS of diverse etiology.35, 36, 37 Furthermore, bedside balloon occlusion pulmonary angiography demonstrated pulmonary artery filling defects of diverse severity and etiology in about one-half of the patients with ARDS.35 Severity of the respiratory insufficiency, pulmonary artery pressure, pulmonary vascular resistance, and mortality were significantly higher in patients with angiographic evidence of vascular obstruction than in those with normal angiography.35 Other authors used the same angiographic technique and found multiple pulmonary artery filling defects in a relevant fraction of patients with severe ARDS.38
Overall, even if understanding the role of pulmonary vascular thrombosis in the development of pulmonary hypertension in the course of ARDS is of paramount importance, there still is uncertainty. Besides functional mechanisms, there is evidence of reduced small pulmonary artery diameter by medial artery thickening, and significant reduction in total artery concentration even after short-course ARDS.39 Because ARDS is a syndrome caused by some primary clinical condition (pneumonia, trauma, shock, sepsis, multiple transfusions, etc.), that could initiate intravascular coagulation; it is not always clear what the main reason is for the observed pulmonary vascular thrombosis in ARDS. The abovementioned studies found a significantly higher rate of thrombotic vascular occlusion in posttraumatic ARDS and in patients with signs of concomitant disseminated intravascular coagulation syndrome.35 , 38
Despite extensive research from the 1970s, the understanding of the basic mechanisms of lung injury and of pulmonary vascular thrombosis in non-COVID-19 ARDS still is uncertain.
As a limitation, the authors acknowledge that this study reported a subset cohort of a larger institutional prospective study. Furthermore, the clinical progression of respiratory distress in this cohort was associated with, but not necessarily caused by, the increased level of D-dimers. On top of this, all consecutive patients undergoing a CT scan for clinical suspicion of pulmonary vascular thrombosis were enrolled; therefore, the overall prevalence in those not presenting clinical suspicion remains unknown. Importantly, in the authors’ hospital, deep vein thrombosis40 was found only in a relatively small percentage of patients with lung thrombosis, and there were no cases in the iliofemoral venous axis, supporting the hypothesis of a lung disease–related complication, due to SARS-CoV2 pathogenicity. The limitation linked to data collected from a single center remains, yet the critical role of the association of disorders in the coagulation pathway, disease severity, and death have been reported in independent cohorts in patients with COVID-19.4 , 5 , 6 , 41 Acute systemic inflammation features previously have been associated with disease severity and mortality.8 , 42 Importantly, D-dimer levels should be considered as an additional predictive tool to stratify patients at risk of adverse outcome and may guide physicians to proceed with CT pulmonary angiography as well as assign anticoagulant administration.
Prospective ongoing randomized trials currently are evaluating the intensity of prophylactic anticoagulation (FREEDOM COVID-19,43 INHIXACOV1944), as well as the use of targeted drugs on endothelium (DEFACOVID,45 DEFI-VID1946). Treatment of COVID-19 pneumonia is changing rapidly with the availability of the results of clinical trials. Immunomodulation therapies to reduce the risk of cytokine- release syndrome induced by SARS-CoV-2 has demonstrated efficacy in single-center and multicenter trials.47 , 48
In conclusion, this study supported the hypothesis of a pathologic relationship between lung inflammation and pulmonary vascular thrombosis, definitively challenging the previous definition of embolism associated with COVID-19 pneumonia and strongly supporting the rationale for an association of anti-inflammatory and anticoagulant treatments in patients with severe COVID-19 pneumonia.
Acknowledgments
Acknowledgments
The authors are indebted to all healthcare personnel who helped during the COVID-19 emergency.
Conflict of Interest
None.
Footnotes
Author's contribution: All authors contributed to conception, data collection, data analysis, and manuscript writing.
Funding: None.
Collaborators to be added in PubMed and Scopus (all from San Raffaele Scientific Institute): Daniele Grippaldi, MD, Roberta Cao, MD, Stefania Calvisi, MD, Valentina Da Prat, MD, Raffaella Scotti, MD, Giorgia Borio, MD, Ludovica Cavallo, MD, Jacopo Castellani, MD, Federica Farolfi, MD, Maria Pascali MD, Diana Canetti, MD, Barbara Castiglioni, MD, Chiara Oltolini, MD, Chiara Tassan Din, MD, Anna Mara Scandroglio, MD, Marianna Sartorelli, MD, Alessandro Belletti, MD, Pasquale Nardelli, MD, Alessandro Ortalda, MD, Gaetano Lombardi, MD, Federica Morselli, MD, Ottavia Pallanch, MD, Carolina Faustini, MD.
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2021.01.011.
Appendix. Supplementary materials
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, Van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashi M, Jacquin A, Dakhil B, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi RM, Solomon SD, Ridker PM, et al. Coronavirus 2019 disease (COVID-19), systemic inflammation, and cardiovascular disease. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerdà P, Ribas J, Iriarte A, et al. Blood test dynamics in hospitalized COVID-19 patients: Potential utility of D-dimer for pulmonary embolism diagnosis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colling ME, Kanti Y. COVID-19-associated coagulopathy: An exploration of mechanisms. Vasc Med. 2020;25:471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54 doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 10.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ai T, Yang Z, Hou H, Zhan C, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotzinger DC, Beigelman-Aubry C, von Garnier C, et al. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grodecki K, Lin A, Cadet S, et al. Quantitative burden of COVID-19 pneumonia on chest CT predicts adverse outcomes: A post-hoc analysis of a prospective international registry. Radiol Cardiothorac Imaging. 2020;2:5. doi: 10.1148/ryct.2020200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: Facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;1(22):91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues JCL, Hare SS, Edey A, et al. An update on COVID-19 for the radiologist - A British Society of Thoracic Imaging statement. Clin Radiol. 2020;75:323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;296:E79–E85. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Zhong Z, Zhao W, et al. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: Relationship to negative RT-PCR testing. Radiology. 2020;296:E41–E45. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 21.Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang R, Li X, Liu H, Zhen Y, et al. Chest CT severity score: An imaging tool for assessing severe COVID-19. Radiol Cardiothoracic Imaging. 2020;2:2. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiumello D. Springer International Publishing AG; Sham, Switzerland: 2017. Acute respiratory distress syndrome (ARDS): Definition, incidence, and outcome. [Google Scholar]
- 25.Hong SB, Kim HJ, Huh JW, et al. A cluster of lung injury associated with home humidifier use: Clinical, radiological and pathological description of a new syndrome. Thorax. 2014;69:694–702. doi: 10.1136/thoraxjnl-2013-204135. [DOI] [PubMed] [Google Scholar]
- 26.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGonagle D, O'Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teuwen LA, Geldhof V, Pasut A, et al. COVID-19. The vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao W, Li T. COVID-19. Towards understanding of pathogenesis. Cell Res. 2020;30:367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaisdell FW. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974;108:44–49. doi: 10.1001/archsurg.1974.01350250036009. [DOI] [PubMed] [Google Scholar]
- 31.Tomashefski Jr JF, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 32.Opal SM. Interactions between coagulation and inflammation. Scand J Infect Dis. 2003;35:545–554. doi: 10.1080/00365540310015638. [DOI] [PubMed] [Google Scholar]
- 33.Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. 2008;34:459–468. doi: 10.1055/s-0028-1092876. [DOI] [PubMed] [Google Scholar]
- 34.Fraser DD, Patterson EK, Slessarev M, et al. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: Implications for microvascular platelet aggregation. Crit Care Explor. 2020;2:e0194. doi: 10.1097/CCE.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene R, Zapol WM, Snider MT, et al. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124:593–601. doi: 10.1164/arrd.1981.124.5.593. [DOI] [PubMed] [Google Scholar]
- 36.Zapol WM, Kobayashi K, Snider MT, et al. Vascular obstruction causes pulmonary hypertension in severe acute respiratory failure. Chest. 1977;71(2 suppl):306–307. doi: 10.1378/chest.71.2_supplement.306. [DOI] [PubMed] [Google Scholar]
- 37.Hill JD, Ratliff JL, Fallat RJ, et al. Prognostic factors in the treatment of acute respiratory insufficiency with long-term extracorporeal oxygenation. J Thorac Cardiovasc Surg. 1974;68:905–917. [PubMed] [Google Scholar]
- 38.Vesconi S, Rossi GP, Pesenti A, et al. Pulmonary microthrombosis in severe adult respiratory distress syndrome. Crit Care Med. 1988;16:111–113. doi: 10.1097/00003246-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Snow RL, Davies P, Pontoppidan H, et al. Pulmonary vascular remodeling in adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126:887–892. doi: 10.1164/arrd.1982.126.5.887. [DOI] [PubMed] [Google Scholar]
- 40.Baccellieri D, Bertoglio L, Apruzzi L, et al. Incidence of deep venous thrombosis in COVID-19 hospitalized patients during the first peak of the Italian outbreak. Phlebology. 2020 Nov 26 doi: 10.1177/0268355520975592. [DOI] [PubMed] [Google Scholar]
- 41.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FREEDOM COVID-19 anticoagulation strategy (FREEDOM COVID). ClinicalTrials.gov identifier: NCT04512079. Available at: https://clinicaltrials.gov/ct2/show/NCT04512079. Accessed December 30, 2020.
- 44.Enoxaparin in COVID-19 moderate to severe hospitalized patients (INHIXACOV19). ClinicalTrials.gov identifier: NCT04427098, Available at: https://clinicaltrials.gov/ct2/show/NCT04427098. Accessed December 30, 2020.
- 45.Defibrotide as prevention and treatment of respiratory distress and cytokine release syndrome of Covid 19 (DEFACOVID). ClinicalTrials.gov identifier: NCT04348383, Available at: https://clinicaltrials.gov/ct2/show/NCT04348383. Accessed December 30, 2020.
- 46.Defibrotide in COVID-19 pneumonia (DEFI-VID19), ClinicalTrials.gov identifier: NCT04335201 Available at: https://clinicaltrials.gov/ct2/show/NCT04335201. Accessed December 30, 2020.
- 47.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders M, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.