Abstract
Background
To evaluate overall ischemic stroke volumes and rates, specific subtypes, and clinical presentation during the COVID-19 pandemic in a multicenter observational study from eight states across US.
Methods
We compared all ischemic strokes admitted between January 2019 and May 2020, grouped as; March-May 2020 (COVID-19 period) and March-May 2019 (seasonal pre-COVID-19 period). Primary outcome was stroke severity at admission measured by NIHSS stratified as mild (0−7), moderate [[8], [9], [10], [11], [12], [13], [14]], and severe (>14). Secondary outcomes were volume of large vessel occlusions (LVOs), stroke etiology, IV-tPA rates, and discharge disposition.
Results
Of the 7969 patients diagnosed with acute ischemic stroke during the study period, 933 (12 %) presented in the COVID-19 period while 1319 (17 %) presented in the seasonal pre-COVID-19 period. Significant decline was observed in the mean weekly volumes of newly diagnosed ischemic strokes (98 ± 3 vs 50 ± 20,p = 0.003), LVOs (16.5 ± 3.8 vs 8.3 ± 5.9,p = 0.008), and IV-tPA (10.9 ± 3.4 vs 5.3 ± 2.9,p = 0.0047), whereas the mean weekly proportion of LVOs (18 % ±5 vs 16 % ±7,p = 0.24) and IV-tPA (10.4 % ±4.5 vs. 9.9 % ±2.4,p = 0.66) remained the same, when compared to the seasonal pre-COVID-19 period. Additionally, an increased proportion of patients presented with a severe disease (NIHSS > 14) during the COVID-19 period (29.7 % vs 24.5 %,p < 0.025). The odds of being discharged to home were 26 % greater in the COVID-19 period when compared to seasonal pre-COVID-19 period (OR:1.26, 95 % CI:1.07–1.49,p = 0.016).
Conclusions
During COVID-19 period there was a decrease in volume of newly diagnosed ischemic stroke cases and IV-tPA administration. Patients admitted to the hospital had severe neurological clinical presentation and were more likely to discharge home.
Keywords: Ischemic stroke, NIHSS, Large vessel occlusion, COVID-19, Coronavirus
1. Introduction
With the rapid spread and high fatality rate of the novel human coronavirus, SARS-CoV-2, [1] it should come as no surprise that community dwellers may have been avoiding hospitals for the treatment of conditions unrelated to this viral infection, including emergencies like acute stroke [[2], [3], [4], [5]], and acute coronary syndromes [[6], [7], [8]]. According to recent published reports, the number of patients presenting to the hospital with acute stroke has fallen by 30–90 % [2,5,9]. In one of the largest observational datasets published to-date, including admission information from 280 centers in China, there was a 40 % decline in stroke admissions in February 2020 when compared to the same month in the previous year [3]. There are also reports of decreased nationwide utilization of perfusion imaging in the United States (US), since the first stay-at-home order was announced [4]. Hospitals across US have also reported a reduction in the use of magnetic resonance imaging (MRI) [2,10], which could potentially lead to fewer diagnoses of acute stroke in patients with mild or no significant neurologic symptoms.
Recently published evidence also report that the median baseline National Institutes of Health Stroke Scale (NIHSS) among stroke patients evaluated during the COVID-19 period was 8 versus 5 in the prior months (p = 0.26) [2]. However, the proportion of patients with large vessel occlusion and cortical signs was significantly greater during this same period [2]. In the present study, we sought to corroborate the findings of reduction in ischemic stroke presentations in a large multicenter US cohort and determine if the absence of mild stroke diagnoses is an effect of the pandemic.
2. Materials and methods
2.1. Study design and participants
Twelve centers representing 8 states in the US participated in this multicenter retrospective observational cohort study as part of the ‘Society of Vascular and Interventional Neurology COVID-19 Multinational Registry’ project. All centers included in the study catered high burden counties or metropolitan cities. High burden are was defined as COVID-19 rate of ≥500 cases per 100 000 people based on the COVID-19 tracker models from Johns Hopkins University (https://coronavirus.jhu.edu/) and the state health departments for the participating centers as of May 2020. Patient-level data were extracted from each center’s prospective stroke registry. All consecutive adult patients 18 years of age or older who were admitted between January 1st 2019 and May 31st 2020 were eligible for inclusion. Only patients with a clinical or radiographic diagnosis of acute cerebral infarction were included in the final analysis. The use of neuroimaging to confirm an ischemic stroke diagnosis was made at the discretion of the treating physician. The presence of a diffusion abnormality on magnetic resonance imaging was not required. The study was not designed to capture the COVID-19 status of stroke patients or the treatment modalities; these findings are reported in a separate publication [11]. This study was approved under the waiver of informed consent by the local institutional review board at each participating center, and it is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [12].
2.2. Data collection
Patient demographic information, including age, sex, race, and ethnicity, as well as pertinent medical history, baseline National Institute of Health Stroke Scale (NIHSS), time from last known well to hospital arrival, stroke etiology, and the presence of large vessel occlusion (LVO) were captured. Limited data sets (including absolute age and relevant event dates) were collected from 10 sites, while de-identified aggregated data sets (including ages binned by decade, and calculated time intervals (e.g., last known well to arrival, arrival to initial imaging) were collected from 2 sites. The mean number of weekly stroke diagnoses, the number of IV-tPA administrations, and the total number of stroke diagnoses were calculated for each institution. Patient’s age was grouped by decade (e.g., 30−39, 40−49) and admission date was binned by weekly and montly epochs between 1/1/2019-5/31/2020, in order to protect the identities of patients in accordance with local regulations. Neuroimaging was interpreted by local investigators and was not centrally adjudicated. Stroke etiology was defined according to the modified Trial of Org 10172 (TOAST) [13] criteria as follows [1]: cardioembolism [2], large vessel disease (cervical or large artery intracranial atherosclerotic disease or unspecified) [3], small vessel disease [4], cryptogenic (due to multiple possible etiologies, undetermined etiology, or unspecified cryptogenic), and [5] other. Treatment decision was made at the discretion of the treating physician.
2.3. Primary and secondary outcomes
Our primary outcome was stroke severity at admission measured by NIHSS stratified as a 3-level categorical variable, with scores of 0−7 being classified as “mild” symptoms, 8−14 as “moderate”, and >14 as “severe” [13,14]. Secondary outcomes included the absolute NIHSS as a continuous variable, the number of weekly acute stroke evaluations, presence of LVO, stroke etiology according to TOAST criteria [15], and discharge disposition. We further explored discharge disposition as “favorable” (home or acute inpatient rehabilitation- which associates with a high probability of an eventual home discharge and functional independence [16,17] or “unfavorable” (all others), as in previous studies [18,19]. To limit the impact of the length-time bias on outcomes for patients who have been recently admitted during the COVID-19 pandemic long-term (e.g., 90 days), their outcome measures were not assessed.
2.4. Statistical analysis
Descriptive statistics were used to summarize the continuous and categorical variables. Normality of continuous data was assessed histographically and confirmed using the Shapiro-Wilk test, while categorical data were reported as absolute event rates and proportions. Continuous variables were reported as medians with interquartile range, or means with standard deviation, and compared using the Wilcoxon rank-sum test, and paired T-test as appropriate. Between-group comparisons for categorical data were made using Chi-square, or Fischer’s exact test when contingency table cell counts were less than five. The COVID-19 period was ostensibly selected as March 1st, 2020-May 31st, 2020 in order to maximize inclusivity of patients evaluated by centers during the beginning of the COVID-19 pandemic. Patients evaluated during the COVID-19 were compared against patients evaluated between March 1st, 2019-May 31st,2019 (seasonal pre-COVID-19 period), in order to account for seasonal variations in treatment patterns and outcome among patients with stroke [[20], [21], [22]]. A separate comparison was made between patients admitted between November 1st, 2019- January 31st, 2020 (immediate pre-COVID-19 period) and the COVID-19 period, while excluding patients admitted during February 2020 as a “wash-in” period to evaluate potential time biases (all results included in the supplementary materials). Weekly epochs were generated for patient events including acute stroke diagnosis, LVO events, IV-tPA volume, and proportion of patients harboring LVOs were compared using One-way ANOVA; Tukey post-hoc test was used for comparisons between the inclusive 12-week epochs of each study period (03/01/2019–5/31//2019 and 11/01/2019–01/31/2020 vs 03/01/2020–05/31/2020).
An ordinal logistic regression model was generated to estimate the effect of the COVID-19 period on stroke severity, the Brant test was used to confirm that the proportional odds assumption was not violated. Logistic regression was used to estimate the effect of the COVID-19 period for secondary binary outcomes of interest. A sensitivity analysis was performed in which logistic regression was used to estimate the effect of the COVID-19 period on the proportions of patients with severe stroke symptoms (NIHSS > 14) versus mild and moderate symptoms (NIHSS 0–13). All the variables that were significant to a p ≤ 0.05 in unadjusted comparisons were included in all logistic regression models for estimation while adjusting for potential confounders. Each model was clustered by the site and was adjusted for age, sex, comorbid conditions including atrial fibrillation/flutter, congestive heart failure, ischemic stroke, diabetes, tobacco use and prior medications. Secondary outcome models were adjusted for age, sex, ethnicity, NIHSS, stroke mechanism, peripheral arterial disease, renal insufficiency, tobacco use, heart failure, arterial fibrillation/flutter, and prior ischemic stroke. These counfounders were selected based on their univariate analysis significance (p < 0.05) (Table 1 ) and clinical relevance (age, atrial fibrillation, ischemic stroke, tobacco) with the outcomes of interest. Missing data were not imputed. Analyses were repeated limiting inclusion to sites that submitted all available data for the study period, regardless of the completeness (excluding one site), in order to optimize the reporting of consecutive patients. All tests were performed at the two-sided level using STATA 15.0 (College Station, TX), with p ≤ 0.05 considered statistically significant.
Table 1.
Demographic during the study periods (March–May 2019 and March–May 2020).
| Seasonal Pre-COVID-19 period | COVID-19 period | |||
|---|---|---|---|---|
| All patients (n = 2252) | Mar – May 2019 (n = 1319) | Mar – May 2020 (n = 933) | p-value | |
| Age group, no. (%) | 0.18 | |||
| <30 | 26 (1.2 %) | 12 (0.9 %) | 14 (1.5 %) | |
| 30−39 | 67 (3.0 %) | 41 (3.1 %) | 26 (2.8 %) | |
| 40−49 | 184 (8.2 %) | 102 (7.7 %) | 82 (8.8 %) | |
| 50−59 | 380 (16.9 %) | 207 (15.7 %) | 173 (18.5 %) | |
| 60−69 | 568 (25.2 %) | 336 (25.5 %) | 232 (24.9 %) | |
| 70−79 | 495 (22.0 %) | 286 (21.7 %) | 209 (22.4 %) | |
| 80−89 | 406 (18.0 %) | 259 (19.6 %) | 147 (15.8 %) | |
| 126 (5.6 %) | 76 (5.8 %) | 50 (5.4 %) | ||
| Sex, no. female (%) | 1051 (46.7 %) | 613 (46.5 %) | 438 (47.0 %) | 0.83 |
| Race, no. (%) | 0.46 | |||
| White | 1362 (60.5 %) | 801 (60.7 %) | 561 (60.1 %) | |
| Black | 511 (22.7 %) | 286 (21.7 %) | 225 (24.1 %) | |
| Asian | 32 (1.4 %) | 19 (1.4 %) | 13 (1.4 %) | |
| Other/unknown | 347 (15.4 %) | 213 (16.2 %) | 134 (14.4 %) | |
| Hispanic, no. (%) | 341/2166 (15.7 %) | 193/1264 (15.3 %) | 148/902 (16.4 %) | 0.47 |
| Medical history, no. (%) | ||||
| Hypertension | 1623 (72.1 %) | 964 (73.1 %) | 659 (70.6 %) | 0.20 |
| Dyslipidemia | 956/2251 (42.5 %) | 616/1318 (46.7 %) | 340/933 (36.4 %) | <0.01 |
| Diabetes mellitus | 774 (34.4 %) | 457 (34.7 %) | 317 (34.0 %) | 0.74 |
| Active tobacco use | 417 (18.6 %) | 242 (18.4 %) | 175 (18.8 %) | 0.78 |
| Atrial fibrillation/flutter | 414 (18.4 %) | 254 (19.3 %) | 160 (17.2 %) | 0.20 |
| Prior ischemic stroke | 402 (17.9 %) | 240 (18.2 %) | 162/933 (17.4 %) | 0.61 |
| Coronary artery disease | 374 (16.6 %) | 213 (16.2 %) | 161 (17.3 %) | 0.49 |
| Congestive heart failure | 246 (10.9 %) | 135 (10.2 %) | 111 (11.9 %) | 0.21 |
| Chronic renal insufficiency | 149 (6.9 %) | 93 (7.4 %) | 56 (6.2 %) | 0.28 |
| Peripheral arterial disease | 62 (2.8 %) | 28 (3.0 %) | 0.55 | |
| Medications prior to admission, no. (%) | ||||
| None | 420/1801 (23.3 %) | 213/1003 (21.2 %) | 207/798 (25.9 %) | 0.02 |
| Antihypertensive | 915/1801 (50.8 %) | 493/1003 (49.2 %) | 422/798 (52.9 %) | 0.12 |
| Antithrombotic (any class) | 813/1801 (45.1 %) | 499/1003 (49.8 %) | 314/798 (39.4 %) | <0.01 |
| Lipid-lowering therapy | 769/1801 (42.7 %) | 466/1003 (46.5 %) | 303/798 (38.0 %) | <0.01 |
| Antidiabetic | 219/1553 (14.1 %) | 114/853 (13.4 %) | 105/700 (15.0 %) | 0.36 |
| Hemoglobin A1c, median % (IQR) | 5.9 % (5.5−6.8 %) | 5.9 % (5.5−6.8) | 5.9 % (5.4−6.9 %) | 0.84 |
| Low-density lipoprotein, median mg/dL (IQR) | 84 (60−112) | 81 (58−109) | 88 (63−114) | 0.02 |
| High-density lipoprotein, median mg/dL (IQR) | 44 (35−54) | 44 (36−53) | 43 (35−55) | 0.41 |
| Total cholesterol, median mg/dL (IQR) | 157 (125−187) | 154 (123−182) | 161 (129−192) | 0.01 |
12-weeks epochs include; COVID-19 period: 03/01/2020−05/31/2020 and seasonal pre-COVID-19 period: 03/01/2019−05/31/2019.
Data will be made available upon reasonable request from the corresponding author.
3. Results
Of the 7969 patients diagnosed with acute ischemic stroke between 1/1/2019 and 5/31/2020, 1319 (17 %) presented in the seasonal pre-COVID-19 period and 933 (12 %) in the COVID-19 period. Table 1 demonstrates the differences in characteristics and comorbid conditions among patients admitted during these periods of interest.
3.1. Primary outcome
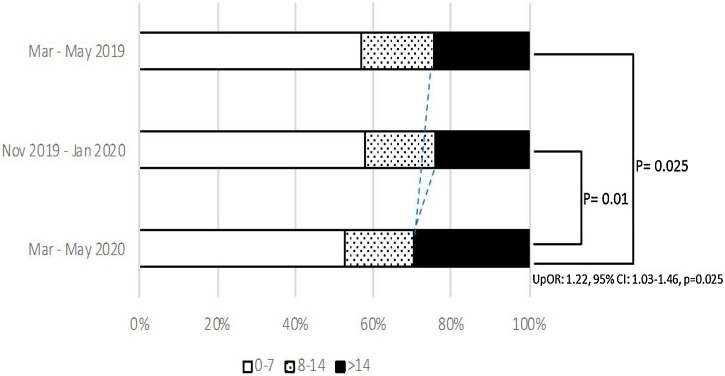
There was a significantly higher proportion (29.7 % vs 24.5 %, p = 0.025) of patients with severe disease (NIHSS > 14) and lesser proportion (52.6 % vs 56.7 %, p = 0.04) of patients with a milder disease (NIHSS < 7), admitted during the COVID-19 period as compared to the seasonal pre-COVID-19 period ( Table 2 and Fig. 1 ). Ordinal regression model showed that the patients presenting during the COVID-19 period were at significantly greater odds of having a more severe stroke (proportional OR: 1.22, 95 % CI: 1.03–1.46, p = 0.025; pBrant = 0.25) when compared with those presenting in the seasonal pre-COVID-19 period. However, after adjusting for all the significant confounders associated with severe NIHSS (>14), this model violated the parallel regression assumption (pBrant = 0.056). Therefore, the standard logistic model was used for the final analysis, which observed a non-significant trend towards increased odds of having a worse neurological presentation (aOR: 0.71, 95 % CI: 0.45–1.11, p = 0.13) during the COVID-19 period (Fig. 1).
Table 2.
Stroke characteristics during the study periods (March-May 2019 and March-May 2020).
| Seasonal Pre-COVID-19 period | COVID-19 period | |||
|---|---|---|---|---|
| All patients (n = 2252) | Mar – May 2019 (n = 1319) | Mar – May 2020 (n = 933) | p-value | |
| NIHSS on admission, median (IQR) | 6 (2−15) | 6 (2−14) | 7 (2−17) | 0.03 |
| NIHSS by severity | 0.04 | |||
| NIHSS 0−7, no. (%) | 1048/1905 (55.0 %) | 636/1121 (56.7 %) | 412/784 (52.6 %) | |
| NIHSS 8−14, no. (%) | 349/1905 (18.3 %) | 210/1121 (18.7 %) | 139/784 (17.7 %) | |
| NIHSS > 14, no. (%) | 508/1905 (26.7 %) | 275/1121 (24.5 %) | 233/784 (29.7 %) | |
| LVO, no. (%) | 401 (17.8 %) | 218 (16.5 %) | 183 (19.6 %) | 0.06 |
| Stroke etiology, no. (%) | <0.01 | |||
| Cardioembolism | 691 (30.7 %) | 447 (33.9 %) | 244 (26.2 %) | |
| Large vessel disease | 428 (19.0 %) | 223 (16.9 %) | 205 (22.0 %) | |
| Cervical atherosclerotic disease | 16 (0.7 %) | 0 (0 %) | 16 (1.7 %) | |
| Intracranial atherosclerotic disease | 22 (1.0 %) | 0 (0 %) | 22 (2.4 %) | |
| Large vessel disease – Unspecified | 390 (17.3 %) | 223 (18.1 %) | 167 (17.9 %) | |
| Small vessel disease | 231 (10.3 %) | 116 (9.4 %) | 115 (12.3 %) | |
| Cryptogenic | 597 (26.5 %) | 376 (28.5 %) | 220 (23.6 %) | |
| Cryptogenic - Undetermined | 233 (10.4 %) | 144 (11.7 %) | 89 (9.7 %) | |
| Cryptogenic – Multiple possible | 153 (6.8 %) | 116 (8.8 %) | 37 (4.0 %) | |
| Cryptogenic – Unspecified | 211 (9.3 %) | 116 (8.8 %) | 94 (10.1 %) | |
| Other etiology | 156 (6.9 %) | 67 (5.5 %) | 89 (9.5 %) | |
| Unknown | 150 (6.7 %) | 90 (6.8 %) | 60 (6.4 %) | |
| Discharge disposition, no. (%) | <0.01 | |||
| Home/Against medical advice | 1049/2232 (47.0 %) | 589/1319 (44.7 %) | 460/895 (51.4 %) | |
| Other healthcare facility | 908/2232 (40.7 %) | 577/1319 (43.8 %) | 331/913 (36.3 %) | |
| Acute rehabilitation facility | 522/2232 (23.4 %) | 348/1319 (26.4 %) | 174/913 (19.1 %) | |
| Skilled nursing facility | 221/2232 (9.9 %) | 154/1319 (11.7 %) | 67/913 (7.3 %) | |
| Long-term acute care facility | 28/2232 (1.3 %) | 17/1319 (1.3 %) | 11/913 (1.2 %) | |
| Other/unspecified health care facility | 130/2232 (5.8 %) | 53/1319 (4.0 %) | 77/913 (8.4 %) | |
| Hospice | 108/2232 (4.8 %) | 64/1319 (4.9 %) | 44/913 (4.8 %) | |
| Expired | 174/2232 (7.8 %) | 94/1319 (7.1 %) | 80/913 (8.8 %) | |
| Favorable discharge disposition*, no. (%) | 1571/2232 (70.4 %) | 937/1319 (71.0 %) | 634/913 (69.4 %) | 0.42 |
| Modified Rankin Scale at discharge, median (IQR) | 4 [2,3,4,5] (n = 995) | 4 [2,3,4] (n = 664) | 4 [2,3,4,5] (n = 331) | 0.02 |
IQR: Interquartile ranges; NIHSS: National Institute of Health Stroke Scale; LVO: Large vessel occlusion.
12-weeks epochs include; COVID-19 period: 03/01/2020−05/31/2020 and seasonal pre-COVID-19 period: 03/01/2019−05/31/2019.
Favorable discharge disposition defined as discharge to home or acute rehabilitation facility.
Fig. 1.
Admission NIHSS by study periods.
Stroke severity measured by NIHSS stratifies as 3-levels, with scores of 0−7 being classified as “mild” symptoms, 8−14 as “moderate”, and >14 as “severe”.
12-weeks epochs include; COVID-19 period: 03/01/2020–05/31/2020, seasonal pre-COVID-19 period: 03/01/2019–05/31/2019 and immediate pre-COVID-19 period: 11/01/2019–01/31/2020.
NIHSS: National Institute of Health Stroke Scale.
UpOR = Unadjusted proportional odds ratio.
3.2. Secondary outcomes
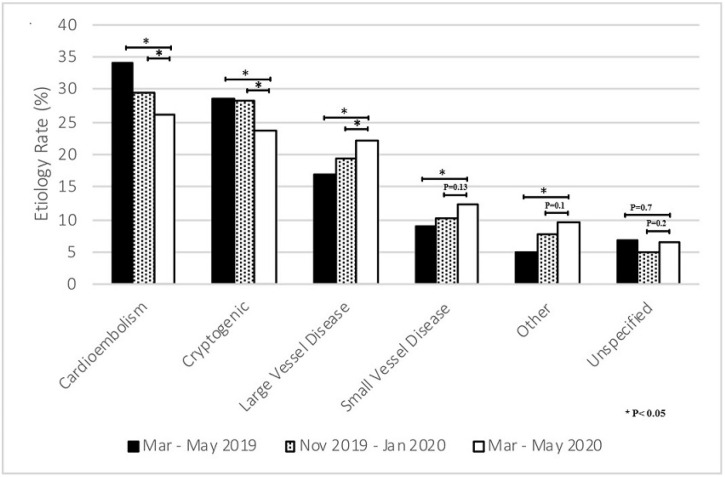
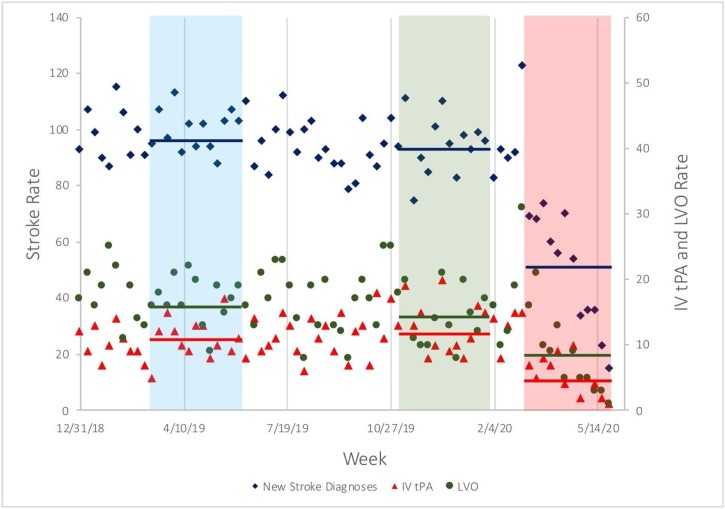
The NIHSS at admission was significantly higher in patients admitted during the COVID-19 period (6; IQR 2−14 vs 7; IQR 2−17; p = 0.039) when compared to the patients admitted durig sesonal pre-COVID-19 period. Among these ischemic stroke patients admitted during the COVID-19 period, there was a significantly higher proportion with stroke due to large vessel disease (22 % vs. 16.9 %, p = 0.02) and lacunar infarct (12.3 % vs 9.4 %, p = 0.01), while the proportion of patients with stroke due to cardioembolism was lower (26.2 % vs. 33.9 %, p = 0.011), when compared to the seasonal pre-COVID-19 period (Table 2 and Fig. 2 ). Compared to the seasonal pre-COVID-19 period, there was a significant decrease in the mean weekly volume of patients diagnosed with ischemic strokes during the COVID-19 period (mean 98 ± 7.3 vs. 50 ± 20.0. p = 0.003) and weekly large vessel occlusions (16.5 ± 3.8 vs. 8.3 ± 5.9, p = 0.008), while the weekly proportions of LVOs remained the same (mean weekly proportion of 18 % ± 5 % vs. 16 % ±7 %, p = 0.24; overall period proportion 18.2 % vs. 19.6 %; p = 0.34) (Fig. 3). Additionally, the mean weekly number of patients treated with IV-tPA was decreased during the COVID-19 period when compared with the seasonal pre-COVID-19 period (mean 10.9 ± 3.4 vs 5.3 ± 2.9, p = 0.0047). Whereas, the proportion of IV-tPA administered relative to the number of strokes during the COVID-19 period remained the same (10.4 % ± 4.5 vs. 9.9 % ± 2.4, p = 0.66) (Fig. 3).
Fig. 2.
Etiology of stroke by study periods.
Criteria stratified by the Trial of Org 10172 for Acute Stroke Treatment.
12-weeks epochs include; COVID-19 period: 03/01/2020–05/31/2020, seasonal pre-COVID-19 period: 03/01/2019–05/31/2019 and immediate pre-COVID-19 period: 11/01/2019–01/31/2020.
Fig. 3.
Weekly new stroke cases during the study periods.
Shown are weekly event rates for new acute ischemic stroke diagnoses (blue diamonds), large vessel occlusions (green bubbles), and treatment rate with intravenous thrombolysis (red triangles). Periods highlighted in blue and red indicate the 12-week periods which were used for t-test comparisons, as described in the Methods. Blue, green, and red bars indicate mean event rates during each red or blue study periods.
IV-tPA denotes intravenous tissue plasminogen activator and LVO large vessel occlusion.
12-weeks epochs include; COVID-19 period: 03/01/2020–05/31/2020, seasonal pre-COVID-19 period: 03/01/2019–05/31/2019 and immediate pre-COVID-19 period: 11/01/2019–01/31/2020.
Lastly, there was no difference in the proportion of patients who had a favorable disposition (home or acute rehabilitation facility), admitted during the COVID-19 period when compared to the seasonal pre-COVID-19 period (69.4 % vs 71 %, p = 0.42). However, interestingly, a higher proportion of patients were discharged to home than to acute rehabilitation during the COVID-19 period (Table 2). The odds of being discharged to home versus all other dispositions were 26 % greater in the COVID-19 period when compared to identical months in 2019 (OR: 1.26, 95 % CI: 1.07–1.49, p = 0.016), and this effect continued to trend but became non-significant after clustering by site and adjusting for all the variables associated with a home discharge in univariate analysis (p < 0.05), including older age, male sex, Hispanic ethnicity (protective), baseline NIHSS, stroke mechanism, history of peripheral arterial disease, renal insufficiency, active tobacco use, heart failure, arterial fibrillation/flutter, and prior ischemic stroke (aOR: 1.48, 95 % CI: 0.96–2.28, p = 0.08).
All the comparison between the immediate pre COVID-19 and COVID-19 periods are summarized in the supplemental material. (Supplemental Tables 1 and 2)
4. Discussion
As the national healthcare system is challenged by the COVID-19 pandemic, timely diagnoses and management of acute conditions including stroke would improve health care resource utilization and could help prevent long term adverse consequences. In this multicenter observational study from 12 US sites, we observed a significant reduction in the weekly new acute ischemic stroke diagnoses and the IV-tPA rates during the COVID-19 period (Mar–May 2020) as compared with the seasonal pre-COVID-19 period (Mar–May 2019). This decrease in hospital admission was observed across all ages (above and below 70 years) and is irrespective of the disease severity. We also observed a higher proportion of patients with large vessel and lacunar strokes as compared to cardioembolic strokes in the patients admitted during the COVID-19 period. Most importantly, our results indicate that the patients evaluated during this period had a more severe stroke presentation, as observed by an increased proportion of patients with a higher NIHSS (>14).
Various studies have reported a decline in stroke cases around the world, during the COVID-19 pandemic [2,5,23]. Similar trends were observed for hospitalizations from acute coronary syndrome [6,24]. In our study, we observed a similar pattern of relative decrease in the newly diagnosed stroke cases and lower proportion of patients with milder strokes (NIHSS ≤ 7) during the COVID-19 period. However, it is likely that the number of overall stroke cases has not declined. Rather, patients are more reluctant to seek medical care during the COVID-19 pandemic due to factors including; the adaptation of social distancing, avoiding crowded emergency departments, and the fear of contracting the disease, especially for individuals with milder stroke symptoms. Although, our study did not account for these psychosocial factors, it was observed that getting infected is the most concerning attribute followed by stay-at-home policies, advice from a healthcare personnel and consiousness of facilitating the overwhelmed healthcare burden [25].
Stroke management guidelines have been updated to optimize health care and resource utilization during the COVID-19 pandemic [[26], [27], [28]]. Therefore, it is imperative to guide and educate patients and providers about alternate services including telehealth (phone ± video), use of urgent care or quick care facilities, and protocols for neuroimaging, emergency medical services (EMS), patient transfer, and treatment, to help mitigate the spread of COVID-19 infection [29,30]. Patients with milder symptoms who are reluctant to come to the hospitals can still be triaged and effectively managed via tele-visits, duly approved by the Centers for Medicare and Medicaid Services (CMS) [31]. This will not only limit the exposure but will also ensure adherence to the practice guidelines for stroke treatment and secondary prevention.
In our study we also observed an increased proportion of stroke patients being discharged home during the COVID-19 period. A smaller proportion was discharged to acute rehabilitation, while an even lower proportion was discharged to other long-term care facilities. Moreover, there was no difference in the functional outcome at discharge among the stroke patients during the COVID-19 period as compared to the pre-COVID period. Despite more severe presentation of stroke during this period, it would be expected that a higher proportion of patients are discharged to a post-acute care facility. It is possible that the patients, their families, and providers are reluctant to transfer patients to these facilities as some of the early published studies reported an increased rate and spread of COVID-19 infection among these post-acute care facilities [32]. Moreover, majority of the residents in these facilities are >60 years, with multiple chronic diseases, making them more susceptible to the infection with a high mortality rate [33,34]. Therefore, to mitigate these challenges, World Health Organization (WHO) and infection prevention experts have published guidelines and infection prevention and control (IPC) polices to better manage these high-risk facilities during this pandemic [[33], [34], [35], [36]].
4.1. Strengths and limitations
This is one of the largest observational cohort studies evaluating the impact of the COVID-19 pandemic on acute stroke treatment in the US. The study includes prospectively maintained patient-level data across eight states, representing a snapshot of the pandemic influence on the diverse US population. The data used for the analysis was maintained on standardized systems across all the participating sites, hence maintaining the integrity of the data collecting process. However, this study was not designed to capture the COVID-19 status of stroke patients and these finding are reported separately [11]. Additionally, findings from this study should be interpreted with caution with reference to the following limitations. Because of the inherent retrospective study design, there is a potential for selection bias. Moreover, due to the urgent nature of this investigation, we could not capture 100 % data across all the variables, whereas aggregate data were included for two participating sites. Also, there is a possibility of missing stroke cases from the study end period, or the admitted patients might still be under investigation when the data was locked. Thus, the unavailability of this real-time data could contribute to the decrease in stroke rates and differences in stroke etiology for the COVID-19 period. However, this does not entirely explain the observations of the current study as other investigators have also reported similar findings [2,4,37]. We surmise that psychosocial factors may contribute to the behavioral patterns leading to the observed findings. However, the current study was not aimed to construe such inferences. Therefore, it is pertinent to explore these findings in prospective studies to better understand these influences, as the healthcare systems evolve and adapt to the pandemic situation. Finally, our study is unable to ascertain any association demonstrating the effect on long-term functional outcomes for the COVID-19 period.
5. Conclusion
This is the largest US study to date, confirming a decline in the volume of acute ischemic stroke admissions and rates of IV-tPA administration since the SARS-CoV-2 pandemic was declared. Stroke care is significantly impacted as healthcare adapts to its new role and the fear of infection possibly affects patient’s behavior. The observations also support the notion that patients with milder symptoms are reluctant to seek healthcare services, while the patients with severe disease were admitted to the hospital. It is therefore, imperative to implement protocols, educate patients and providers, and organize healthcare services to mitigate long term consequences from stroke, during this COVID-19 pandemic.
Funding
None.
CRediT authorship contribution statement
Santiago Ortega-Gutierrez: Conceptualization, Data curation, Investigation, Validation, Writing - original draft, Writing - review & editing. Mudassir Farooqui: Data curation, Validation, Writing - original draft, Writing - review & editing. Alicia Zha: Data curation, Validation, Writing - review & editing. Alexandra Czap: Data curation, Validation, Writing - review & editing. Jacob Sebaugh: Data curation, Validation, Writing - review & editing. Shashvat Desai: Data curation, Validation, Writing - review & editing. Ashutosh Jadhav: Data curation, Validation, Writing - review & editing. Nirav Vora: Data curation, Validation, Writing - review & editing. Vivek Rai: Data curation, Validation, Writing - review & editing. Tudor G. Jovin: Conceptualization, Investigation, Supervision, Writing - review & editing. Jesse M. Thon: Data curation, Validation, Writing - review & editing. Mark Heslin: Data curation, Validation, Writing - review & editing. Lauren Thau: Data curation, Validation, Writing - review & editing. Cynthia Zevallos: Data curation, Validation, Writing - review & editing. Darko Quispe-Orozco: Data curation, Validation, Writing - review & editing. Dinesh V. Jillella: Data curation, Validation, Writing - review & editing. Fadi Nahab: Data curation, Validation, Writing - review & editing. Mahmoud H. Mohammaden: Data curation, Validation, Writing - review & editing. Raul G. Nogueira: Conceptualization, Investigation, Validation, Writing - review & editing. Diogo C. Haussen: Conceptualization, Investigation, Validation, Writing - review & editing. Thanh N. Nguyen: Conceptualization, Investigation, Validation, Writing - review & editing. Jose Rafael Romero: Data curation, Validation, Writing - review & editing. Hugo J. Aparicio: Data curation, Validation, Writing - review & editing. Mohamed Osman: Data curation, Validation, Writing - review & editing. Israr Ul Haq: Data curation, Validation, Writing - review & editing. David Liebeskind: Conceptualization, Investigation, Validation, Writing - review & editing. Ameer E. Hassan: Conceptualization, Investigation, Validation, Writing - review & editing. Osama Zaidat: Conceptualization, Investigation, Validation, Writing - review & editing. James E. Siegler: Conceptualization, Data curation, Investigation, Validation, Formal analysis, Writing - original draft, Writing - review & editing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.clineuro.2020.106436.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegler J., Heslin M., Thau L., Smith A., Jovin T. Falling stroke rates during COVID-19 pandemic at a Comprehensive Stroke Center: cover title: falling stroke rates during COVID-19. J. Stroke Cerebrovasc. Dis. 2020:104953. doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J., Li H., Kung D., Fisher M., Shen Y., Liu R. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;120:030225. doi: 10.1161/STROKEAHA.120.030225. STROKEAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kansagra A.P., Goyal M.S., Hamilton S., Albers G.W. Collateral effect of Covid-19 on stroke evaluation in the United States. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morelli N., Rota E., Terracciano C., et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur. Neurol. 2020:1. doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Filippo O., D’Ascenzo F., Angelini F., et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccolo R., Bruzzese D., Mauro C., et al. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huet F., Prieur C., Schurtz G., et al. One train may hide another: acute cardiovascular diseases could be neglected because of the COVID-19 pandemic. Arch. Cardiovasc. Dis. 2020 doi: 10.1016/j.acvd.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchino K., Kolikonda M.K., Brown D., et al. Decline in stroke presentations during COVID-19 surge. Stroke. 2020;10:030331. doi: 10.1161/STROKEAHA.120.030331. 1161/STROKEAHA.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegler J.E., Cardona P., Arenillas J.F., et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int. J. Stroke. 2020 doi: 10.1177/1747493020959216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Logallo N., Novotny V., Assmus J., et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781–788. doi: 10.1016/S1474-4422(17)30253-3. [DOI] [PubMed] [Google Scholar]
- 14.Saver J.L., Yafeh B. Confirmation of tPA treatment effect by baseline severity-adjusted end point reanalysis of the NINDS-tPA stroke trials. Stroke. 2007;38(2):414–416. doi: 10.1161/01.STR.0000254580.39297.3c. [DOI] [PubMed] [Google Scholar]
- 15.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Heinemann A.W., Roth E.J., Cichowski K., Betts H.B. Multivariate analysis of improvement and outcome following stroke rehabilitation. Arch. Neurol. 1987;44(11):1167–1172. doi: 10.1001/archneur.1987.00520230051013. [DOI] [PubMed] [Google Scholar]
- 17.Yagura H., Miyai I., Seike Y., Suzuki T., Yanagihara T. Benefit of inpatient multidisciplinary rehabilitation up to 1 year after stroke. Arch. Phys. Med. Rehabil. 2003;84(11):1687–1691. doi: 10.1053/s0003-9993(03)00286-7. [DOI] [PubMed] [Google Scholar]
- 18.Boehme A.K., Siegler J.E., Mullen M.T., et al. Racial and gender differences in stroke severity, outcomes, and treatment in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014;23(4):e255–61. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belagaje S.R., Zander K., Thackeray L., Gupta R. Disposition to home or acute rehabilitation is associated with a favorable clinical outcome in the SENTIS trial. J. Neurointerv. Surg. 2015;7(5):322. doi: 10.1136/neurintsurg-2014-011132. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Zhou Z., Chen N., He L., Zhou M. Seasonal variation in the occurrence of ischemic stroke: a meta-analysis. Environ. Geochem. Health. 2019;41(5):2113–2130. doi: 10.1007/s10653-019-00265-y. [DOI] [PubMed] [Google Scholar]
- 21.Lanska D.J., Hoffmann R.G. Seasonal variation in stroke mortality rates. Neurology. 1999;52(5):984–990. doi: 10.1212/wnl.52.5.984. [DOI] [PubMed] [Google Scholar]
- 22.Lichtman J.H., Jones S.B., Wang Y., Leifheit-Limson E.C., Goldstein L.B. Seasonal variation in 30-day mortality after stroke: teaching versus nonteaching hospitals. Stroke. 2013;44(2):531–533. doi: 10.1161/STROKEAHA.112.670547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diegoli H., Magalhães P.S., Martins S.C., et al. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke. 2020;120:030481. doi: 10.1161/STROKEAHA.120.030481. STROKEAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzler B., Siostrzonek P., Binder R.K., Bauer A., Reinstadler S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur. Heart J. 2020;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S., Lorenzi N., Smith T., Carlson B.R., Sternberg Jr P. Critical insights from patients during the COVID-19 pandemic. NEJM Catalyst Innovations in Care Delivery. 2020;1(4) [Google Scholar]
- 26.Lyden P. Temporary emergency guidance to US stroke centers during the COVID-19 pandemic on behalf of the AHA/ASA Stroke Council Leadership. Stroke. 2020:10. [Google Scholar]
- 27.Qureshi A.I., Abd-Allah F., Al-Senani F., et al. Management of acute ischemic stroke in patients with COVID-19 infection: insights from an international panel. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.05.018. S0735-6757(20)30356-30359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dafer R.M., Osteraas N.D., Biller J. Elsevier; 2020. Acute Stroke Care in the Coronavirus Disease 2019 Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leira E.C., Russman A.N., Biller J., et al. Preserving stroke care during the COVID-19 pandemic: potential issues and solutions. Neurology. 2020 doi: 10.1212/WNL.0000000000009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen T.N., Abdalkader M., Jovin T.G., et al. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams: a guidance statement from the Society of vascular and Interventional Neurology. Stroke. 2020;51(6):1896–1901. doi: 10.1161/STROKEAHA.120.030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medicare Cf, Services M. 2020. Physicians and Other Clinicians: CMS Flexibilities to Fight COVID‐19. [Google Scholar]
- 32.McMichael T.M., Currie D.W., Clark S., et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comas-Herrera A., Zalakaín J., Litwin C., Hsu A.T., Lane N., Fernández J.-L. LTCcovid org, International Long-Term Care Policy Network; 2020. Mortality Associated With COVID-19 Outbreaks in Care Homes: Early International Evidence. [Google Scholar]
- 34.Covid C., Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai C.-C., Wang J.-H., Ko W.-C., et al. COVID-19 in long-term care facilities: an upcoming threat that cannot be ignored. J. Microbiol. Immunol. Infect. 2020;53(3):444–446. doi: 10.1016/j.jmii.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Organization W.H. World Health Organization; 2020. Infection Prevention and Control Guidance for Long-term Care Facilities in the Context of COVID-19: Interim Guidance, 21 March 2020. Contract No.: Document Number. [Google Scholar]
- 37.Yaghi S., Ishida K., Torres J., et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;120:030335. doi: 10.1161/STROKEAHA.120.030335. STROKEAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.