Abstract
Background
Probiotic treatments might contribute to the prevention of ventilator-associated pneumonia (VAP). Due to its unclear clinical effects, here we intend to assess the preventive effect and safety of probiotics on intensive care unit (ICU) patients.
Methods
Eligible randomised controlled trials were selected in databases until 30 September 2019. The characteristics of the studies were extracted, including study design, definition of VAP, probiotics intervention, category of included patients, incidence of VAP, mortality, duration of mechanical ventilation (MV) and ICU stay. Heterogeneity was evaluated by Chi-squared and I2 tests.
Results
15 studies involving 2039 patients were identified for analysis. The pooled analysis suggests significant reduction on VAP (risk ratio, 0.68; 95% Cl, 0.60 to 0.77; p<0.00001) in a fixed-effects model. Subgroup analyses performed on the category of clinical and microbiological criteria both support the above conclusion; however, there were no significant differences in duration of MV or length of ICU stay in a random-effects model. Also, no significant differences in total mortality, overall mortality, 28-day mortality or 90-day mortality were found in the fixed-effects model.
Conclusions
The probiotics helped to prevent VAP without impacting the duration of MV, length of ICU stay or mortality.
Short abstract
Pooled analysis suggests significant reduction in VAP in probiotic-treated patients but no change in the length of ICU stay, duration of mechanical ventilation or mortality https://bit.ly/30FfDTD
Background
Ventilator-associated pneumonia (VAP) is a nosocomial infection characterised by onset after 48 h of the application of mechanical ventilation (MV) [1]. VAP is among the most common infections in hospitalised patients and affects between 9% and 27% of intubated patients [2]. VAP remains a serious problem in the intensive care unit (ICU), which has considerable consequences, including significant morbidity, mortality, markedly prolonged ICU length of stay, and increased ventilator days [3–5]. Patients who develop VAP cost US$40 000 more to treat than those who do not [6]. Currently, initial appropriate antimicrobial therapy with a length of 7 or 8 days for most patients is a critical strategy of VAP treatment. However, multidrug-resistant (MDR) or extensive-drug resistant (XDR) pathogens are becoming prevalent as antibiotic drugs continue to be used inappropriately, which means our current antibiotic strategies are becoming ineffective [7]. Some scientists have now devoted themselves to developing a new generation of synthetic antimicrobials, aiming to minimise the risks of spontaneous resistance and toxicity associated with currently available antibiotics [8]. The major cause of VAP is aspiration of either microorganism from the oropharynx or fragments of biofilms from the endotracheal tube, and inhibition of the colonisation and formation of the biofilm are viewed as a feasible strategy [9, 10]. Probiotics are living microorganisms and considered as an option for the prevention of VAP through minimising the colonisation by virulent species or modulating the host immune defence [11]. The conclusions of randomised controlled trials (RCTs) conducted in succession are inconsistent. Meta-analysis performed by Siempos et al. [12], Bo et al. [13], van Ruissen et al. [14] and Su et al. [15] supported the efficacy of probiotics in prevention of VAP, while other meta-analyses conducted by Gu et al. [16] and Wang et al. [17] denied the positive effect of probiotics. Due to the limited number of studies, low quality of the evidence and the wide confidence interval (Cl) of the estimated effect, these meta-analyses cannot provide sufficient evidence to draw a conclusion supporting the efficacy of probiotics in preventing VAP. We further standardised the inclusion criteria with the objective of improving the quality of evidence. For example, we excluded the RCTs conducted by Kotzampassi et al. [18], which just illustrated the reduction of respiratory tract infection without a define of VAP. Then, we performed a meta-analysis after including the most recent RCTs and present results in order to summarise the effect and limitation of probiotics for preventing VAP in the critically ill.
Methods
Search strategies for data
This systematic meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions [19], and the International prospective register of systematic reviews (PROSPERO) [20]. Although it was not registered, the entire process was in accordance with the above requirements. The protocol has not been previously published. All of the articles reporting the prophylactic effect of probiotics for VAP in critically ill patients were systematically retrieved through databases, including PubMed, Medline, EMBASE, the Cochrane Controlled Trials Register databases, and the China National Knowledge Infrastructure database until 30 September 2019. The database search was conducted with the following Medical Subject Headings entry terms and thesaurus vocabulary for indexing articles: “probiotic”, “probiotics”, “prebiotic”, “prebiotics”, “symbiotic”, “symbiotics”, “pneumonia”, “pneumonias”, “ventilator”, “ventilation”, mechanical ventilation”, “ventilator-associated pneumonia”, “random”, “randomised”, “control”, “controlled”, “trial”, “trials”, “clinical trial”, “clinical trials”, “randomised controlled trial”, “randomised controlled trials”, “RCT”, “RCTs”. No language restriction was applied. To identify other potentially eligible articles, studies were searched manually on primary researches, review articles, and manufacturers' websites for trial information by reviewing titles, abstracts, and full texts.
Inclusion and exclusion criteria
Eligible articles were selected according to the Cochrane Handbook for Systematic Reviews of Interventions and the PICOS model for the definition of inclusion criteria [19]: 1) population: all of the participants had an expected need of MV for at least 48 h; 2) intervention: any type of probiotics regimen; 3) comparison: with placebo or other therapies; 4) outcomes: incidence of VAP, mortality within 28 days, ICU and hospital length of stay, duration of MV, complications, number of free days of antibiotic use for VAP at day 28; and 5) study design: RCTs. However, probiotic formulation and dose or time of administration, minimum sample size, and age of participators were not included as criteria. Studies were excluded if they had any of the following characteristics: 1) text without data about participant characteristics or outcome, such as comment, guideline, case, poster, and review; 2) studies were not performed in humans, or conducted in ex vivo cells or animals; 3) studies only analysed participants with a special occupation; 4) studies involving patients already with evidence of previous history of pneumonia; and 5) studies involving patients with immunosuppression. The eligible articles were judged and selected by two researchers independently.
Data extraction and risk of bias assessment
Two investigators extracted the following information independently in related articles: study design, general characteristics of patients (sample size, age, number of females or males in each trial, sex, baseline of general condition, definition of VAP, therapeutic dosages and process of probiotics, the administration on ICU patients in placebo groups), and the primary or secondary outcomes, including the incidence of VAP, infectious complications, the proportions of eradication of colonisation, ICU, and hospital length of stay, duration of MV, mortality, faecal bacterial counts, organic acid concentration, length of antibiotic use for VAP, prevalence of microorganisms in surveillance cultures, and adverse events, among others. Some data were calculated with available data by Review Manager (RevMan, version 5.3.0., Cochrane Collaboration, Oxford, UK) if they were not provided directly in texts. Any discrepancies were resolved by a third reviewer after assessing the original literature. Heterogeneity among the included trials was assessed via the Chi-squared test and quantitatively measured through the I2 statistic. In situations of significant heterogeneity, the source was explored through sensitivity analysis. Subgroup analyses were made to evaluate the effect of probiotics on short-term mortality and long-term mortality. The risk of bias for the included papers was assessed using the Cochrane Handbook for Systematic Reviews of Interventions criteria [19]. A different definition of VAP for trial enrolment is an important source of heterogeneity and bias. Subanalysis was performed on the clinically confirmed VAP, quantitative microbiological confirmed VAP and nonquantitative microbiological confirmed VAP. The evaluation criteria were based on sample selection, allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias), statistical analysis and outcome validation, selective reporting and free of source of funding (reporting bias) measured the degree of bias, definition of inclusion and exclusion criteria. They were categorised as low risk, high risk, and unclear risk. Funnel plots were used to identify possible instances of publication bias or other bias.
Statistical analysis
The incidence of VAP in patients exposed to probiotics, duration of MV, length of ICU stay, and mortality was analysed in this systematic review. For continuous variables, we calculated the standardised mean difference (SMD) with 95% CI. The dichotomous data variables were measured by risk ratio and corresponding 95% CI. The p-value for the comparison between the groups was calculated, and a p-value ≤0.05 was considered as statistically significant. Heterogeneity was considered significantly statistical (p<0.10 or I2 ≥50%) evaluated by Chi-squared and I2 tests in a fixed-effects model or random-effects model. Publication bias was assessed by visually inspecting funnel plots in this meta-analysis. The comparison of the outcome between the probiotic and placebo was conducted using Review Manager 5.3 (Revman, The Cochrane Collaboration, Oxford, UK) [19]. P-values <0.05 were considered statistically significant.
Results
Characteristics and risk of bias assessment of the included studies
This systematic analysis was conducted according to PROSPERO, and the PRISMA checklist is provided in supplemental table 1. A total of 342 studies were selected by searching PubMed, Medline, EMBASE, the Cochrane Database and the China National Knowledge Infrastructure database, and 29 publications remained after removing duplicated texts and excluding inappropriate articles (identified as a comment, guideline, case, poster, review, abstract without data, editorials, erratum via reviewing the titles and abstracts). As shown in figure 1, after full-text reviewing of the selected 29 articles, 15 studies with 2039 participants were identified for quantitative analysis and the systematic review. All of the studies were designed as RCTs, among which 13 trials were carried out on adults [9, 21–32] (age ≥16 years), and two studies were conducted on children [33, 34]. Only one article did not mention the detailed blind design [33]. Seven studies and two studies were designed as double-blind trials [23, 26–29, 31, 32] and single-blind trials [22, 24], respectively, while five studies were open-label trials [9, 21, 25, 30, 34]. All of the articles made the statistical analysis of the occurrence of VAP in patients, although the works by Shimizu et al. [24] did not provide definite details about diagnosis criteria of VAP [31]. The characteristics of the trials are classified in table 1 and supplemental table 2. The risk of bias assessment of each included trial is displayed in figure 2. Briefly, the bias of analysis mostly originated from the bias of performance and outcome assessment among the included trials, in which only five studies showed low risk [23, 27, 28, 31, 32]. The studies conducted by Knight et al. [27] and Mahmoodpoor et al. [32] can be viewed as high-quality studies, which show low risk in every procedure of the clinical trial.
FIGURE 1.
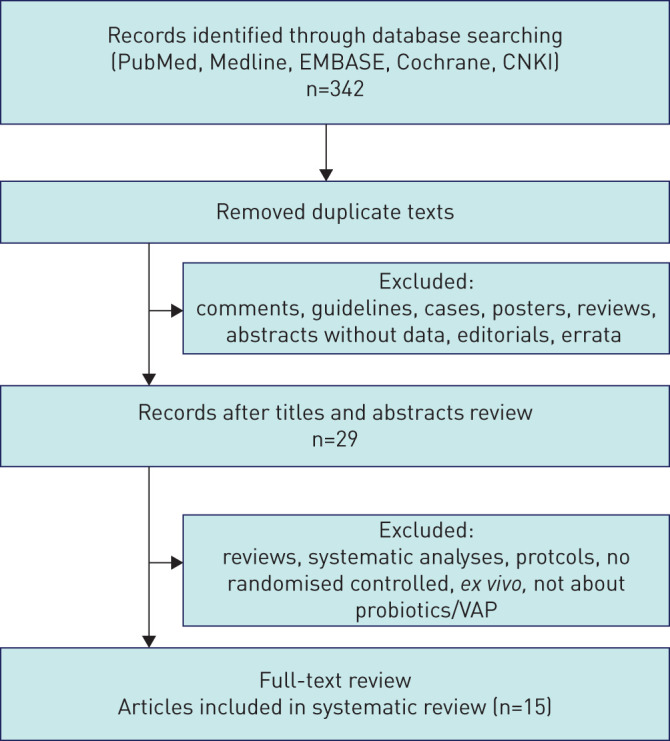
Outline of literature strategy, yielding 15 randomised publications that compared probiotics with conventional treatment strategies.
TABLE 1.
Characteristics and designs of the included studies
| First author year [ref.] | Study design | Male/patients | Age years | Inclusion criteria | Definition of VAP | Probiotics intervention | Primary outcomes | Secondary outcomes |
| Mahmoodpoor 2019 [32] | Prospective, double-blind, randomise, controlled trial | 55/102 | Probiotics: 59.1±12.9/ Control: 57.5±14.5 |
Patients ≥18 years old and had been undergoing MV for >48 h | A new or persistent infiltration on chest radiography with 2 of the 3 criteria of 1) temperature >38°C or <36°C; 2) leukocytosis or leukopenia; 3) purulent sputum underwent BAL 4) the quantitative BAL cultures had at least 104 CFU·mL−1 | Lac+Bif+Str: 1 capsule every 12 h daily for 14 days. Each capsule contained 1010 bacteria consisting of Lactobacillus species (casei, acidophilus, rhamnosus, bulgaricus), Bifidobacterium species (breve, longum) and Streptococcus thermophilus | VAP frequency | ICU and hospital length of stay, duration of MV, complications |
| Klarin 2018 [30] | A multicentre, prospective, randomised controlled open trial | 76/137 | Probiotics: 66 (57–76)/ Control: 65.5 (54–75) |
Patients ≥18 years old, critically ill with an anticipated need for MV of at least 24 h, not moribund, not having pneumonia, no fractures in the facial skeleton or the base of the skull, no oral ulcers, not immune deficient, not a carrier of HIV or viral hepatitis, not being tracheotomised, and no standard oral care before | A new, persistent or progressive infiltrate on chest radiograph combined with at least 3 of the other 4 criteria: 1) a purulent tracheal aspirate; 2) positive culture of tracheal aspirates occurring after 48 h of MV; 3) rectal or urine bladder temperature >38.0°C or <35.5°C; 4) WBC>12×103 or <3×103·mm−3 or a rapid increase in WBC count without suspicion of infection in another organ | Lac: Lp299 (Lactobacillus plantarum 299) were applied to the mucosal surface of the oral cavity twice a day, the subsequent cleansing was performed with gauze swabs soaked in carbonated bottled water | VAP frequency, Duration of ICU stay, duration of MV | Number of patients with findings of emerging microorganisms, positive findings of bacteria and fungi species |
| Shimizu 2018 [24] | Single-blind, randomised, controlled trial | 47/72 | Probiotics: 74 (64–82)/ Control: 74 (64–81) |
Patients >16 years old and had been undergoing MV for >72 h after admission to the ICU, diagnosed as sepsis | Not mentioned | Lac+Bif: Yakult BL Seichoyaku (3 g·day−1) contained 1×108 living bacteria of the B. breve strain Yakult/g and 1×108 living bacteria of the L. casei strain Shirota/g and galactooligo-saccharides (10 g·day−1) were administered via nasal tube and were continued until oral intake was initiated | Infectious complications including enteritis, VAP, and bacteraemia within 4 weeks from admission | Mortality within 4 weeks, faecal bacterial counts, organic acid concentration |
| Zeng 2016 [25] | Prospective, open-label, randomised, controlled multicentre trial | 138/235 | Probiotics: 50.2±18.2/ Control: 54.6±17.9 |
Patients ≥18 years with an expected need of MV for at least 48 h | A new, persistent or progressive infiltrate on chest radiographs that persisted for at least 48 h combined with at least 2 of the 3 criteria: 1) temperature >38.0°C or <35.5°C; 2) WBC>12×103 or <3×103·mm−3 and/or left shift; 3) purulent tracheal aspirates | Bac+Ent: Capsules containing active Bacillus subtilis and Enterococcus faecalis (4.5×109/0.25 g and 0.5×109/0.25 g, respectively) 0.5 g three times daily, for a maximum of 14 days | Microbiologically confirmed VAP incidence, the proportions eradication of colonisation, acquired colonisation with PPMOs in the oropharynx and stomach | Days on MV, days in the ICU and in the hospital after ICU admission, mortality (in ICU, in hospital), days of antibiotic use for VAP, antibiotic-free days at day 28, carbapenem-free days at day 28 and glycopeptide or linezolid-free days at day 28 |
| Rongrungruang 2015 [21] | Prospective, randomised, open-label, controlled trial | 62/150 | Probiotics: 73±13.16 (30–94)/ Control: 69±18.45 (20–97) |
Adult patients who were expected to receive MV at least 72 h and had no VAP at enrolment | A new, persistent, or progressive infiltrate visible on a chest radiograph in combination with at least 3 of the 4 criteria: 1) temperature >38 °C or <35.5°C; 2) WBC >12×103 or <3×103·mm−3; 3) purulent tracheal aspirate; 4) semi-quantitative culture of tracheal aspirate samples positive for pathogenic bacteria | Lac: 80 mL of commercially- available fermented dairy product containing 8×109 CFU of Lactobacillus casei, oral care after the standard oral care once daily, additional 80 mL of the aforementioned fermented dairy product was given once daily for 28 days | Incidence of VAP, incidence rate of VAP episodes per 1000 ventilator days | Length of hospital stay, mortality at day 28 and 90, incidence of diarrhoea, presence of resistant bacteria in oropharyngeal and rectal swab samples on day 0, 7 and 28 |
| Banupriya 2015 [34] | Open-label, randomised, controlled trial | 91/150 | Probiotics: 2.9±3.41/ Control: 2.93±3.77 |
Aged 12 years or less, likely to need MV>48 h | A new, persisting radiographical infiltrate combined with radiographical evidence of pulmonary abscess formation, or 2 of the 3 criteria: 1) fever (increase in the temperature of at least 1°C and a core temperature >38.3°C); 2) leukocytosis (25% increase in circulating leukocytes from baseline or WBC>10×103·mm−3); 3) purulent tracheal aspirate, a positive blood or pleural fluid culture with the microorganisms | Lac+Bif+Str: One capsule containing 2 billion CFU of Lactobacillus, 1 billion CFU of Bifidobacterium, and 300 million CFU of Streptococcus thermophilus were used twice a day | Incidence of VAP | Duration of hospital stay, mortality |
| Li 2012 [33] | Randomised, control trail | 92/165 | Probiotics: 32.3±1.5/ Control: 31.6±1.4 (weeks) |
MV≥48 h, no respiratory tract infection before orotracheal intubation, no history of using a large number of adrenal cortex hormone and immunosuppressive agents within 48 h before orotracheal intubation, no immune deficiency disease | After 48 h of MV, airway secretion culture was positive or new pathogenic bacteria appeared, new infiltration shadow appeared on chest radiography accompanied by increased pulmonary, clinical fever, WBC >10.0×109·L−1 | Bif: Probiotics (Bifidobacterium triple viable powder, Shanghai Xinyi Pharmaceutical) at a dose of 0.33 g·day−1 after the start of micro feeding and continued until the end of the study, if feeding intolerance occurs, immediately fast and stop oral probiotics, and continue oral probiotics when milk is reopened, probiotics fed for 3 days before blood bacterial culture | The number of the bacterial strain of VAP | The time of bacterial colonisation, VAP occurrence |
| Tan 2011 [22] | Prospective, randomised, single-blind study | 40/52 | Probiotics: 40.5±13.0/ Control: 40.8±12.8 |
Closed head injury alone, admission within 24 h after trauma, GCS score between 5 and 8, aged 18 to 60 years old, able to be fed via nasogastric tube within 48 h after admission | Pneumonia occurring ≥48 h after endotracheal intubation, a new or progressive radiographic infiltrate, at least two clinical features: 1) temperature >38.0°C; 2) WBC>12×109·L−1 or WBC count <4×109·L−1; 3) purulent tracheobronchial secretions and positive cultures of tracheobronchial secretion | Bif+Lac+Str: Seven sachets of viable probiotics (each sachet containing 0.5×108 Bifidobacterium longum, 0.5×107 Lactobacillus bulgaricus, and 0.5×107 Streptococcus thermophilus) three times a day, which provided a total of 109 bacteria | Multiple infections in the same patient | The use of antibiotics, length of ICU stay and the 28-day, mortality rate |
| Morrow 2010 [23] | Prospective, randomised, double-blind, placebo-controlled trial | 86/146 | Probiotics: 52.5±19.3/ Control: 54.6±16.3 |
Adults ≥19 years old require MV with an endotracheal tube ≥72 h | A new and persistent infiltrate on chest radiographs with 2 of 3 criteria:1) temperature >38.5°C or <35.0°C; 2) WBC>10 000·mm−3 or <3000·mm−3; 3) purulent sputum 4) quantitative BAL culture with at least 104 CFU·mL−1 in patients intubated for 48 h or longer | Lac: 2×109 CFU of Lactobacillus rhamnosus GG on a twice-daily basis, the contents of one capsule containing 109 CFU of Lactobacillus were suspended in sterile, water-based surgical lubricant and administered as a slurry to the oropharynx, the contents of a second capsule containing 109 of CFU Lactobacillus were suspended in sterile water and given through the nasogastric tube | Microbiologically confirmed VAP incidence | Mortality, the time to occurrence of VAP, durations of MV, ICU stay and hospital stay, clostridium difficile-associated diarrhoea and another ICU-associated diarrhoea, antibiotic consumption, and hospital charges |
| Barraud 2010 [28] | Double-blind, concealed randomised, placebo-controlled trial | 68/167 | 60.7±15.8 | Adult patients under MV for a predicted period of at least 48 h | A new and persistent infiltrate on chest radiograph associated with at least one of the following: 1) purulent tracheal secretions, temperature ≥38.3°C and WBC count ≥10×103·μL−1; 2) positive quantitative cultures of distal pulmonary secretions obtained from BAL (significant threshold more than 104 CFU·mL−1) | Lac+Bif: 2×1010 of revivable bacteria (mainly Lactobacillus rhamnosus GG, but also Lactobacillus casei, Lactobacillus acidophilus, and Bifidobacterium bifidum) once a day until successful weaning | 28-day mortality | Infection and diarrhoea, length of stay in ICU of hospital, resolution of organ failure at 28 days |
| Knight 2009 [27] | Prospective, randomised, double-blind, placebo-controlled trial | 161/259 | Probiotics: 49.5±19.6/ Control: 50.0±18.5 |
Expected to require MV for at least 48 h, no contraindications to enteral nutrition | A new progressive, or persistent infiltration on chest radiograph plus at least two of the following: 1) temperature >38.0°C; 2) WBC>12×103·μL−1 or <4 ×103·μL−1; 3) purulent tracheobronchial secretions | Synbiotic 2000 FORTE: At least 2 days of either Synbiotic 2000 FORTE twice a day, or a crystalline cellulose based placebo, Synbiotic 2000 FORTE contains Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp. paracasei and Lactobacillus plantarum as probiotics | Incidence of VAP | Oropharyngeal flora, duration of MV and VAP rates per 1000 ventilator days, length of ICU stay, mortality in ICU and hospital |
| Giamarellos-Bourboulis 2009 [26] | Double-blind, placebo-controlled, multicentre, randomised clinical trial | ?/72 | Probiotics: 52.9/ Control: 55.9 |
Patients with severe multiple organ injuries necessitating emergency tracheal intubation and ventilation support and subsequent hospitalisation in ICU | Patients presenting with all of the following: 1) new or persistent consolidation in lung radiograph; 2) purulent TBS; 3) clinical pulmonary infection score (CPIS)>6 | Synbiotic 2000 FORTE: The formula Synbiotic 2000FORTE was diluted in 100 mL of tap water and administered by a nasogastric tube or through gastrostomy once daily for 15 consecutive days after admission | Incidence of VAP | Incidence of bloodstream infections, incidence and comparative time of primary bacteraemia, comparative serum levels of WBCs and CRP of patients with primary bacteraemia and with VAP |
| Forestier 2008 [29] | Prospective, randomised, double-blind, placebo-controlled pilot study | 146/208 | Probiotics: 60 (18–91)/ Control: 57 (18–80) |
Patients ≥18 years with a stay longer than 48 h and a nasogastric feeding tube | According mostly to CDC's NHSN criteria: 1) at least one positive sample (bronchoalveolar mini-lavage >103 CFUs·mL−1 or endotracheal aspirate with >105 CFUs·mL−1) 2) the presence of one or several new abnormal radiographical and progressive parenchymatous infiltrates; 3) one of the following signs: purulent sputum production, fever (temperature >38.5°C), pathogenic bacteria in blood culture without other infection source, and BAL>5% cells with intracellular bacteria | Lac: L. casei rhamnosus (109 CFU), twice daily | The time of first P. aeruginosa acquisition | Whether respiratory tract infection or colonisation due to P. aeruginosa, to evaluate the ability of L. casei rhamnosus to persist in the stomach |
| Klarin 2008 [9] | Randomised, controlled, open pilot study | 22/44 | Probiotics: 70 (20–87)/ Control: 70 (43–81) |
Patients ≥18 years, critically ill with an anticipated need for MV of at least 24 h, not moribund, not suffering from pneumonia at admission, no fractures in the facial skeleton or the base of the skull, no oral ulcers, not immune deficient, not a carrier of HIV or viral hepatitis | A new, persistent or progressive infiltrate on chest radiograph combined with at least 3 of the other 4 criteria: 1) a purulent tracheal aspirate; 2) positive culture of tracheal aspirates occurring after 48 h of MV; 3) rectal or urine bladder temperature >38.0°C or <35.5°C; 4) WBC >12×109·L−1 or <3×109·L−1 | Lac: Lp299 (Lactobacillus plantarum 299) twice a day, subsequent cleansing was performed with gauze swabs soaked in carbonated bottled water | Pathogenic bacteria analysis in oropharynx and trachea | |
| Spindler-Vesel 2007 [31] | Prospective, randomised, double-blind study | 88/113 | 41±18.9 | Multiple injured patients with an ISS of >18 and at least a 4-day ICU stay | Not mentioned | Synbiotic 2000 FORTE: The contents of the sachets (1010 Pediococcus pentosaceus 5–33:3, 1010 Lactococcus raffinolactis 32–77:1, 1010 Lactobacillus paracasei subsp. paracasei 19, 1010 Lactobacillus plantarum 2362 and the fibres) were dissolved in 100 mL of lukewarm sterile water | Incidence of infection (such as VAP), duration of MV, multiple organ failure scores, length of ICU stay | |
VAP: ventilation-associated pneumonia; MV: mechanical ventilation; BAL: bronchoalveolar lavage; Lac: Lactobacillus (casei, plantarum, rhamnosus, bulgaricus; acidophilus); Bif: Bifidobacterium (breve, longum, bifidum); Str: Streptococcus thermophilus; ICU: intensive care unit; WBC: white blood cell; Bac: Bacillus subtilis; Ent: Enterococcus (faecalis); PPMO: potentially pathogenic microorganism; CFU: colony-forming unit; GCS: Glasgow coma score; TBS: tracheobronchial secretions; CPIS: clinical pulmonary infection score; CRP: C-reactive protein; CDC: the US Centers for Disease Control and Prevention; NHSN: National Healthcare Safety Network; ISS: injury severity score. Ages are presented as median (range) or mean±sd.
FIGURE 2.
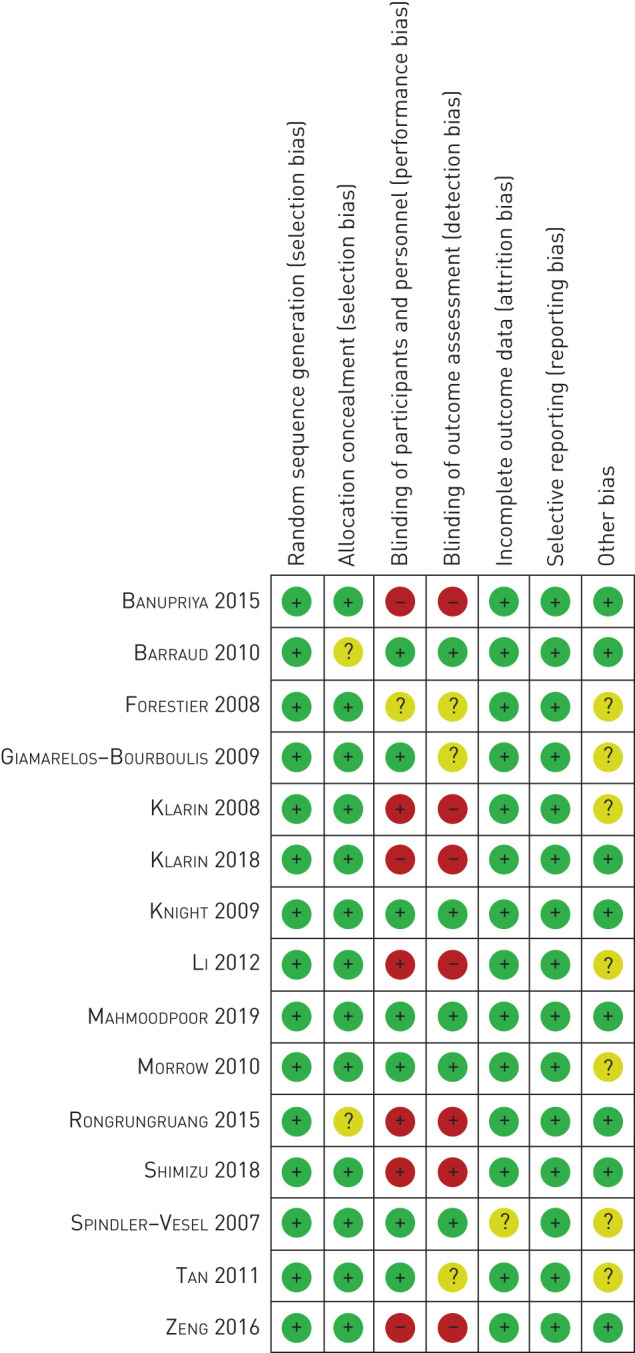
The risk of bias summary of 15 randomised control trials based on bias assessment of selection, allocation concealment, performance, detection, attrition and publication.
Probiotics decreased the incidence of VAP
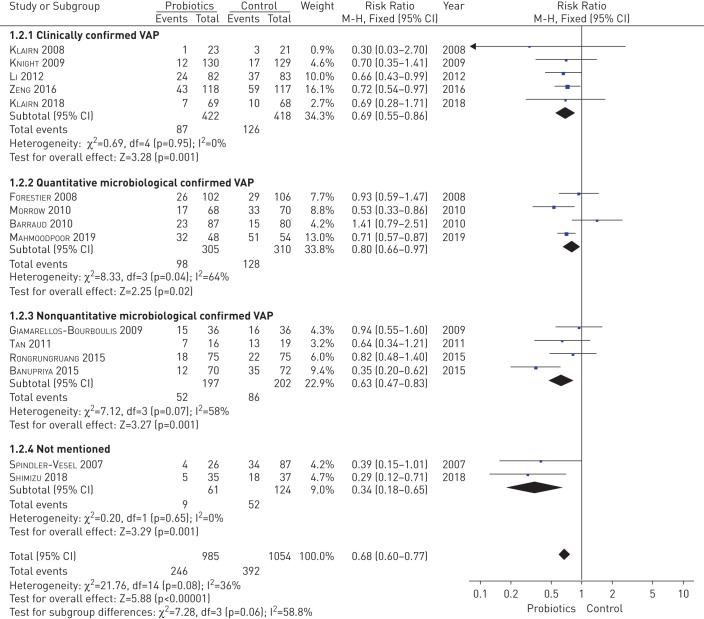
The pooled analysis with a fixed-effects model suggested significantly decreased morbidity of VAP in the probiotics group compared with the placebo group (risk ratio, 0.68; 95% Cl, 0.60 to 0.77; p<0.00001) based on 15 studies involving 2039 patients [9, 21–34]. Chi-squared and I2 tests were used to evaluate the statistical heterogeneity of these studies. As shown in figure 3, mild statistical heterogeneity was observed (p=0.08, I2=36%). However, among the included trials, eight studies did not detect a significant preventive effect for probiotics compared to a control group [9, 21, 22, 26–30]. The sample size was too small to detect minor discrepancy between groups, which might account for the two trails reporting a different result [9, 22]. Oudhuis et al. [35] reported that the effect of probiotics in preventing VAP was not superior to the selective decontamination of the digestive tract (SDD) (p=0.25), while isolates of antibiotic resistance strains were significantly higher in the probiotics group than in the SDD group (p<0.05) [36]. Forestier et al. [29] observed a decreased VAP frequency but without reaching statistical significance (p>0.05), while VAP occurrence was significantly delayed in the probiotics group (11 days versus 50 days, p=0.01). The absence of probiotics treatment (adjusted hazard ratio, 3.2; 95% CI, 1.1 to 9.1) was found to be an independent factor associated with increased risk for Pseudomonas aeruginosa respiratory infection [29]. Rongrungruang et al. [21] carried out their trial with a basic oral administration of chlorhexidine (CHX) for all the patients and found no significant difference in VAP frequency (p=0.46) [37, 38]. However, we cannot ignore the interfering impact of CHX, which might impair the viability of the probiotics. Interestingly, Klarin et al. [9, 30] performed an RCT comparing oral application of probiotics and CHX in 2008 and subsequently continued the study after expanding the sample size 10 years later. Both trials found that probiotics were not superior to CHX (p>0.05) [37–39]. Giamarellos-Bourboulis et al. [26] have reported that probiotics did not help to prevent VAP with diverse pathogens (p>0.05), but contributed to the reduction of VAP of Acinetobacter baumanii (p=0.047), which suggests that the effects of probiotics depend on the strain of bacteria. A subgroup analysis was performed based on category of diagnostic criteria of VAP, which was reported to have associations with VAP occurrence, duration of ICUs and mortality [40]. The subgroup analysis of clinically confirmed VAP (risk ratio, 0.69; 95% Cl, 0.55 to 0.86; p=0.001) and quantitative microbiological confirmed VAP (risk ratio, 0.80; 95% Cl, 0.66 to 0.97; p=0.02) and nonquantitative microbiological confirmed VAP (risk ratio, 0.63; 95% Cl, 0.47 to 0.83; p=0.001) all suggested a reduction of VAP incidence (figure 3). Sensitivity analysis by removing each trial also showed a significant reduction of VAP incidence similarly to the overall analysis. The funnel plot analysis was seemingly symmetric, suggesting no publication bias (supplemental figure 1).
FIGURE 3.
The pooled and subgroup analysis for the effect of probiotics on morbidity of ventilator-associated pneumonia based on 15 studies, involving 2039 patients compared with the placebo group.
Probiotics did not affect the duration of mechanical ventilation
Comprehensive analysis based on the eight studies involving 1200 participators that evaluated duration of MV indicated that there was no significant reduction in the probiotic group compared to the control group (SMD, −0.20; 95% Cl, −0.41 to 0.01; p=0.07) in a random-effects model (supplemental figure 2) [9, 23, 25, 27, 28, 31, 32, 34]. The results were limited by high heterogeneity (p=0.003; I2=67%), indicating possible bias. The subgroup analysis of clinically confirmed VAP (SMD, −0.11; 95% Cl, −0.53 to 0.31; p=0.61; I2=80%), quantitative microbiological confirmed VAP (SMD, −0.19; 95% Cl, −0.49 to 0.11; p=0.22; I2=56%) both indicated no significant reduction of duration of MV with high heterogeneity. However, the nonquantitative microbiological confirmed VAP subgroup suggested probiotics significantly reduced the duration of MV (SMD, −0.61; 95% Cl, −0.95 to −0.27; p=0.004). Only three studies reported a significantly statistical difference in the duration of MV [9, 32, 34]. In addition, Klarin et al. [9, 30] detected a significant difference (p<0.0001) in 2008 but reported opposite conclusions after analysing a larger sample size as part of a multicentre collaboration in 2018. Generally, duration of MV is a variable influenced by multiple factors, such as diverse complications, the basic physic status, age, and the reasons for ICU admission of the patients. Patients with thoracic trauma and respiratory failure are likely to have a long length of MV, which likely accounts for the high heterogeneity. For instance, Oudhuis et al. [35] reported longer length of MV (mean difference (MD)±sd, 16.7±23.6) in patients with higher APACHE score (MD±sd, 23±7.7), mostly diagnosed as respiratory insufficient and sepsis shock, than the study by Knight et al. [27] (MD±sd, 5±5.19), in which patients were mostly assessed after surgery (with a median APACHE score of 17; range, 12 to 23) [27]. Sensitivity analysis by removing each trial suggested the same results as in the overall analysis. The funnel plot analysis was unsymmetrical, suggesting that a publication bias exists (supplemental figure 3).
No change of the length of ICU stay in probiotics treated patients
Eleven studies assessed length of ICU stay, and the pooled meta-analysis with random-effects model indicated no significant reduction of ICU stay in the probiotics group compared to the control group (SMD, −0.20, 95% Cl, −0.46 to 0.06; p=0.13) [9, 21–25, 27, 28, 31, 32, 34]. However, the results were limited due to the high heterogeneity (p<0.00001, I2=83%) (supplemental figure 4). We conducted a subgroup analysis and found that no significant reduction of ICU stay in the probiotics group compared to control group for patients diagnosed by clinical (SMD, −0.12, 95% Cl, −0.29 to 0.05; p=0.17; I2=0%) or quantitative microbiological criteria (SMD, −0.39, 95% Cl, −1.03 to 0.26; p=0.24; I2=90%) and nonquantitative microbiological criteria (SMD, −0.19, 95% Cl, −1.12 to 0.74; p=0.68; I2=94%). A previous study indicated that patients with positive microbiology had more frequently decreased consciousness as the cause of ICU admission and longer ICU stays before the diagnosis of pneumonia [40]. Patients with different aetiology have various rehabilitation abilities, which impact the duration of ICU stay. The findings of Mahmoodpoor et al. [32], Banupriya et al. [34], and Tan et al. [22] are inconsistent with our analysis, and we found that their subjects were gathered from a specific population, such as adults with surgical reasons of neurological manifestations (SMD, −1.13; 95%Cl, −1.15 to −0.71; p=0.007), those with severe traumatic brain injury (SMD, −0.66; 95%Cl, −1.22 to −0.10; p=0.034), and children with neurological problems (SMD, −0.62; 95% Cl, −0.96 to −0.28; p<0.001). The remaining eight studies all found no significant difference in duration of ICU stay [9, 21, 23–25, 27, 28, 31]. Rongrungruang et al. [21], who assessed the data of probiotics effect on patients with basic oral application of CHX and found that the length of ICU stay in the probiotics group was longer than in the control group (MD, 30.5 versus 19), although without reaching statistically significance (SMD, 0.67; 95% Cl, 0.34 to 0.99; p=0.46). Sensitivity analysis by removing one trail suggested an obvious change of ICU stay length after prebiotics treatment (SMD, −0.29, 95%Cl, −0.51 to −0.08; p=0.007). After removing the data of Rongrungruang et al. [21], the nonquantitative microbiologically confirmed VAP subgroup also suggested a significant reduction of ICU duration after probiotic treatment (SMD, −0.63, 95% Cl, −0.92 to −0.34; p<0.0001, I2=0%). The subjects of the trial of Rongrungruang et al. [21] were older, female, and had diverse severe comorbidities, of whom the length of the ICU stay was not just associated with VAP but influenced by complex individual status. Therefore, this trial's result was not of sufficiently high quality to evaluate the effect of probiotics on duration of ICU stay. No obvious publication bias was observed by funnel plot analysis (supplemental figure 5).
Mortality analysis of critically ill patients
Eleven studies assessed the mortality of patients and the meta-analysis suggested that there was no significant difference in total mortality in the probiotic group (145 of 716, 20.25%) versus the control group (160 of 684, 23.39%) in the fixed-effects model (supplemental figure 6) [9, 21–28, 32, 34]. Rongrungruang et al. [21] and Barraud et al. [28] recorded the 28-day mortality and 90-day mortality of the participations, whereas Tan et al. [22] only evaluated the 28-day mortality of their patients. As shown in supplemental figure 6, the eight remaining trials and that of Barraud et al. [28] all evaluated overall mortality [9, 23–27, 32, 34]. Due to such a distinction in recorded statistics, a subgroup analysis was carried out to achieve a more convincible conclusion. Our meta-analysis result was that there was no significant difference in 28-day mortality between probiotic (43 of 188, 22.9%) and control (41 of 181, 22.7%) groups (risk ratio, 1.01; 95% Cl, 0.69 to 1.47; p=0.97), 90-day mortality in the probiotic (52 of 162, 32%) and control (50 of 155, 32.3%) groups (risk ratio, 1.00; 95% Cl, 0.72 to 1.37; p=0.99), and overall mortality in the probiotic (111 of 615, 18%) versus control (126 of 583, 21.6%) groups (risk ratio, 0.84; 95% Cl, 0.67 to 1.05; p=0.13). At the same time, heterogeneity was not observed in 28-day mortality (I2=0%, p=0.72), 90-day mortality (I2=0%, p=0.82), and overall mortality (I2=0%, p=0.56). All of the above meta-analysis results suggested that prophylactic use of probiotics was not beneficial in decreasing critically ill patients' mortality. Interestingly, Barraud et al. [28] performed a subgroup analysis based on different severities of sepsis and observed a significant mortality reduction among these severe septic patients treated with probiotics (odds ratio, 0.38; 95% CI, 0.16 to 0.93; p=0.035). By contrast, nonsevere septic patients administered by probiotics had higher mortality at 28 and 90 days than placebo-treated patients, which could be confirmed by a survival analysis: the hazard ratio for 90-day death was 3.40 (95% Cl, 1.18 to 7.64; p=0.02). This result, opposite to previous studies that deleterious effects of probiotics are only expected to appear among the more severe patients, makes the population and time point of probiotic regimen controversial [41]. Besides, fatal adverse effects were not observed among all studies. Funnel plot analysis indicated no obvious publication bias regarding the total mortality (supplemental figure 7).
Discussion
Many studies have suggested probiotics' preventive effect, among which Giamarellos-Bourboulis et al. [26] reported the prophylactic effect for VAP of Acinetobacter baumannii [26], and Forestier et al. [29] reported that probiotics delayed respiratory tract infection by P. aeruginosa. However, Knight et al. [27], Barraud et al. [28], and Oudhuis et al. [35] found no evidence that probiotics help to prevent VAP. As Mahmoodpoor et al. [32] have described, their result was inconclusive due to many influencing factors, such as different definitions of VAP occurrence, the sample size, the number of centres, degree of compliance to VAP prevention bundles, and types of probiotic species. A recent network meta-analysis conducted by Fan et al.[42] suggested that “Bifidobacterium longum+Lactobacillus bulgaricus+Streptococcus thermophiles” have higher efficacy in decreasing the incidence of VAP than other probiotic regimens. As illustrated in table 1, the probiotics regimens of the included studies varied, which might be a potential source of the heterogeneity and deserving attention. This systemic review comprehensively analysed the data of ICU patients from 15 RCTs to assess the preventive effect of probiotics for VAP. We found that the administration of probiotics played a significant role in reducing VAP incidence, but did not lead to a statistically significant reduction in length of ICU stay, duration of MV, mortality short- (28-day) or long-term (90-day), or overall mortality. Serious adverse effects or fatal complications were not observed among the studies. Recently, two larger multicentre RCTs have been registered and the recruitment as well as analysis are ongoing for now [43, 44], which will further enrich our understanding. So why is it that probiotics still cannot reduce mortality, despite significantly preventing VAP? The results of clinical trials and animal experiments both supported the benefits of probiotics in immunomodulation and fighting against pathogens. In our opinion, the direct effect of probiotics is to maintain the balance of microorganism. Additionally, probiotics might improve patients' overall status by systemic immunomodulation, an indirect method that influenced by individual immune system. Critically ill patients always have severe primary aetiology and multiple comorbidities, and the recovery of the most severe symptom determined the duration of MV, duration of ICU stay and mortality. Besides, the different efficacy of the dissimilar probiotic regimens should also be taken consideration. Synbiotic 2000FORTE was suggested to have potential effect in reducing mortality according to a network meta-analysis of 14 RCTs [42].
Among the studies included in our meta-analysis, only two RCTs applied the Synbiotic 2000FORTE. This might be an explanation why probiotics can reduce incidence of VAP but cannot decrease mortality.
The protective role of gut microbiota in host protection against bacterial pneumonia has been demonstrated using animal models. Schuijt et al. [45] found increased bacterial dissemination, inflammation, organ failure, and increased mortality following infection with Streptococcus pneumoniae in a mouse gut-bacteria-depleted model. These authors also observed that faecal microbiota transplantation to gut microbiota-depleted mice resulted in decreased bacterial counts and tumour necrosis factor-α and interleukin (IL)-10 levels in the lung early after pneumococcal infection compared to the gut-bacteria-depleted group. Using a Staphylococcus aureus pneumonia model, Gauguet et al. [46] found that mice in their segmented filamentous bacteria (SFB)-deficit group had a higher bacterial burden in the lungs, lung inflammation, and mortality than mice colonised with SFB. Vieira et al. [47] reported that live probiotics release metabolites (e.g. acetate) that modulate the inflammatory response against infection in a mice model of Klebsiella pneumoniae, and that these effects involve reactive oxygen species generation by alveolar macrophages, IL-10 generation, interference with nuclear factor-κB pathway, and transduction of the Mal/TIRAP signal pathway. Khailova et al. [48] observed reduced bacterial counts, reduced histological lung injury, lower number of infiltrating neutrophils, and decreased mice mortality in a Pseudomonas pneumonia model after administration of Lactobacillus rhamnosus GG (LGG), which appeared to be mediated by immunomodulatory cells and molecules.
Although the mechanisms by which probiotics produce a preventive effect are still poorly understood, multiple valuable scientific advances have been published [49, 50]. Several studies have reported that probiotics play a positive role in fighting against pathogens via immunomodulatory activities. Probiotics can facilitate protection against influenza virus infection by enhancing mucosal secretory IgA production and activating humoral and cellular immune response [51–53]. When comparing a gut-microbe-depleted group and control group, there was a difference in the super pathway of cholesterol biosynthesis and zymosterol biosynthesis canonical signal pathways, which are associated with antibacterial effector functions of alveolar macrophages [45]. The gut microbiota enhances the response of alveolar macrophages to bacterial virulence factors and phagocytosis capacity. The administration of LGG can increase gene expression of Foxp3, and the Foxp3 transcription factor controls the expression of CD4 and CD25, the maker of regulatory T (Treg) cells. Traditionally, Treg cells can regulate inflammation directly or through releasing anti-inflammatory cytokines, such as IL-10 [48]. However, the protective effect of Lactobacillus is demonstrated as not only irrelevant with IL-10 in respiratory virus infection but is also independent of specific interaction with pattern recognition receptors, Toll-like receptor (TLR2) and NOD2 in vitro, which suggests that more possible mechanisms needed to be explored [53]. Type 17 helper T (TH17) cell innate immune response is also activated by probiotics as bronchoalveolar lavage fluid levels of IL-22 (one of the main TH17 effector cytokines) were significantly increased in a mouse model, subsequently contributing to neutrophil attraction and production of antimicrobial peptides during pulmonary S. aureus infection [46]. Interestingly, the intestinal microbiota, including SFB, can regulate pulmonary adaptive immune responses during acute fungal infection in the lung [54, 55]. In summary, all findings described above prove the immunomodulatory properties of the antimicrobial activity of probiotics and indicate probiotics might play a positive role in treating pneumonia. Studies about animals and cells fail to show systemic conclusions of comprehensive spectrum pathogens of pneumonia so far. Therefore, further analyses are required. Nevertheless, because of heterogeneity of age, type and degree of disease, treatment duration, dose, type of strains, and outcome measures, the effect of probiotics in clinical trials involving ICU patients remains unclear. A trial performed on patients with predicted severe acute pancreatitis by Besselink et al. [56] suggested that probiotic prophylaxis did not reduce the risk of infectious complications and was associated with an increased risk of mortality. Barraud et al. [28] prematurely stopped their trial due to the risk reported by Besselink et al. [56], which might impact the reliability of the study. None of the included studies reported fatal side effects of administration of probiotics, which might be a result of researchers avoiding selecting this category of ICU patients. Probiotics showed a preventive effect on severe septic patients but were associated with a high risk of death in nonsevere septic patients, as Barraud et al. [28] observed. These findings might be explained by the fact that septic patients were sicker than nonseptic patients and that a treatment effect might have been only apparent in the more severe patients. Age is likely a factor that needs to be considered when interpreting these results because two studies were conducted on children [33, 34], to whom probiotics have a different effect compared to adults. It is well-known that children's microflora is dynamic and begins to resemble that of adults at around 2 years of age [34]. Besides, diverse controlled conditions, such as definitions of VAP, the sample size, the number of centres enrolled in the study, compliance to the prevention bundles, different species, or combinations of bacteria lead to the heterogeneity among the results. The quality and quantity of randomised evidence remain insufficient to draw firm conclusion about the clinical effects of probiotics, neither supporting nor discouraging their systematic administration in critically ill patients.
Our meta-analysis review has several limitations: 1) among the included studies, only seven were double-blind trials, and the remainder were single-blind or open-label trials; 2) two studies were performed on children, whereas the remaining studies were all carried out on adults; 3) the heterogeneity derived from age, race, baseline treatment, dose, and type of strains possibly affected the precision of our conclusions; 4) possible publication bias should not be ignored as we failed to identify articles showing neutral or negative outcomes and unpublished studies; 5) we cannot exclude the possibility that our conclusions are erroneous because some critical information was ignored; and 6) the diverse diagnosis criteria of VAP and admission criteria of the ICU might have contributed to inconsistency. Though there are some limitations to this review, we attempted to eradicate selection bias by selecting the articles that were strictly subjected to inclusion and exclusion criteria. Also, we performed this study by strictly following the Cochrane Handbook for Systematic Reviews of Interventions 5.1 guidelines, which should minimise bias as much as possible.
Conclusion
Our systematic review and meta-analysis demonstrated that probiotics played a positive role in preventing VAP without impacting duration of MV, length of ICU stay, or mortality. Well-designed multicentre trials are also needed to further establish the efficacy of probiotics in prevention of VAP.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00302-2020.SUPPLEMENT (685.5KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com
Author contributions: Bao-ping Tian contributed to the conception and design of this study. Article selection was performed by Jie Zhao and Lei-qing Li. The data from each eligible study were extracted by Cheng-yang Chen and Gen-sheng Zhang. Bao-ping Tian and Wei Cui performed the data analysis. Any dissenting opinion was resolved by a third investigator. All authors reviewed the draft and approved the submission.
Conflict of interest: Jie Zhao has nothing to disclose.
Conflict of interest: Lei-qing Li has nothing to disclose.
Conflict of interest: Cheng-yang Chen has nothing to disclose.
Conflict of interest: Gen-sheng Zhang has nothing to disclose.
Conflict of interest: Wei Cui has nothing to disclose.
Conflict of interest: Bao-ping Tian has nothing to disclose.
Support statement: This work was supported by the National Natural Science Foundation of China (81700023), the Zhejiang Provincial Natural Science Foundation of China (LY20H010002), and the Medical and Health Research Program of Zhejiang Province (2019RC181) to Bao-ping Tian. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Metersky ML, Kalil AC. Management of ventilator-associated pneumonia: guidelines. Clin Chest Med 2018; 39: 797–808. doi: 10.1016/j.ccm.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 2.Schreiber MP, Shorr AF. Challenges and opportunities in the treatment of ventilator-associated pneumonia. Expert Rev Anti Infect 2017; 15: 23–32. doi: 10.1080/14787210.2017.1250625 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Yao Z, Zhan S, et al. Disease burden of intensive care unit-acquired pneumonia in China: a systematic review and meta-analysis. Int J Infect Dis 2014; 29: 84–90. doi: 10.1016/j.ijid.2014.05.030 [DOI] [PubMed] [Google Scholar]
- 4.Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 2013; 13: 665–671. doi: 10.1016/S1473-3099(13)70081-1 [DOI] [PubMed] [Google Scholar]
- 5.Muscedere J, Sinuff T, Heyland DK, et al. The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest 2013; 144: 1453–1460. doi: 10.1378/chest.13-0853 [DOI] [PubMed] [Google Scholar]
- 6.Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 2012; 33: 250–256. doi: 10.1086/664049 [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, de Souza Barros D, Cianferoni S. Diagnosis, management and prevention of ventilator-associated pneumonia: an update. Drugs 2010; 70: 1927–1944. doi: 10.2165/11538080-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 8.Motos A, Yang H, Yang M, et al. Perspectives on synthetic pharmacotherapy for the treatment of nosocomial pneumonia. Expert Opin Pharmacother 2019; 20: 1439–1448. doi: 10.1080/14656566.2019.1617852 [DOI] [PubMed] [Google Scholar]
- 9.Klarin B, Molin G, Jeppsson B, et al. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Crit Care 2008; 12: R136. doi: 10.1186/cc7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loo CY, Lee WH, Young PM, et al. Implications and emerging control strategies for ventilator-associated infections. Expert Rev Anti Infect Ther 2015; 13: 379–393. doi: 10.1586/14787210.2015.1007045 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S, van Heel D, Playford RJ. Probiotics in inflammatory bowel disease: is it all gut flora modulation? Gut 2004; 53: 620–622. doi: 10.1136/gut.2003.034249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med 2010; 38: 954–962. doi: 10.1097/CCM.0b013e3181c8fe4b [DOI] [PubMed] [Google Scholar]
- 13.Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014; 10: CD009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Ruissen MCE, Bos LD, Dickson RP, et al. Manipulation of the microbiome in critical illness-probiotics as a preventive measure against ventilator-associated pneumonia. Intensive Care Med Exp 2019; 7: 37. doi: 10.1186/s40635-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su M, Jia Y, Li Y, et al. Probiotics for the prevention of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Respir Care 2020; 65: 673–685. doi: 10.4187/respcare.07097 [DOI] [PubMed] [Google Scholar]
- 16.Gu WJ, Wei CY, Yin RX. Lack of efficacy of probiotics in preventing ventilator-associated pneumonia probiotics for ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Chest 2012; 142: 859–868. doi: 10.1378/chest.12-0679 [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Liu KX, Ariani F, et al. Probiotics for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of high-quality randomized controlled trials. PLoS ONE 2013; 8: e83934. doi: 10.1371/journal.pone.0083934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, et al. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg 2006; 30: 1848–1855. doi: 10.1007/s00268-005-0653-1 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Chandler J, Cumpston M, et al. eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). https://training.cochrane.org/handbook/archive/v6 [Google Scholar]
- 20.Liberati A, Moher D, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rongrungruang Y. Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J Med Assoc Thai 2015; 98: 253–259. [PubMed] [Google Scholar]
- 22.Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care 2011; 15: R290. doi: 10.1186/cc10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med 2010; 182: 1058–1064. doi: 10.1164/rccm.200912-1853OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu K, Yamada T, Ogura H, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care 2018; 22: 239. doi: 10.1186/s13054-018-2167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng J, Wang CT, Zhang FS, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med 2016; 42: 1018–1028. doi: 10.1007/s00134-016-4303-x [DOI] [PubMed] [Google Scholar]
- 26.Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, et al. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma 2009; 67: 815–821. doi: 10.1097/TA.0b013e31819d979e [DOI] [PubMed] [Google Scholar]
- 27.Knight DJ, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med 2009; 35: 854–861. doi: 10.1007/s00134-008-1368-1 [DOI] [PubMed] [Google Scholar]
- 28.Barraud D, Blard C, Hein F, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med 2010; 36: 1540–1547. doi: 10.1007/s00134-010-1927-0 [DOI] [PubMed] [Google Scholar]
- 29.Forestier C, Guelon D, Cluytens V, et al. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care 2008; 12: R69. doi: 10.1186/cc6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klarin B, Adolfsson A, Torstensson A, et al. Can probiotics be an alternative to chlorhexidine for oral care in the mechanically ventilated patient? A multicentre, prospective, randomised controlled open trial. Crit Care 2018; 22: 272. doi: 10.1186/s13054-018-2209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spindler-Vesel A, Bengmark S, Vovk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr 2007; 31: 119–126. doi: 10.1177/0148607107031002119 [DOI] [PubMed] [Google Scholar]
- 32.Mahmoodpoor A, Hamishehkar H, Asghari R, et al. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: a prospective double-blind randomized controlled trial. Nutr Clin Pract 2019; 34: 156–162. doi: 10.1002/ncp.10191 [DOI] [PubMed] [Google Scholar]
- 33.Li XC, Wang JZ, Liu YH. Effect of probiotics on respiratory tract pathogen colonization in neonates undergoing mechanical ventilation. Chin J Contemp Pediatr 2012; 14: 406–408. [PubMed] [Google Scholar]
- 34.Banupriya B, Biswal N, Srinivasaraghavan R, et al. Probiotic prophylaxis to prevent ventilator associated pneumonia (VAP) in children on mechanical ventilation: an open-label randomized controlled trial. Intensive Care Med 2015; 41: 677–685. doi: 10.1007/s00134-015-3694-4 [DOI] [PubMed] [Google Scholar]
- 35.Oudhuis GJ, Bergmans DC, Dormans T, et al. Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med 2011; 37: 110–117. doi: 10.1007/s00134-010-2002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003; 362: 1011–1016. doi: 10.1016/S0140-6736(03)14409-1 [DOI] [PubMed] [Google Scholar]
- 37.Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med 2006; 173: 1348–1355. doi: 10.1164/rccm.200505-820OC [DOI] [PubMed] [Google Scholar]
- 38.Wittekamp BHJ, Plantinga NL, Leleu K, et al. Oral mucosal adverse events with chlorhexidine 2% mouthwash in ICU. Intensive Care Med 2016; 42: 620–621. doi: 10.1007/s00134-016-4217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua F, Xie H, Worthington HV, et al. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev 2016; 10: CD008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giunta V, Ferrer M, Esperatti M, et al. ICU-acquired pneumonia with or without etiologic diagnosis: a comparison of outcomes. Crit Care Med 2013; 41: 2133–2143. doi: 10.1097/CCM.0b013e31828a453b [DOI] [PubMed] [Google Scholar]
- 41.Besselink MG, van Santvoort HC, Renooij W, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg 2009; 250: 712–719. doi: 10.1097/SLA.0b013e3181bce5bd [DOI] [PubMed] [Google Scholar]
- 42.Fan QL, Yu XM, Liu QX, et al. Synbiotics for prevention of ventilator-associated pneumonia: a probiotics strain-specific network meta-analysis. J Int Med Res 2019; 47: 5349–5374. doi: 10.1177/0300060519876753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook DJ, Johnstone J, Marshall JC, et al. Probiotics: prevention of severe pneumonia and endotracheal colonization trial-PROSPECT: a pilot trial. Trials 2016; 17: 377. doi: 10.1186/s13063-016-1495-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnstone J, Heels-Ansdell D, Thabane L, et al. Evaluating probiotics for the prevention of ventilator-associated pneumonia: a randomised placebo-controlled multicentre trial protocol and statistical analysis plan for PROSPECT. BMJ Open 2019; 9: e025228. doi: 10.1136/bmjopen-2018-025228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuijt TJ, Lankelma JM, Scicluna BP, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016; 65: 575–583. doi: 10.1136/gutjnl-2015-309728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauguet S, D'Ortona S, Ahnger-Pier K, et al. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect Immun 2015; 83: 4003–4014. doi: 10.1128/IAI.00037-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira AT, Rocha VM, Tavares L, et al. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 5(1A). Microbes Infect 2016; 18: 180–189. doi: 10.1016/j.micinf.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 48.Khailova L, Baird CH, Rush AA, et al. Lactobacillus rhamnosus GG improves outcome in experimental Pseudomonas aeruginosa pneumonia: potential role of regulatory T cells. Shock 2013; 40: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tok D, Ilkgul O, Bengmark S, et al. Pretreatment with pro- and synbiotics reduces peritonitis-induced acute lung injury in rats. J Trauma 2007; 62: 880–885. doi: 10.1097/01.ta.0000236019.00650.00 [DOI] [PubMed] [Google Scholar]
- 50.Asahara T, Takahashi A, Yuki N, et al. Protective effect of a synbiotic against multidrug-resistant Acinetobacter baumannii in a murine infection model. Antimicrob Agents Chemother 2016; 60: 3041–3050. doi: 10.1128/AAC.02928-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YN, Youn HN, Kwon JH, et al. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antiviral Res 2013; 98: 284–290. doi: 10.1016/j.antiviral.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 52.Song JA, Kim HJ, Hong SK, et al. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J Microbiol Immunol Infect 2016; 49: 16–23. doi: 10.1016/j.jmii.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 53.Percopo CM, Rice TA, Brenner TA, et al. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antiviral Res 2015; 121: 109–119. doi: 10.1016/j.antiviral.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 2018; 48: 39–49. doi: 10.1002/eji.201646721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAleer JP, Nguyen NL, Chen K, et al. Pulmonary Th17 antifungal immunity is regulated by the gut microbiome. J Immunol 2016; 197: 97–107. doi: 10.4049/jimmunol.1502566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371: 651–659. doi: 10.1016/S0140-6736(08)60207-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00302-2020.SUPPLEMENT (685.5KB, pdf)