Hereditary haemorrhagic telangiectasia (HHT) is a rare autosomal dominant vascular disorder. The prevalence of pulmonary hypertension (PH) in the course of the disease is considered to be lower than 10% [1–4]. As previously reported, the increase in pulmonary arterial pressure in this setting may result from different mechanisms: isolated high flow state; PH due to left heart disease secondary to high cardiac output in the presence of hepatic arteriovenous malformations (post-capillary PH, clinical classification group 2); or pulmonary vascular remodelling (pre-capillary PH) [1–3]. For the latter mechanism, a diagnosis of heritable pulmonary arterial hypertension (group 1.2) could be applicable, as genetic mutations in the transforming growth factor-β (TGF-β) signalling pathway (ALK1, ENG) are always found [5].
Short abstract
Multiple clinical conditions are combined with genetic mutations to contribute to the development of pulmonary vascular remodelling in hereditary haemorrhagic telangiectasia. A systematic aetiological evaluation is required for these patients. https://bit.ly/34V7HPy
To the Editor:
Hereditary haemorrhagic telangiectasia (HHT) is a rare autosomal dominant vascular disorder. The prevalence of pulmonary hypertension (PH) in the course of the disease is considered to be lower than 10% [1–4]. As previously reported, the increase in pulmonary arterial pressure in this setting may result from different mechanisms: isolated high flow state; PH due to left heart disease secondary to high cardiac output in the presence of hepatic arteriovenous malformations (post-capillary PH, clinical classification group 2); or pulmonary vascular remodelling (pre-capillary PH) [1–3]. For the latter mechanism, a diagnosis of heritable pulmonary arterial hypertension (group 1.2) could be applicable, as genetic mutations in the transforming growth factor-β (TGF-β) signalling pathway (ALK1, ENG) are always found [5].
We hypothesised that several causes or risk factors may be associated in this genetically susceptible population to promote the occurrence of pre-capillary PH. Therefore, a study was conducted in HHT patients from a large single-centre cohort. During the study period (1995 to 2018), all eligible patients with a definite diagnosis of HHT referred to the French National Reference Centre for HHT (Lyon, France) were invited to participate in a prospective cohort. Data from pre-capillary PH patients were studied. This registry was set up in agreement with French bioethics laws.
Patients who underwent right heart catheterisation (RHC) had been classified into three haemodynamic profile subgroups according to current definitions of PH [6]: pre-capillary PH (mean pulmonary arterial pressure (mPAP) >20 mmHg, pulmonary artery wedge pressure (PAWP) ≤15 mmHg, pulmonary vascular resistance (PVR) ≥3 Wood Units (WU)), isolated post-capillary PH (mPAP >20 mmHg, PAWP >15 mmHg, PVR <3 WU) and combined pre- and post-capillary PH (mPAP >20 mmHg, PAWP >15 mmHg and PVR ≥3 WU). Clinical data of patients with pre-capillary and combined PH have been reviewed with a focus on aetiological evaluation (pulmonary function tests, arterial blood gases, ventilation/perfusion lung scan, computed tomography, abdominal ultrasound scan and genetic testing).
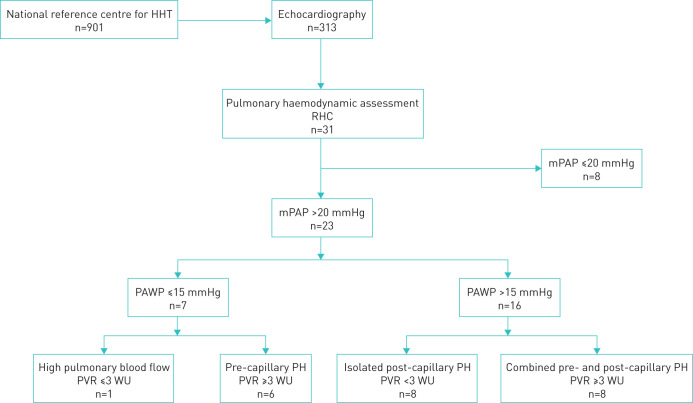
Out of 901 consecutive patients with HHT, 313 underwent echocardiography for arteriovenous malformation screening or for dyspnoea. Depending on the echographic outcomes, 31 RHCs were performed at baseline. Among the 23 patients who had a mPAP>20 mmHg, one had isolated high pulmonary blood flow, eight out of 31 (25.8%) had isolated post-capillary PH, eight out of 31 (25.8%) had combined pre- and post-capillary PH, and six out of 31 (19.3%) had isolated pre-capillary PH (figure 1). Of 14 patients with pre-capillary and combined PH, 12 were female: male-to-female ratio 0.17 versus 0.68 in HHT patients without PH (p=0.047). The mean (sd) age at PH diagnosis was 66.3 (9.9) years. PH was discovered 38 (±52) months after HHT diagnostics were performed.
FIGURE 1.
Flow chart. HHT: hereditary haemorrhagic telangiectasia; RHC: right heart catheterisation; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; PH: pulmonary hypertension: WU: Wood units.
Multiple clinical conditions that could have contributed to PH pathogenesis were identified in 12 out of 14 patients with pre-capillary PH (86%) (table 1): 1) Genetic mutations in the TGF-β signalling pathway (ALK1 n=12, ENG n=2) were present in all cases. Mutation ALK1, exon 8, c.1112 dup was found in eight out of 14 patients (57%). 2) Chronic lung disease with a forced expiratory volume in 1 s (FEV1) <60% or a forced vital capacity (FVC) <70% was found in six patients (linked to three restrictive and three obstructive pulmonary diseases, associated with severe hypoxaemia in two of them). 3) Portal hypertension related to hepatic fibrosis was present in one patient. 4) Four patients also had significant perfusion defects on ventilation – perfusion lung scintigraphy and thromboembolic patterns on computed tomography. 5) A hyperkinetic state was observed in three patients. 6) Left heart failure was further noted in eight individuals (table 1). Overall, six patients (43%) had three or more potential risk factors for PH.
TABLE 1.
Haemodynamic, genetic and clinical characteristics of patients with pre-capillary and combined pulmonary hypertension
| Sex/age years | mPAP mmHg | PAWP mmHg | Cardiac index L·min−1·m−2 | PVR WU | Hb g·dL−1 | Mutation gene, exon, nucleotide, change | AVM | FEV1 L (% pred) | FVC L (% pred) | TLC L (% pred) |
PaO2 mmHg |
CTEPH |
Classification subgroups |
| F/60 | 34 | 14 | 3.8 | 3.3 | 10.6 | ALK1, 8, c.1112, dup | P+H | 0.72 (31%) | 1.58 (56%) | 3.94 (80%) | 50.2 | • | 1.2+3+4 |
| F/67 | 36 | 10 | 4.1 | 3.8 | 10.7 | ENG, 4, c.446, G>A | H | 1.93 (94%) | 2.71 (105%) | 4.33 (91%) | 72.7 | • | 1.2+HS |
| F/46 | 29 | 9 | 3.2 | 3.1 | 14.8 | ALK1, 4, c.470, C>T | H | 1.54 (53%) | 2.01 (58%) | 4.92 (93%) | 93 | 1.2+3 | |
| F/82 | 48 | 9 | 3.1 | 6.8 | 13.8 | ALK1, 8, c.1112, dup | H | 1.80 (84%) | 2.80 (103%) | 5.20 (102%) | 99 | • | 1.2+4 |
| F/70 | 41 | 6 | 2.7 | 8.7 | 9.4 | ALK1, 8, c.1112, dup | H | 1.2 | |||||
| F/68 | 24 | 13 | 2.3 | 3.2 | 11.3 | ALK1, 3, c.102, C>A | P+H | 1.56 (92%) | 2.03 (93%) | 3.68 (85%) | 89.2 | 1.2 | |
| M/77 | 34 | 20 | 1.9 | 4.8 | 12.4 | ENG, 12, c.1522, C>T | 0.86 (43%) | 2.34 (86%) | 4.75 (88%) | 81 | • | 1.2+2+3+4 | |
| M/55 | 53 | 23 | 3.7 | 4.9 | 9.2 | ALK1, 8, c.1112, dup | P | 0.49 (17%) | 0.80 (21.5%) | 2.67 (45%) | 44.2 | 1.2+2+3 | |
| F/71 | 49 | 21 | 4.6 | 4.0 | ALK1, 8, c.1112, dup | P+H | 0.79 (37%) | 1.13 (44%) | 1.2+2+3+HS | ||||
| F/57 | 30 | 18 | 2.1 | 3.4 | 13.1 | ALK1, 7, c.956, G>A | 2.28 (97%) | 3.33 (117%) | 5.66 (117%) | 81 | 1.2+2 | ||
| F/80 | 31 | 18 | 2.6 | 3.1 | 10.9 | ALK1, 8, c.1112, dup | P | 1.2+2 | |||||
| F/65 | 45 | 20 | 2.9 | 4.3 | 8.9 | ALK1, 8, c.1112, dup | H | 2.61(101%) | 3.63 (115%) | 5.97 (111%) | 77.2 | 1.2+2 | |
| F/67 | 38 | 22 | 2.4 | 3.6 | ALK1, 8, c.1112, dup | H | 0.64 (29%) | 0.86 (33%) | 88.5 | 1.2+2+3 | |||
| F/63 | 53 | 28 | 4.3 | 3.1 | 9 | ALK1, 3, c.140, G>C | H | 1.2+1.4.3+2 +HS |
mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR : pulmonary vascular resistance; WU: Wood units; Hb: haemoglobin; AVM: arteriovenous malformation; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; PaO2: arterial oxygen tension; CTEPH: chronic thromboembolic pulmonary hypertension; F: female; M: male; dup: duplication; H: hepatic; P: pulmonary; 1.2: heritable pulmonary hypertension; 1.4.3: portal hypertension; 2: left heart diseases; 3: lung diseases; 4: pulmonary artery obstruction; HS: hyperkinetic state.
The combination of multiple potential risk factors for PH was striking since the vast majority (86%) of patients with PH exhibited multifactorial mechanisms. Therefore, a systematic aetiological evaluation is required to guide management. Even if genetic mutations in the TGF-β signalling pathway can participate in PH development, additional factors might have contributed, especially frequent left heart failure (group 2 PH). Indeed, chronic increase of pulmonary flow due to left-to-right shunt may lead, as in Eisenmenger syndrome, to pulmonary vascular remodelling in the long term [7]. Chronic lung disease and chronic thromboembolic disease may also have significantly increased the risk of PH in this HHT population (group 3 and 4 PH, respectively). It is noteworthy that HHT patients have an increased risk of thrombosis [8, 9]. It has been reported that environmental risk factors such as methamphetamine or dexfenfluramine exposure had contributed to the development of PH in HHT patients [10, 11]. In our study, exposure to drugs and toxins was not systematically assessed and may have been underestimated. These findings are reminiscent of other aetiological contexts in patients with PH, in which several comorbidities and cofactors can contribute to increase the risk of PH – patients with HIV infection, hepatitis liver disease, interferon therapy and illicit drug intake, for example [12].
The large female predominance noticed here has previously been reported [4]. Epidemiological data of both heritable PH and HHT demonstrate female predominance. In some studies, hepatic arteriovenous malformations and ALK1 mutations were also more common in women than in men with HHT [13], leading to the hypothesis that female sex hormones may affect vascular remodelling.
Limitations in our study included the retrospective design, although the data were collected prospectively in a national HHT registry at the time of each hospitalisation. The study was monocentric but included a relatively large cohort of patients from a tertiary referral HHT centre. In this large cohort, only a few subjects underwent an RHC. This was probably due to the fact that transthoracic echocardiography was not performed systematically but only when arteriovenous malformation screening was conducted or when PH was clinically suspected. Therefore, it cannot be excluded that pre-capillary PH could have been underestimated, although results were consistent with previous data [1–4]. Finally, the ALK1 gene mutation c.1112 dup was frequently found due to the founder effect described in the Rhône-Alpes region [14].
In conclusion, multiple clinical conditions combine with genetic mutations to contribute to the development of PH in HHT patients. This finding highlights how critical a systematic aetiological evaluation could be, and various management strategies could result from it.
Acknowledgments
This study was presented as a presentation/abstract publication at the CPLF conference in January 2019, in Marseille, France, and the European Respiratory Society International Congress in September 2019, in Madrid, Spain.
Footnotes
Conflict of interest: V. Margelidon-Cozzolino reports non-financial support for travel to medical meetings from Actelion and Vitalaire outside the submitted work.
Conflict of interest: V. Cottin reports advisory board and lecture fees, and travel support from Actelion; grants, consultancy and lecture fees, and travel support from Boehringer Ingelheim and Roche; advisory board and data safety monitoring board (DSMB) fees from Bayer/MSD and Galapagos; advisory board and lecture fees from Novartis; lecture fees from Sanofi; DSMB and steering committee fees from Promedior; and DSMB fees from Celgene and Galecto, all outside the submitted work.
Conflict of interest: S. Dupuis-Girod has nothing to disclose.
Conflict of interest: J. Traclet reports travel to medical meetings supported by Roche, Boehringer Ingelheim, CSL Behring and Actelion, and lecture fees from Actelion, outside the submitted work.
Conflict of interest: K. Ahmad reports lecture fees and invitations to medical meetings from Actelion and from Roche, and invitations to medical meetings from Boehringer Ingelheim, outside the submitted work.
Conflict of interest: J.F. Mornex reports consultancy fees from LFB Biomedicaments and CSL Behring, lecture fees and travel support from Actelion, consultancy fees from Adene, travel support from Agiradom and Amgen, travel support and lecture fees from Boehringer Ingelheim and Roche, and travel support from Novartis, Pfizer and Vitalaire, outside the submitted work.
Conflict of interest: S. Turquier reports lecture fees and a congress invitation from Actelion, and a congress invitation from Vitalaire, outside the submitted work.
References
- 1.Revuz S, Decullier E, Ginon I, et al. Pulmonary hypertension subtypes associated with hereditary hemorrhagic telangiectasia: hemodynamic profiles and survival probability. PLoS One 2017; 12: e0184227. doi: 10.1371/journal.pone.0184227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyle MA, Fenstad ER, McGoon MD, et al. Pulmonary hypertension in hereditary hemorrhagic telangiectasia. Chest 2016; 149: 362–371. doi: 10.1378/chest.15-0535 [DOI] [PubMed] [Google Scholar]
- 3.Dupuis-Girod S, Cottin V, Shovlin CL. The lung in hereditary hemorrhagic telangiectasia. Respiration 2017; 94: 315–330. doi: 10.1159/000479632 [DOI] [PubMed] [Google Scholar]
- 4.Harder EM, Fares WH. Hospitalizations with hereditary hemorrhagic telangiectasia and pulmonary hypertension in the United States from 2000 to 2014. Respir Med 2019; 147: 26–30. doi: 10.1016/j.rmed.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 5.Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345: 325–334. doi: 10.1056/NEJM200108023450503 [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinovitch M. Pulmonary hypertension: pathophysiology as a basis for clinical decision making. J Heart Lung Transplant 1999; 11: 1041–1053. doi: 10.1016/S1053-2498(99)00015-7 [DOI] [PubMed] [Google Scholar]
- 8.Shovlin CL, Sulaiman NL, Govani FS, et al. Elevated factor VIII in hereditary haemorrhagic telangiectasia (HHT): association with venous thromboembolism. Thromb Haemost 2007; 98: 1031–1039. doi: 10.1160/TH07-01-0064 [DOI] [PubMed] [Google Scholar]
- 9.Riera-Mestre A, Mora-Lujan JM, Trujillo-Santos J, et al. Natural history of patients with venous thromboembolism and hereditary hemorrhagic telangiectasia. Findings from the RIETE registry. Orphanet J Rare Dis 2019; 14: 196. doi: 10.1186/s13023-019-1172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayala E, Kudelko KT, Haddad F, et al. The intersection of genes and environment: development of pulmonary arterial hypertension in a patient with hereditary hemorrhagic telangiectasia and stimulant exposure. Chest 2012; 141: 1598–1600. doi: 10.1378/chest.11-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaouat A, Coulet F, Favre C, et al. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax 2004; 59: 446–448. doi: 10.1136/thx.2003.11890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traclet J, Khouatra C, Piegay F, et al. Pulmonary arterial hypertension in heroin users. J Heart Lung Transplant 2016; 35: 932–934. doi: 10.1016/j.healun.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 13.Girerd B, Montani D, Coulet F, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med 2010; 181: 851–861. doi: 10.1164/rccm.200908-1284OC [DOI] [PubMed] [Google Scholar]
- 14.Lesca G, Genin E, Blachier C, et al. Hereditary hemorrhagic telangiectasia: evidence for regional founder effects of ACVRL1 mutations in French and Italian patients. Eur J Hum Genet 2008; 16: 742–749. doi: 10.1038/ejhg.2008.3 [DOI] [PubMed] [Google Scholar]