Abstract
Background
Although the benefits of sodium–glucose cotransporter 2 inhibitors (SGLT2i) on cardiovascular events have been reported in patients with type 2 diabetes mellitus (T2DM) with or without heart failure (HF), the impact of SGLT2i on cardiac remodelling remains to be established.
Methods
We searched the PubMed, Embase, Cochrane Library and Web of Science databases up to November 16th, 2020, for randomized controlled trials reporting the effects of SGLT2i on parameters of cardiac structure, cardiac function, plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) level or the Kansas City Cardiomyopathy Questionnaire (KCCQ) score in T2DM patients with or without chronic HF. The effect size was expressed as the mean difference (MD) or standardized mean difference (SMD) and its 95% confidence interval (CI). Subgroup analyses were performed based on the stage A–B or stage C HF population and HF types.
Results
Compared to placebo or other antidiabetic drugs, SGLT2i showed no significant effects on left ventricular mass index, left ventricular end diastolic volume index, left ventricular end systolic volume index, or left atrial volume index. SGLT2i improved left ventricular ejection fraction only in the subgroup of HF patients with reduced ejection fraction (MD 3.16%, 95% CI 0.11 to 6.22, p = 0.04; I2 = 0%), and did not affect the global longitudinal strain in the overall analysis including stage A–B HF patients. SGLT2i showed benefits in the E/e’ ratio (MD − 0.45, 95% CI − 0.88 to − 0.03, p = 0.04; I2 = 0%), plasma NT-proBNP level (SMD − 0.09, 95% CI − 0.16 to − 0.03, p = 0.004; I2 = 0%), and the KCCQ score (SMD 3.12, 95% CI 0.76 to 5.47, p = 0.01; I2 = 0%) in the overall population.
Conclusion
The use of SGLT2i was associated with significant improvements in cardiac diastolic function, plasma NT-proBNP level, and the KCCQ score in T2DM patients with or without chronic HF, but did not significantly affect cardiac structural parameters indexed by body surface area. The LVEF level was improved only in HF patients with reduced ejection fraction.
Keywords: Sodium–glucose cotransporter 2 inhibitors, Type 2 diabetes mellitus, Chronic heart failure, Cardiac remodelling
Background
Heart failure (HF) is one of the leading causes of morbidity and mortality worldwide. Type 2 diabetes mellitus (T2DM) can cause diabetic cardiomyopathy, which typically manifests first as left ventricular hypertrophy, diastolic dysfunction, and impaired systolic reserve before gradually showing clinical indications of heart failure with preserved ejection fraction (HFpEF), followed by systolic dysfunction and heart failure with reduced ejection fraction (HFrEF) [1]. T2DM also increases the risk of coronary heart disease and subsequent HF, especially HFrEF [2]. Besides, both in HFrEF and HFpEF patients, comorbid T2DM is associated with a worse prognosis [3–5].
The effects of sodium–glucose cotransporter 2 inhibitors (SGLT2i) on the prognosis (including all-cause death, cardiovascular death, and HF hospitalization) of T2DM [6–9] patients with or without HF [10–12] have been demonstrated in large-scale randomized controlled trials (RCTs) and meta-analyses. Based on clinical evidence, SGLT2i was recommended by the latest guidelines of the American Diabetes Association and the European Association for the Study of Diabetes in patients with T2DM and HF [13], and several agents were recommended by the Heart Failure Association of the European Society of Cardiology in T2DM patients at high cardiovascular risk or with established cardiovascular disease, especially symptomatic HFrEF [14]. However, the mechanism and intermediate links of the drugs remain to be clarified.
Cardiac anatomical and functional parameters partially predict the prognosis and quality of life of patients with T2DM and patients with HF and serve as important surrogate endpoints. Experiments in rodent T2DM models revealed the benefits of SGLT2i on left ventricular hypertrophy [15] and dilation [16], as well as cardiac systolic [15] and diastolic functions [15, 17]. In rodent and porcine nondiabetic HFrEF models, SGLT2i improved left ventricular ejection fraction (LVEF) [18–20] but not diastolic function [20], and showed conflicting results in left ventricular structure [18–21]. In animal models of HFpEF with or without T2DM, SGLT2i improved left ventricular structure [22] and diastolic function [22, 23], but did not affect LVEF [23].
Recent clinical studies have also reported conflicting results. In T2DM patients, the DAPA-LVH trial showed that SGLT2i reversed left ventricular hypertrophy compared to placebo [24], but the EMPA-HEART CardioLink-6 trial showed nonsignificant results [25]. The impacts on LVEF [25, 26], global longitudinal strain (GLS) [24, 27], and diastolic function [25, 28] were also inconsistent in different studies. Similarly, in patients with T2DM and HF, the effects of SGLT2i on left ventricular hypertrophy [27, 29], cardiac function [27, 30, 31], and neurohormonal parameters [32, 33] were inconsistent. Whether such diversity was due to insufficient sample size or heterogeneity among studies remains to be explored.
To make better use of up-to-date clinical evidence, we conducted this meta-analysis to further clarify the effect of SGLT2i on cardiac structure, cardiac function, plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) level and the Kansas City Cardiomyopathy Questionnaire (KCCQ) score in T2DM patients with or without chronic HF. Subgroup analyses were performed based on the stage A–B or stage C HF population and HF types.
Methods
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34].
Search strategy and selection criteria
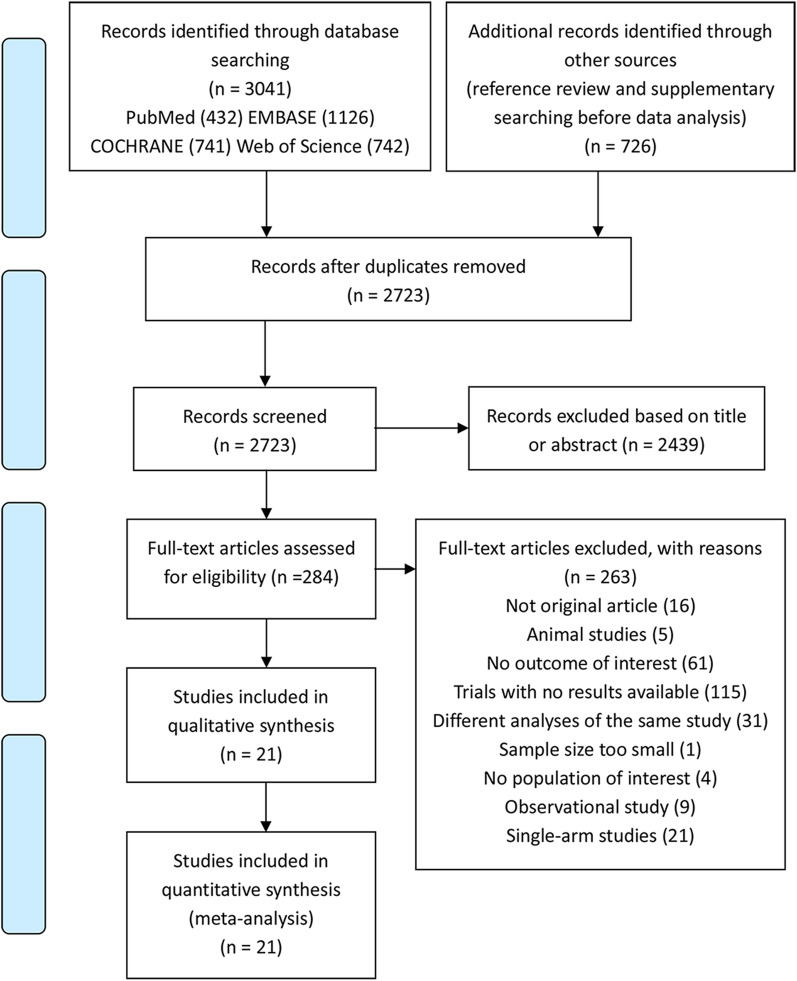
We systematically searched PubMed, Embase, Cochrane Library and Web of Science databases up to November 16th, 2020, using specific MeSH terms and random words with no restriction of language or publication status. The inclusion criteria were as follows: (1) reported the effect of SGLT2i in adult T2DM patients (≥ 18 years) with or without chronic HF; (2) placebo or other antidiabetic agents were accepted as comparison; (3) reported the outcomes of interest; (4) was an RCT; and (5) had complete data for extraction. Observational studies, single-arm studies, studies in acute heart failure patients and studies with a sample size of < 10 were excluded. The reference lists of eligible studies and related articles were reviewed manually to identify additional studies. The main search was conducted on April 21st, 2020, and the supplementary search was performed before data analysis with the same strategy in case of omission. We also sent data request letters by email to the authors of articles with insufficient data for analysis. In the case of two independent reports of the same study, only the one with more complete data was included. Searching details and the flow diagram (including the exclusion criteria) are available in Additional file 1: Data S1 and Fig. 1.
Fig. 1.
PRISMA flow diagram of study selection
Data extraction
The extracted data included (1) general information: title, author, publication year, trial name, eligibility and the reasons; (2) clinical information: age, sex, country or area of the participants; specific agent of the SGLT2 inhibitor given to the experiment group; therapy for the control group; whether the participant was diagnosed as HF at baseline; HF types by reduced or preserved ejection fraction; (3) data for overall effect size calculation: the sample size of each group, as well as the mean value and standard deviation (SD) of the change of outcomes before and after treatment in each group; and (4) methodological information. Data were extracted from the main article reporting the included studies, related articles reporting the same study, and the study registry websites. T2DM patients with an established diagnosis of HF was classified as stage C HF, and those without were classified as stage A–B HF.
Quality assessment of eligible studies
We used the revised Cochrane risk-of-bias tool to assess the quality of the RCTs (see Additional file 2: Figure S1). Publication bias was evaluated by funnel plots (see Additional file 3: Figure S2). Egger’s regression asymmetry test was conducted to assess the significance of funnel plot asymmetries.
We assessed the certainty of the evidence for each outcome using the Grading Recommendations Assessment, Development and Evaluation (GRADE) approach. We used the Guideline Development Tool (https://www.gradepro.org) to formulate the evidence profile table.
Literature search, study selection, data extraction, quality assessment of eligible studies and the GRADE assessment were performed by two researchers (YWY and YHW) independently, and disagreements were resolved by consensus.
Outcomes
The outcomes of this meta-analysis were (1) cardiac anatomic changes including left ventricular mass indexed by body surface area (LVMI), left ventricular end-diastolic volume indexed by body surface area (LVEDVI), left ventricular end-systolic volume indexed by body surface area (LVESVI), and left atrial volume indexed by body surface area (LAVI); (2) cardiac functional changes including LVEF, GLS, and the mitral inflow to mitral relaxation velocity ratio (E/eʹ); (3) changes in plasma NT-proBNP level; and (4) the KCCQ score, or the score of any scale in the questionnaire including the symptom section.
Data analysis
All the variables of interest were continuous and expressed as the mean ± SD. Data reported as the median and interquartile range were transformed to the mean and SD according to the methods suggested by McGrath [35] and Wan [36]. The SD was calculated according to the Cochrane Handbook [37] if results were reported in other forms [p values or confidence intervals (CI)]. The NT-proBNP level reported as the geometric means or geometric mean ratio and 95% CI in three studies were converted to log-transformed scale and analyzed by the generic inverse variance method [38], as sensitivity analysis for the studies reported in the raw scale. The KCCQ score was also analyzed by the generic inverse variance method due to incomplete reporting of the mean ± SD in each group. We used a random-effects model for all the analyses. The effects of SGLT2i on the outcomes were compared between the intervention and comparison arms. Pooled results were expressed as the mean difference (MD) or standardized mean difference (SMD) and its 95% CI. A two-sided P < 0.05 was considered significant. The heterogeneity of the results was assessed using I2 statistics. Sensitivity analyses included heterogeneity analysis using the leave-one-out method, analysis of only high-quality studies, and analysis of only studies using placebo as the control group. Subgroup analyses were performed if each subgroup contains two or more studies, basing on the stage A–B or stage C HF population and the LVEF level in stage C HF patients. All analyses were performed using Review Manager software version 5.4 (The Cochrane Collaboration), R version 3.6.1 (R Foundation for Statistical Computing), and STATA software version 15.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 21 RCTs [10, 24, 25, 29–31, 33, 39–52] were recognized eligible in this meta-analysis, including 3 in crossover design [41, 44, 50]. A total of 10,978 participants were enrolled, including 6236 in the SGLT2i group and 4821 in the control group. Seventy percent of the participants were male, and the mean age ranged from 56 to 73 years old. The mean follow-up period ranged from 14 days to one year, including three studies [41, 50, 51] less than 3 months. Participants with T2DM that were mostly in stage A–B HF were enrolled in 10 studies [24, 25, 39, 40, 44, 46, 48, 49, 51, 52], and patients with T2DM and stage C HF were enrolled in 11 studies [10, 29–31, 33, 41–43, 45, 47, 50]. LVMI, LVEDVI, LVESVI, LAVI, LVEF, GLS, the E/e’ ratio, plasma NT-proBNP level, and the KCCQ score were reported in 6 [24, 25, 29, 44, 45, 52], 3 [25, 29, 30], 3 [25, 29, 30], 4 [25, 29, 45, 52], 9 [24, 25, 29–31, 39, 40, 44, 45], 4 [24, 39, 44, 51], 8 [24, 25, 30, 31, 44, 45, 49, 52], 11 [10, 24, 25, 31, 41, 44–46, 48–50] and 3 [10, 42, 43] studies, respectively. Cardiac structure and function were evaluated by magnetic resonance imaging in 4 studies [24, 25, 29, 51], echocardiography in 8 studies [24, 30, 31, 39, 44, 45, 49, 52], and impedance cardiography in 1 study [40]. The treatment for the control group was placebo in 15 studies [10, 24, 25, 29, 33, 40–44, 46, 47, 50–52], and conventional treatment or other antidiabetic drugs in 6 studies [30, 31, 39, 45, 48, 49]. Baseline characteristics of the eligible studies were presented in Table 1.
Table 1.
Baseline characteristics of eligible studies
| Author | Publication year | Study design | Trial | Country/area | Intervention in treatment and control arms | Population | Overall sample size (n) | Follow-up period | Age (year, mean ± SD) | Sex (male%) | Imaging | Parameters |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Januzzi | 2017 | RCT | – | Multiple countries and areas | Canagliflozin 100 mg/day or 300 mg/day; placebo | Older patients with T2DM | 666 | 52 weeks | 63.74 ± 6.31 | 57.27% | – | NT-proBNP |
| Bonora | 2019 | RCT | DAPA-HDL | Italy | Dapagliflozin 10 mg/day; placebo | T2DM, excluding HF patients with NYHA classes III-IV | 30 | 12 weeks | 63.4 ± 6.9 | 66.70% | ICG | LVEF |
| Brown | 2020 | RCT | DAPA-LVH | UK | Dapagliflozin 10 mg/day; placebo | T2DM, excluding patients diagnosed as clinical HF | 66 | 12 months | 65.53 ± 6.87 | 57.60% | MRI; ECHO | LVMI, LVEF, GLS, E/e', NT-proBNP |
| Ikonomidis | 2020 | RCT | – | Greece | SGLT2i; standard care without SGLT2i | T2DM | 160 | 12 months | 58 ± 10 | 72% | ECHO | LVEF, GLS |
| Katakami | 2020 | RCT | UTOPIA | Japan | Tofogliflozin 20 mg/day; conventional drugs | T2DM | 340 | 52 weeks | 61.10 ± 9.49 | 58.40% | – | NT-proBNP |
| Kayano | 2020 | RCT | – | Japan | Dapagliflozin 5 mg/day; conventional therapy | T2DM candidates with hypertension (grade 1 or 2) and/or a history of ischemic heart disease | 74 | 6 months | 67.65 ± 8.53 | 89.18% | ECHO | E/eʹ, NT-proBNP |
| Oldgren | 2020 | RCT | DAPACARD | Sweden and Finland | Dapagliflozin 10 mg/day; placebo | T2DM with normal left ventricular ejection fraction (≥ 50%) assessed within 1 year | 49 | 6 weeks | 64.4 ± 7.2 | 53% | MRI | GLS |
| Shim | 2020 | RCT | IDDIA | Korea | Dapagliflozin 10 mg/day; placebo | T2DM and LV diastolic dysfunction | 60 | 24 weeks | – | – | ECHO | LVMI, LAVI, E/eʹ |
| Verma | 2019 | RCT | EMPA-HEART CardioLink-6 | Canada | Empagliflozin 10 mg/day; placebo | T2DM and CAD, excluding patients with an LVEF < 30%, NYHA class IV or hospitalized for decompensated HF within the preceding 3 months | 97 | 6 months | 67.6 ± 6.6 | 80% | MRI | LVMI, LVEDVI, LVESVI, LAVI, LVEF, E/eʹ, NT-proBNP |
| Anker | 2020 | RCT | EMPEROR-Reduced | Multiple countries and areas | Empagliflozin 10 mg/day; placebo | T2DM and CHF | 1856 | 52 weeks | 66.70 ± 10.15 | 76.90% | – | KCCQ, NT-proBNP |
| Bhatt | 2020 | RCT | SOLOIST-WHF | Multiple countries and areas | Sotagliflozin 200 mg/day; placebo | T2DM recently hospitalized for worsening heart failure | 1222 | 4 months | 69.90 ± 9.34 | 66.24% | – | KCCQ |
| Carbone | 2020 | RCT | CANA-HF | US | Canagliflozin 100 mg/day; sitagliptin 100 mg/day | T2DM and HFrEF | 36 | 12 weeks | 56.1 ± 7.8 | 77.77% | ECHO | LVEDVI, LVESVI, LVEF, E/eʹ |
| de Boer | 2020 | RCT | – | Multiple countries and areas | Empagliflozin 25 mg/day; placebo | T2DM and CHF | 63 | 12 weeks | 68.02 ± 9.10 | 61.93% | – | NT-proBNP |
| Eickhoff | 2020 | RCT (crossover) | DapKid | Denmark | Dapagliflozin 10 mg/day; placebo | T2DM and CHF | 40 | 12 weeks | 64 ± 8 | 89% | ECHO | LVMI, LVEF, GLS, E/eʹ, NT-proBNP |
| Ejiri | 2020 | RCT | MUSCAT-HF | Japan | Luseogliflozin 2.5 mg/day; voglibose | T2DM and HFpEF | 165 | 12 weeks | 73.1412 ± 7.8130 | 62.52% | ECHO | LVMI, LAVI, LVEF, E/eʹ, NT-proBNP |
| Griffin | 2020 | RCT (crossover) | – | US | Empagliflozin 10 mg/day; placebo | T2DM and HF | 20 | 14 days | 60 ± 12 | 75% | – | NT-proBNP |
| Januzzi | 2020 | RCT | CANVAS; CANVAS-R | Multiple countries and areas | Canagliflozin 100 or 300 mg/day; placebo | T2DM and high risk for cardiovascular events | 3587 | 1 year | 62.6999 ± 7.8670 | 67% | – | NT-proBNP |
| Mordi | 2020 | RCT (crossover) | RECEDE-CHF | UK | Empagliflozin 25 mg/day; placebo | T2DM and CHF | 23 | 6 weeks | 69.8 ± 5.7 | 73.90% | – | NT-proBNP |
| Petrie | 2020 | RCT | DAPA-HF | Multiple countries and areas | Dapagliflozin 10 mg/day; placebo | T2DM and HFrEF | 2139 | 8 months | 66.50 ± 9.85 | 77.70% | – | NT-proBNP, KCCQ |
| Singh | 2020 | RCT | REFORM | UK | Dapagliflozin 10 mg/day; placebo | T2DM and CHF | 56 | 1 year | 67.1 | 66.10% | MRI | LVMI, LVEDVI, LVESVI, LAVI, LVEF |
| Tanaka | 2020 | RCT | CANDLE | Japan | Canagliflozin 100 mg/day; glimepiride 0.5 to 6.0 mg/day | T2DM and HF | 233 | 24 weeks | 68.6 ± 10.1 | 74.71% | ECHO | LVEF, E/e', NT-proBNP |
RCT randomized controlled trial, SGLT2i sodium–glucose cotransporter 2 inhibitors, T2DM type 2 diabetes mellitus, HF heart failure, CHF chronic heart failure, HFrEF heart failure with reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, LVEF left ventricular ejection fraction, CAD coronary artery disease, CV cardiovascular, NYHA New York Heart Association, MRI magnetic resonance imaging, ECHO echocardiography, ICG impedance cardiography, LVMI left ventricular mass indexed by body surface area, LVEDVI left ventricular end diastolic volume indexed by body surface area, LVESVI left ventricular end systolic volume indexed by body surface area, LAVI left atrial volume indexed by body surface area, GLS global longitudinal strain: E/e' mitral inflow to mitral relaxation velocity ratio, NT-proBNP N-terminal pro-brain natriuretic peptide, KCCQ the Kansas City Cardiomyopathy Questionnaire, UK the United Kingdom, US the United States
Results of the main analyses and sensitivity analyses
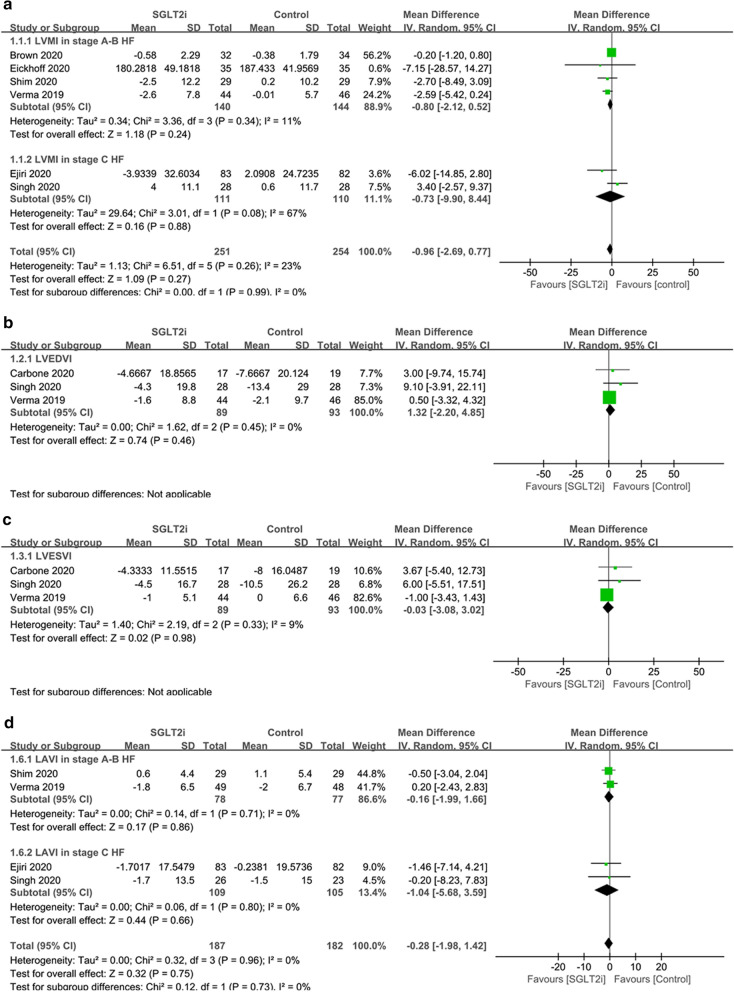
The use of SGLT2i showed no significant effect on LVMI compared with placebo or other antidiabetic drugs in T2DM patients with or without HF (MD -0.96 g/m2, 95% CI − 2.69 to 0.77, p = 0.27; I2 = 23%) (Fig. 2). LVEDVI, LVESVI and LAVI were also not significantly changed by the use of SGLT2i compared to the control group in the overall population (MD 1.32 ml/m2, 95% CI −2.20 to 4.85, p = 0.46; I2 = 0%; MD −.03 ml/m2, 95% CI −3.08 to 3.02, p = 0.98; I2 = 9%; MD −.28 ml/m2, 95% CI − 1.98 to 1.42, p = 0.75; I2 = 0%) (Fig. 2).
Fig. 2.
Forest plots of the effects of SGLT2i on cardiac structure indexed by body surface area. a LVMI; b LVEDVI; c LVESVI; d LAVI. SGLT2i sodium–glucose cotransporter 2 inhibitors, T2DM type 2 diabetes mellitus, HF heart failure, LVMI left ventricular mass indexed by body surface area, LVEDVI left ventricular end diastolic volume indexed by body surface area, LVESVI left ventricular end systolic volume indexed by body surface area, LAVI left atrial volume indexed by body surface area
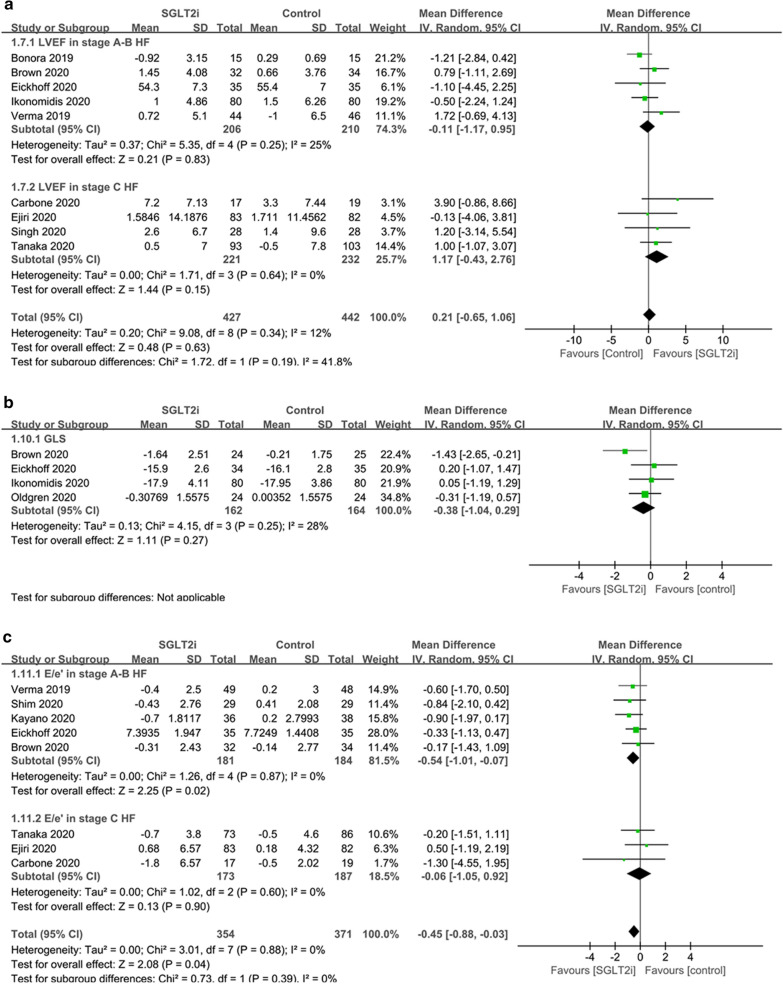
As for systolic function, SGLT2i did not have a significant effect on LVEF (MD 0.21%, 95% CI − 0.65 to 1.06, p = 0.63; I2 = 12%) (Fig. 3) or GLS (MD − 0.38%, 95% CI − 1.04 to 0.29, p = 0.27; I2 = 28%) (Fig. 3) in the overall population. For left ventricular diastolic function, the use of SGLT2i was associated with a reduction of the E/eʹ ratio (MD − 0.45, 95% CI − 0.88 to − 0.03, p = 0.04; I2 = 0%) (Fig. 3). Sensitivity analysis including only the 7 high-quality studies showed a similar reduction of the E/eʹ ratio by SGLT2i, and analysis including the 4 placebo-controlled studies showed insignificant results.
Fig. 3.
Forest plots of the effects of SGLT2i on cardiac function. a LVEF; b GLS; c E/eʹ. SGLT2i sodium–glucose cotransporter 2 inhibitors, T2DM type 2 diabetes mellitus, HF heart failure, LVEF left ventricular ejection fraction, GLS global longitudinal strain, E/eʹ mitral inflow to mitral relaxation velocity ratio
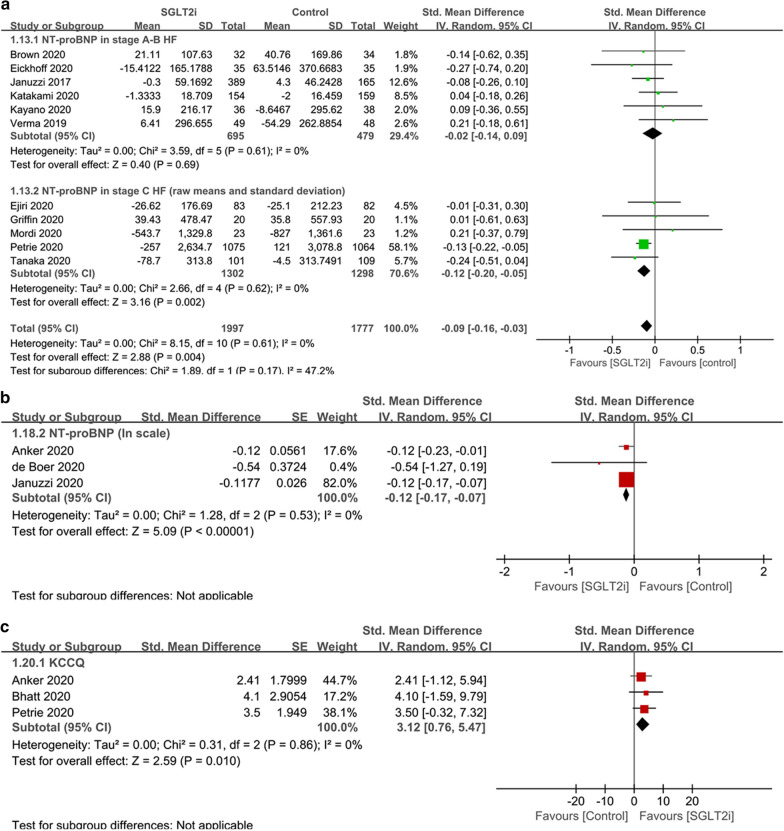
The use of SGLT2i reduced the plasma NT-proBNP levels (SMD − 0.09, 95% CI − 0.16 to − 0.03, p = 0.004; I2 = 0%) (Fig. 4) in the overall population. The three studies reporting data in the geometric scales could not be pooled with those reporting data in the raw scale, thus served as sensitivity analysis, and showed consistent results as in the main analysis (SMD − 0.12, 95% CI − 0.17 to − 0.07, p < 0.00001; I2 = 0%) (Fig. 4). Other sensitivity analyses included only the 9 high quality studies and only the 7 placebo-controlled studies, both showed consistent results with the main analysis.
Fig. 4.
Forest plots of the effects of SGLT2i on a NT-proBNP and b KCCQ score. SGLT2i sodium–glucose cotransporter 2 inhibitors, T2DM type 2 diabetes mellitus, HF heart failure, NT-proBNP N-terminal pro-brain natriuretic peptide, KCCQ the Kansas City Cardiomyopathy Questionnaire
The KCCQ score was significantly improved by SGLT2i compared with placebo or other antidiabetic drugs (SMD 3.12, 95% CI 0.76 to 5.47, p = 0.01; I2 = 0%) (Fig. 4). The KCCQ items used were different among the three eligible trials, including the total symptom score in the DAPA-HF trial, the total symptom score and physical limitation score in the EMPEROR-Reduced trial, and the KCCQ-12 items score in the SOLOIST-WHF trial. All the trials were placebo-controlled and of high quality, so sensitivity analysis was not conducted.
Results of subgroup analyses
Subgroup analyses of LVMI and LAVI based on stage A–B or stage C HF population showed insignificant results. We did not conduct subgroup analysis in LVEDVI and LVESVI because only three studies reported the outcomes.
LVEF was not significantly changed by the use of SGLT2i compared to placebo or other antidiabetic drugs in subgroup analysis based on stage A–B or stage C HF population. Nevertheless, in subgroup analyses in stage C HF patients based on HF types, SGLT2i was related to improved LVEF in HFrEF patients (MD 3.16%, 95% CI 0.11 to 6.22, p = 0.04; I2 = 0%), but was insignificant in HFpEF patients (MD 0.19%, 95% CI − 1.76 to 2.15, p = 0.85; I2 = 0%) (Additional file 4: Figure S3). All the studies reporting GLS were in stage A–B HF patients with T2DM, so subgroup analysis was not conducted. SGLT2i improved the E/e’ ratio in stage A–B HF population (MD − 0.54, 95% CI − 1.01 to − 0.07, p = 0.02; I2 = 0%) but not in stage C HF population (MD − 0.06, 95% CI − 1.05 to 0.92, p = 0.9; I2 = 0%) (Fig. 3). In stage C HF patients, SGLT2i did not significantly affect the E/e’ ratio in both the HFrEF (MD − 0.33, 95% CI − 2.76 to 2.10, p = 0.79; I2 = 0%) and HFpEF (MD − 0.19, 95% CI − 1.23 to 0.85, p = 0.72; I2 = 2%) groups (Additional file 4: Figure S3).
The use of SGLT2i reduced the NT-proBNP level in stage C HF population (SMD − 0.12, 95% CI − 0.20 to − 0.05, p = 0.002; I2 = 0%) but not in stage A–B HF population (SMD − 0.02, 95% CI − 0.14 to 0.09, p = 0.69; I2 = 0%) (Fig. 4). In stage C HF patients, SGLT2i significantly improved the NT-proBNP level in the HFrEF subgroup (SMD − 0.14, 95% CI − 0.22 to − 0.05, p = 0.001; I2 = 0%) but not in the HFpEF subgroup (SMD − 0.07, 95% CI − 0.29 to 0.14, p = 0.51; I2 = 0%) (Additional file 4: Figure S3).
All the three studies reporting the KCCQ score were conducted in stage C HF patients with T2DM and subgroup analysis was not performed.
Quality assessment and publication bias
Quality assessments of each of the RCTs are shown in Additional file 2: Figure S1. Among the 21 RCTs included in this meta-analysis, 14 were considered to be at low risk, 3 with some concerns, and 4 were at high risk, which was mainly driven by the open-label design in the studies by Tanaka et al. and Katakami et al., and the high missing rate in the studies by Ikonomidis et al. and de Boer et al. The results of publication bias assessment are shown in Additional file 3: Figure S2. According to the results of Egger’s asymmetry test, there was no obvious publication bias in any of the analyses (p > 0.05).
According to the GRADE evidence profile (Table 2), the certainty of the evidence was moderate for most of the outcomes, except for LVEF in HFrEF population, which showed a low certainty mostly driven by the high risk of bias in the study by Tanaka et al.; and for the KCCQ score, which showed a high certainty.
Table 2.
GRADE evidence profile
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | SGLT2i | Control | Relative (95% CI) | Absolute (95% CI) | ||
| LVMI | ||||||||||||
| 6 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 251 | 254 | – | MD 0.96 lower (2.69 lower to 0.77 higher) |
⨁⨁⨁◯ Moderate |
Important |
| LVEDVI | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 89 | 93 | – | MD 1.32 higher (2.20 lower to 4.85 higher) |
⨁⨁⨁◯ Moderate |
Important |
| LVESVI | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 89 | 93 | – | MD 0.03 lower (3.08 lower to 3.02 higher) |
⨁⨁⨁◯ Moderate |
Important |
| LAVI | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 187 | 182 | – | MD 0.28 lower (1.98 lower to 1.42 higher) |
⨁⨁⨁◯ Moderate |
Important |
| LVEF | ||||||||||||
| 9 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 427 | 442 | - | MD 0.21 higher (0.65 lower to 1.06 higher) |
⨁⨁⨁◯ Moderate |
Important |
| LVEF in HFrEF | ||||||||||||
| 2 | Randomized trials | Serious b | Not serious | Not serious | Seriousa | None | 70 | 74 | – | MD 3.16 higher (0.11 higher to 6.22 higher) |
⨁⨁◯◯ Low |
Important |
| LVEF in HFpEF | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 151 | 158 | – | MD 0.19 higher (1.76 lower to 2.15 higher) |
⨁⨁⨁◯ Moderate |
Important |
| GLS | ||||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 162 | 164 | – | MD 0.38 lower (1.04 lower to 0.29 higher) |
⨁⨁⨁◯ Moderate |
Important |
| E/eʹ | ||||||||||||
| 8 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 354 | 371 | – | MD 0.45 lower (0.88 lower to 0.03 lower) |
⨁⨁⨁◯ Moderate |
Important |
| NT-proBNP | ||||||||||||
| 11 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 1997 | 1777 | – | SMD 0.09 lower (0.16 lower to 0.03 lower) |
⨁⨁⨁◯ Moderate |
Critical |
| NT-proBNP (ln scale) | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Seriousa | None | 2702 | 1629 | – | SMD 0.12 lower (0.17 lower to 0.07 lower) |
⨁⨁⨁◯ Moderate |
Critical |
| KCCQ | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 2610 | 2607 | – | SMD 3.12 higher (0.76 higher to 5.47 higher) |
⨁⨁⨁⨁ High |
Critical |
SGLT2i sodium–glucose cotransporter 2 inhibitors, MD mean difference, SMD standardized mean difference, HFrEF heart failure with reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, LVMI left ventricular mass indexed by body surface area, LVEDVI left ventricular end diastolic volume indexed by body surface area, LVESVI left ventricular end systolic volume indexed by body surface area, LAVI left atrial volume indexed by body surface area, LVEF left ventricular ejection fraction, GLS global longitudinal strain, E/e' mitral inflow to mitral relaxation velocity ratio, NT-proBNP N-terminal pro-brain natriuretic peptide, KCCQ the Kansas City Cardiomyopathy Questionnaire
aSample size below optimal information size contributing to imprecision which lowers our certainty in effect
bOne in the two studies is of high risk according to the Cochrane risk of bias tool. And excluding the study would cause change of the results
Discussion
This meta-analysis comprehensively and quantitively analyses the effects of SGLT2i on cardiac structure, cardiac function, plasma NT-proBNP level and the KCCQ score in T2DM patients with or without chronic HF. The main findings of this study included the following: (1) SGLT2i showed no significant effects on LVMI, LVEDVI, LVESVI, and LAVI; (2) SGLT2i improved LVEF in HFrEF patients but not in HFpEF patients or stage A–B HF patients with T2DM, and showed no significant effects on GLS in stage A–B HF patients with T2DM; (3) SGLT2i reduced the E/e’ ratio in the overall population and stage A–B HF patients but not in stage C HF patients; (4) SGLT2i improved the plasma NT-proBNP level in the overall population and stage C HF patients, and showed no significant results in stage A–B HF patients; and (5) SGLT2i improved the KCCQ score in stage C HF patients with T2DM.
Our searching and analysis results on the LVM, LVEDV, and LVESV were the same as those reported in a recently published meta-analysis [53], thus were not presented in this article. Pooled analysis of two studies [24, 25] reporting LVM measured by MRI in stage A–B HF population showed a significant reduction after the use of SGLT2i compared to placebo or other antidiabetic drugs (MD − 3.04 g, 95% CI − 5.14 to − 0.94, p = 0.005; I2 = 0%). The inconsistency in the results of SGLT2i regarding LVM and LVMI may be attributed to the concomitant effect of weight loss, which was also observed in studies included in our analysis and a previous meta-analysis [24, 29, 31, 39, 54]. Since LVMI was calculated by LVM indexed by body surface area (BSA), which was influenced by both temporal height and weight of the individual, weight loss would obscure the estimation of the actual anatomical change of the heart. This was previously discussed in the study by Brown et al. [24], showing that SGLT2i significantly reduced LVM as well as LVM indexed by height or baseline BSA but not that indexed by real-time BSA. LVM was demonstrated to be a risk factor for the decline of LVEF [55] as well as all-cause and cardiovascular mortality [56] in stage A–B HF. The decrease of LVM might be related to the reduction of the incidence of stage C HF observed in previous RCTs. Despite larger sample sizes than the studies reporting LVM, the use of SGLT2i showed no significant effects on LVEDV, LVESV, LVEDVI, LVESVI, and LAVI, suggesting a null or faint effect of the drug on the dilation of cardiac chambers. Since the increase of LVM usually reflects both enlargement of the left ventricle and thickening of the walls, the results above may imply an effect of SGLT2i on the wall thickness rather than on the ventricle volume, which is to be demonstrated in future studies.
Taken together, the results of the overall and subgroup analyses suggested that SGLT2i significantly reduced LVEF in HFrEF patients but not in HFpEF patients, showing benefits in patients with obvious systolic dysfunction. However, the results in HFrEF subgroup suffered from a low certainty in the GRADE evidence profile, calling for more future studies in the population. The effect of SGLT2i on GLS, a more sensitive parameter reflecting even mild systolic dysfunction [57–59], was not significant in the pooled analysis. Nevertheless, GLS was reported in four RCTs in stage A–B HF patients with T2DM but not yet in stage C HF patients. Ongoing trials such as ERTU-GLS (NCT03717194) in the T2DM and stage C HF population would provide more evidence. As for diastolic dysfunction, the E/eʹ ratio was reduced by SGLT2i in the overall population and stage A–B HF patients, but not in stage C HF patients. The discrepancy between the subgroups could be due to the mild and more reversible impairment of the diastolic dysfunction in stage A–B HF patients, whereas large-scale trials are still needed.
The use of SGLT2i significantly reduced the plasma NT-proBNP level in the stage C HF population. However, the effect on NT-proBNP level between the SGLT2i and control group was − 333 pg/ml in the T2DM subgroup in the DAPA-HF trial [10] (median baseline level in the SGLT2i group: 1479 pg/ml), and − 103 pg/ml in the whole population of EMPEROR-Reduced trial [42] (median baseline level in the SGLT2i group: 1894 pg/ml) declaring no significant difference in patients with and without T2DM. Those changes were moderate and inconsistent with the remarkable influence of SGLT2i on the cardiovascular events [60], suggesting that NT-proBNP could not be considered to be a satisfying surrogate endpoint for efficacy assessment in this case.
In the pre-SGLT2i age, the change of NT-proBNP level used to be expected to predict the effect size of HF therapy on cardiovascular outcomes. One meta-analysis [61] suggested a significant association between changes in NT-proBNP level and the risk of hospital stay for HF worsening. In the PARADIGM-HF trial [62], the use of angiotensin receptor-neprilysin inhibitor in HFrEF patients induced a 30% decline in NT-proBNP level after the run-in period of 4–6 weeks, and the reduction was associated with the change in cardiovascular mortality and HF hospitalization rate. However, the relationship was less strong in the PARAGON-HF trial [63] in HFpEF patients, which showed a considerable effect of SGLT2i on the reduction of NT-proBNP but a moderate effect on the primary outcome in the subgroups of men and patients with higher LVEF. Moreover, the termination of the GUIDE-IT trial [64] due to futility suggested against the add-on NT-proBNP-guided strategy versus guideline-directed medical therapy alone in HFrEF patients. Updated evidence from the trials in SGLT2i further supported the view that the NT-proBNP could not be used generally as a predictor of the hard endpoints, but may be indicative for specific drugs or in certain subgroups of patients.
Pooled results of the three large-scale RCTs reporting the KCCQ score showed significant improvement by SGLT2i compared with placebo in T2DM patients with stage C HF. As for the magnitude of the effect, analysis of the T2DM subgroup in DAPA-HF trial [10] showed that more patients reported an increase of at least 5 points in the SGLT2i group compared with the placebo group (58.9% vs 49.9%), yielding a number needed to treat of 14 patients with dapagliflozin for one to be clinically better in eight months, which showed a considerable benefit [65]. The MD in the change of the KCCQ score was 4.1 points (95% CI 1.3 to 7.0) in the SOLOIST-WHF trial and 2.41 (95% CI 0.64 to 4.17) in the T2DM subgroup in EMPEROR-Reduced trial, but the numbers needed to treat were not calculable. The benefit on symptoms and quality of life associated with SGLT2i was consistent with the noteworthy reduction in the risk of hospitalization for heart failure in the T2DM subgroup of the DAPA-HF and EMPEROR-Reduced trials.
Despite the clinically significant improvement of quality of life and cardiovascular outcomes by SGLT2i, the debate on the underlying mechanism of the drug is still on the way. The most known mechanism of SGLT2i is based on excess excretion of fluid and glucose and modest removal of sodium [66]. Diuresis alleviates cardiac preload, leading to reduced blood pressure [67], left ventricular wall stress, and left ventricular filling pressure. This could be the reason for the reduction of the NT-proBNP level and the E/e’ ratio that we have observed. However, the significant effect of SGLT2i on LVM but not ventricular volume could not be fully interpreted by the theory above. Other possible mechanisms such as more efficient energy source of ketone bodies and fatty acids rather than glucose [19, 68], relieving inflammation [69, 70], and reducing fibrosis and oxidative stress [15], may also play a role. The previously prompted hypothesis of the inhibition of cardiac Na+–H+ Exchanger-1 was however challenged in a recent in vitro study [71]. Still, further research is required to illuminate the complete picture.
Previous systemic and narrative reviews [72–75] summarized completed and ongoing studies available on the same topic as ours. However, they were mostly conducted before the releasing of results of several important recent studies and thus lacked sufficient data for quantitative analyses. This meta-analysis included only RCTs but not observational studies to minimize the possible risk of bias, and used the GRADE tool to assess the certainty of the evidence for each outcome. Although conducted strictly following the PRISMA guidelines, the meta-analysis still has some limitations. First, in subgroup analyses, we stratified the T2DM population as stage A–B and stage C HF patients. But in some studies recognized as stage A–B HF, HF patients were not fully excluded. Second, heterogeneity in clinical characteristics and study methods was not completely avoidable, in consideration of which we used a random-effects model for all the analyses. Third, subgroup analyses based on the dosage forms of SGLT2i and the modality of imaging were not conducted due to insufficient data, which remain to be clarified in future studies.
Large-scale RCTs focusing on the effects of SGLT2i in different populations are required to provide more evidence for individualized intervention. The results of the ongoing EMPA-TROPISM (NCT03485222) [76], EMPA-HEART (EUDRACT 2016-0022250-10) [77], ERTU-GLS (NCT03717194), NATRIURETIC (NCT04535960), VERTICAL (NCT04490681), EMPERIAL-Preserved and EMPERIAL-Reduced (NCT03448406, NCT03448419) [78] trials would enhance knowledge of this topic. Although the efficacy and safety of SGLT2i in several dosage forms have been repeatedly verified in T2DM patients with or without HF to support the clinical application, the underlying mechanism remains to be clarified to achieve a more comprehensive understanding.
Conclusion
We found in this meta-analysis that SGLT2i improves the parameters of cardiac diastolic function, plasma NT-proBNP level, and the KCCQ score in T2DM patients with or without chronic HF, but did not significantly affect cardiac structural parameters indexed by body surface area. The LVEF level was improved only in HF patients with reduced ejection fraction. Future studies are anticipated to further elucidate the mechanisms and intermediate links in the effect of SGLT2i.
Supplementary Information
Additional file 1: Data S1. Strategy for the main search conducted on April 21st, 2020.
Additional file 2: Figure S1. Quality assessment of RCTs using the revised Cochrane risk-of-bias tool. (a) Risk of bias graph; (b) Risk of bias summary.
Additional file 3: Figure S2. Funnel plots for publication bias assessment. (a) LVMI; (b) LVEDVI; (c) LVESVI; (d) LAVI; (e) LVEF; (f) GLS; (g) E/e’; (h) NT-proBNP; (i) KCCQ.
Additional file 4: Figure S3. Subgroup analyses of the effects of SGLT2i on (a) LVEF in HFrEF vs. HFpEF patients; (b) E/e’ in HFrEF vs. HFpEF patients; (c) NT-proBNP in HFrEF vs. HFpEF patients. Abbreviations: SGLT2i: sodium-glucose cotransporter 2 inhibitors; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; LVEF: left ventricular ejection fraction; E/e': mitral inflow to mitral relaxation velocity ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Acknowledgements
We would like to express our sincere gratitude to Tian-Yu Xu and You-Nan Yao from the Heart Failure Center, Fuwai Hospital, for providing helpful comments on the manuscript; and Yang Wang from the Medical Research and Biometrics Center, National Center for Cardiovascular Diseases, for constructive advice on the statistical analysis.
Abbreviations
- HF
Heart failure
- T2DM
Type 2 diabetes mellitus
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- SGLT2i
Sodium–glucose cotransporter 2 inhibitors
- RCT
Randomized controlled trial
- LVEF
Left ventricular ejection fraction
- GLS
Global longitudinal strain
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- KCCQ
The Kansas City Cardiomyopathy Questionnaire
- PRISMA
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SD
Standard deviation
- GRADE
The Grading Recommendations Assessment, Development and Evaluation
- LVMI
Left ventricular mass indexed by body surface area
- LVEDVI
Left ventricular end diastolic volume indexed by body surface area
- LVESVI
Left ventricular end systolic volume indexed by body surface area
- LAVI
Left atrial volume indexed by body surface area
- E/e'
The mitral inflow to mitral relaxation velocity ratio
- CI
Confidence interval
- MD
Mean difference
- SMD
Standardized mean difference
- BSA
Body surface area
Authors’ contributions
YWY designed the study, conducted the data collection and analysis, and wrote the manuscript. XMZ assisted in study design, data analysis and interpretation. YHW assisted in data collection and analysis. QZ, YH, and MZ substantively reviewed and edited the intellectual content. JZ revised the study design and the manuscript critically, and gave final approval of the version to be published. All authors read and approved the final manuscript.
Funding
This work was supported by the Key Projects in the National Science and Technology Pillar Program of the 13th Five-Year Plan Period (grant number 2017YFC1308300 to Jian Zhang), Beijing, People’s Republic of China.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-020-01209-y.
References
- 1.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 3.McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, et al. Heart failure with preserved ejection fraction and diabetes: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;73(5):602–611. doi: 10.1016/j.jacc.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 5.Dauriz M, Mantovani A, Bonapace S, Verlato G, Zoppini G, Bonora E, et al. Prognostic impact of diabetes on long-term survival outcomes in patients with heart failure: a meta-analysis. Diabetes Care. 2017;40(11):1597–1605. doi: 10.2337/dc17-0697. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 9.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 10.Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8(3):192–205. doi: 10.1016/S2213-8587(19)30422-X. [DOI] [PubMed] [Google Scholar]
- 12.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet (London, England) 2020;396(10254):819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 13.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seferovic PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, Jhund PS, et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur J Heart Fail. 2020;22(2):196–213. doi: 10.1002/ejhf.1673. [DOI] [PubMed] [Google Scholar]
- 15.Li CG, Zhang J, Xue M, Li XY, Han F, Liu XY, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18:15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TI, Chen YC, Lin YK, Chung CC, Lu YY, Kao YH, et al. Empagliflozin attenuates myocardial sodium and calcium dysregulation and reverses cardiac remodeling in streptozotocin-induced diabetic rats. Int J Mol Sci. 2019;20(7):1680. doi: 10.3390/ijms20071680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16(1):9. doi: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurista SR, Sillje HHW, Oberdorf-Maass SU, Schouten EM, Giani MGP, Hillebrands JL, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21(7):862–873. doi: 10.1002/ejhf.1473. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Gallego CG, Requena-Ibanez JA, Antonio RS, Ishikawa K, Watanabe S, Picatoste B, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G, et al. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci. 2017;2(4):347–354. doi: 10.1016/j.jacbts.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connelly KA, Zhang Y, Desjardins JF, Nghiem L, Visram A, Batchu SN, et al. Load-independent effects of empagliflozin contribute to improved cardiac function in experimental heart failure with reduced ejection fraction. Cardiovasc Diabetol. 2020;19(1):13. doi: 10.1186/s12933-020-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connelly KA, Zhang Y, Visram A, Advani A, Batchu SN, Desjardins JF, et al. Empagliflozin improves diastolic function in a nondiabetic rodent model of heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2019;4(1):27–37. doi: 10.1016/j.jacbts.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pabel S, Bollenberg H, Bengel P, Tirilomis P, Mustroph J, Wagner S, et al. Empagliflozin directly improves diastolic function in human heart failure. Eur Heart J. 2018;39:284–285. doi: 10.1093/eurheartj/ehy565.P1509. [DOI] [PubMed] [Google Scholar]
- 24.Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020;41:3421–3432. doi: 10.1093/eurheartj/ehaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- 26.Braha A, Timar B, Diaconu L, Lupusoru R, Vasiluta L, Sima A, et al. Dynamics of epicardiac fat and heart function in type 2 diabetic patients initiated with SGLT-2 inhibitors. Diabetes Metab Syndr Obes Targets Ther. 2019;12:2559–2566. doi: 10.2147/DMSO.S223629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang IC, Cho GY, Yoon YE, Park JJ, Park JB, Lee SP, et al. Different effects of SGLT2 inhibitors according to the presence and types of heart failure in type 2 diabetic patients. Cardiovasc Diabetol. 2020;19(1):69. doi: 10.1186/s12933-020-01042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. doi: 10.1186/s12933-018-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. 2020;43:1356–1359. doi: 10.2337/dc19-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbone S, Billingsley HE, Canada JM, Bressi E, Rotelli B, Kadariya D, et al. The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: results of the CANA-HF study. Diabetes/metabolism research and reviews. 2020;36:e3335. doi: 10.1002/dmrr.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka A, Hisauchi I, Taguchi I, Sezai A, Toyoda S, Tomiyama H, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE) ESC heart failure. 2020;7:1585–1594. doi: 10.1002/ehf2.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 33.de Boer RA, Nunez J, Kozlovski P, Wang Y, Proot P, Keefe D. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozinvsplacebo or empagliflozin in patients with type 2 diabetes and heart failure. Br J Clin Pharmacol. 2020;86(7):1346–1356. doi: 10.1111/bcp.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020 doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.JPT H, (editors) GS. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org.
- 38.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27(29):6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. 2020;9(9):e015716. doi: 10.1161/JAHA.119.015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonora BM, de Kreutzenberg SV, Avogaro A, Fadini GP. Effects of the SGLT2 inhibitor dapagliflozin on cardiac function evaluated by impedance cardiography in patients with type 2 diabetes Secondary analysis of a randomized placebo-controlled trial. Cardiovasc Diabetol. 2019;18(1):106. doi: 10.1186/s12933-019-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, et al. Empagliflozin in heart failure: diuretic and cardio-renal effects. Circulation. 2020;142:1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status—results from the EMPEROR-reduced trial. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.051824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2030183. [DOI] [PubMed]
- 44.Eickhoff MK, Olsen FJ, Frimodt-Moller M, Diaz LJ, Faber J, Jensen MT, et al. Effect of dapagliflozin on cardiac function in people with type 2 diabetes and albuminuria—a double blind randomized placebo-controlled crossover trial. J Diabetes Complications. 2020;34(7):107590. doi: 10.1016/j.jdiacomp.2020.107590. [DOI] [PubMed] [Google Scholar]
- 45.Ejiri K, Miyoshi T, Kihara H, Hata Y, Nagano T, Takaishi A, et al. Effect of luseogliflozin on heart failure with preserved ejection fraction in patients with diabetes mellitus. J Am Heart Assoc. 2020;9(16):e015103. doi: 10.1161/JAHA.119.015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Januzzi JL, Jr, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. 2017;70(6):704–712. doi: 10.1016/j.jacc.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Januzzi JL, Jr, Xu J, Li J, Shaw W, Oh R, Pfeifer M, et al. Effects of canagliflozin on amino-terminal pro-b-type natriuretic peptide: implications for cardiovascular risk reduction. J Am Coll Cardiol. 2020;76(18):2076–2085. doi: 10.1016/j.jacc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol. 2020;19(1):1–16. doi: 10.1186/s12933-019-0977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kayano H, Koba S, Hirano T, Matsui T, Fukuoka H, Tsuijita H, et al. Dapagliflozin influences ventricular hemodynamics and exercise-induced pulmonary hypertension in type 2 diabetes patients—a randomized controlled trial. Circ J. 2020;84(10):1807–1817. doi: 10.1253/circj.CJ-20-0341. [DOI] [PubMed] [Google Scholar]
- 50.Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the recede-chf trial. Circulation. 2020;142(18):1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldgren J, Laurila S, Akerblom A, Latva-Rasku A, Rebelos E, Isackson H, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes. Diabetologia. 2020;63(SUPPL 1):S62–S63. doi: 10.1111/dom.14363. [DOI] [PubMed] [Google Scholar]
- 52.Shim CY, Seo J, Cho I, Lee CJ, Cho IJ, Lhagvasuren P, et al. Randomized, controlled trial to evaluate the effect of dapagliflozin on left ventricular diastolic function in patients with type 2 diabetes mellitus: the IDDIA trial. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.051992. [DOI] [PubMed] [Google Scholar]
- 53.Patoulias D, Papadopoulos C, Katsimardou A, Kalogirou M-S, Doumas M. Meta-analysis assessing the effect of sodium-glucose co-transporter-2 inhibitors on left ventricular mass in patients with type 2 diabetes mellitus. Am J Cardiol. 2020;134:149–152. doi: 10.1016/j.amjcard.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, et al. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2016;11(11):e0166125. doi: 10.1371/journal.pone.0166125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43(12):2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 56.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 57.Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8(12):1351–1359. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu JH, Chen Y, Yuen M, Zhen Z, Chan CW, Lam KS, et al. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:22. doi: 10.1186/s12933-016-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velazquez EJ, Reinhardt SW. Limitations of natriuretic peptide levels in establishing SGLT-2 inhibitors for heart failure care. J Am Coll Cardiol. 2020;76(18):2086–2088. doi: 10.1016/j.jacc.2020.09.538. [DOI] [PubMed] [Google Scholar]
- 61.Savarese G, Musella F, D'Amore C, Vassallo E, Losco T, Gambardella F, et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: a meta-analysis. JACC Heart failure. 2014;2(2):148–158. doi: 10.1016/j.jchf.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, et al. Prognostic implications of changes in n-terminal pro-b-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68(22):2425–2436. doi: 10.1016/j.jacc.2016.09.931. [DOI] [PubMed] [Google Scholar]
- 63.Cunningham JW, Vaduganathan M, Claggett BL, Zile MR, Anand IS, Packer M, et al. Effects of sacubitril/valsartan on N-terminal Pro-B-type natriuretic peptide in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8(5):372–381. doi: 10.1016/j.jchf.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318(8):713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas city cardiomyopathy questionnaire in clinical trials and clinical care: JACC state-of-the-art Review. J Am Coll Cardiol. 2020;76(20):2379–2390. doi: 10.1016/j.jacc.2020.09.542. [DOI] [PubMed] [Google Scholar]
- 66.Garg V, Verma S, Connelly K. Mechanistic insights regarding the role of SGLT2 inhibitors and GLP1 agonist drugs on cardiovascular disease in diabetes. Prog Cardiovasc Dis. 2019;62(4):349–357. doi: 10.1016/j.pcad.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136(12):1167–1169. doi: 10.1161/CIRCULATIONAHA.117.029529. [DOI] [PubMed] [Google Scholar]
- 68.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME Trial: a "Thrifty Substrate" hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 69.Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metab Clin Exp. 2018;85:32–37. doi: 10.1016/j.metabol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, et al. Empagliflozin Blunts worsening cardiac dysfunction associated with reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13(1):e006277. doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 71.Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, et al. Off-target effects of SGLT2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar K, Kheiri B, Simpson TF, Osman M, Rahmouni H. Sodium-glucose cotransporter 2 inhibitors in heart failure: a meta-analysis of randomized clinical trials. Am J Med. 2020;133:e625–e630. doi: 10.1016/j.amjmed.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Matsumura K, Sugiura T. Effect of sodium glucose cotransporter 2 inhibitors on cardiac function and cardiovascular outcome: a systematic review. Cardiovasc Ultrasound. 2019;17(1):26. doi: 10.1186/s12947-019-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lan NSR, Fegan PG, Yeap BB, Dwivedi G. The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail. 2019;6(5):927–935. doi: 10.1002/ehf2.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka H, Hirata KI. Potential impact of SGLT2 inhibitors on left ventricular diastolic function in patients with diabetes mellitus. Heart Fail Rev. 2018;23(3):439–444. doi: 10.1007/s10741-018-9668-1. [DOI] [PubMed] [Google Scholar]
- 76.Santos-Gallego CG, Garcia-Ropero A, Mancini D, Pinney SP, Contreras JP, Fergus I, et al. Rationale and design of the EMPA-TROPISM Trial (ATRU-4): are the “Cardiac Benefits” of empagliflozin independent of its hypoglycemic activity? Cardiovasc Drugs Ther. 2019;33(1):87–95. doi: 10.1007/s10557-018-06850-0. [DOI] [PubMed] [Google Scholar]
- 77.Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovasc Diabetol. 2017;16(1):130. doi: 10.1186/s12933-017-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abraham WT, Ponikowski P, Brueckmann M, Zeller C, Macesic H, Peil B, et al. Rationale and design of the EMPERIAL-Preserved and EMPERIAL-Reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail. 2019;21(7):932–942. doi: 10.1002/ejhf.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Data S1. Strategy for the main search conducted on April 21st, 2020.
Additional file 2: Figure S1. Quality assessment of RCTs using the revised Cochrane risk-of-bias tool. (a) Risk of bias graph; (b) Risk of bias summary.
Additional file 3: Figure S2. Funnel plots for publication bias assessment. (a) LVMI; (b) LVEDVI; (c) LVESVI; (d) LAVI; (e) LVEF; (f) GLS; (g) E/e’; (h) NT-proBNP; (i) KCCQ.
Additional file 4: Figure S3. Subgroup analyses of the effects of SGLT2i on (a) LVEF in HFrEF vs. HFpEF patients; (b) E/e’ in HFrEF vs. HFpEF patients; (c) NT-proBNP in HFrEF vs. HFpEF patients. Abbreviations: SGLT2i: sodium-glucose cotransporter 2 inhibitors; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; LVEF: left ventricular ejection fraction; E/e': mitral inflow to mitral relaxation velocity ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Data Availability Statement
Not applicable.