Abstract
The aim was to determine whether losartan reduces cigarette smoke (CS)-induced airway inflammation and mucus hypersecretion in an in vitro model and a small clinical trial.
Primary human bronchial epithelial cells (HBECs) were differentiated at the air–liquid interface (ALI) and exposed to CS. Expression of transforming growth factor (TGF)-β1 and the mucin MUC5AC, and expression or activity of matrix metalloproteinase (MMP)-9 were measured after CS exposure. Parameters of mucociliary clearance were evaluated by measuring airway surface liquid volumes, mucus concentrations, and conductance of cystic fibrosis transmembrane conductance regulator (CFTR) and large conductance, Ca2+-activated and voltage-dependent potassium (BK) channels. Nasal cells were collected from study participants and expression of MUC5AC, TGF-β1, and MMP-9 mRNAs was measured before and after losartan treatment.
In vitro, CS exposure of HBECs caused a significant increase in mRNA expression of MUC5AC and TGF-β1 and MMP-9 activity and decreased CFTR and BK channel activities, thereby reducing airway surface liquid volumes and increasing mucus concentrations. Treatment of HBECs with losartan rescued CS-induced CFTR and BK dysfunction and caused a significant decrease in MUC5AC expression and mucus concentrations, partially by inhibiting TGF-β signalling. In a prospective clinical study, cigarette smokers showed significantly reduced mRNA expression levels of MUC5AC, TGF-β1, and MMP-9 in the upper airways after 2 months of losartan treatment.
Our findings suggest that losartan may be an effective therapy to reduce inflammation and mucus hypersecretion in CS-induced chronic airway diseases.
Short abstract
Inflammation and mucus hypersecretion, associated with cigarette smoke-induced chronic airway diseases, is ameliorated by losartan in human airway epithelial cells in vitro and in the upper airways of human subjects in a small clinical trial https://bit.ly/2G0aM8j
Introduction
The consequences of cigarette smoking are well documented, with smoking-related diseases causing over six million deaths per year worldwide [1]. COPDs include chronic bronchitis and mostly result from long-term smoking. Chronic bronchitis, even without obstruction, is characterised by persistent airway inflammation and mucus hypersecretion [2], which both decrease mucociliary clearance and accelerate disease progression. Unfortunately, no effective therapies exist, but their goal would be to decrease mucus hyperconcentration by controlling inflammation and facilitating the removal of mucus from the airways. One of the primary gel-forming mucins in the airways, MUC5AC [3], is induced by cigarette smoke (CS) in vivo and in primary human airway epithelial cells in vitro [4]. In fact, recent reports have found increased levels of MUC5AC in the sputum of smokers when compared to nonsmokers [5], with MUC5AC expression levels correlating with COPD progression [6]. Transforming growth factor (TGF)-β1 and matrix metalloproteinase (MMP)-9 are also known to be major players in the pathogenesis of COPD [7–9] as well as other chronic airway diseases such as cystic fibrosis (CF) [10–12]. They have been proposed to serve as relevant biomarkers in COPD [13, 14].
Losartan is a US Food and Drug Administration (FDA)-approved angiotensin II receptor type 1 (AGTR1) blocker (ARB), widely used to treat hypertension. However, losartan also exhibits anti-inflammatory properties, possibly independent of its ARB activity [7, 12, 15, 16]. We showed that losartan can increase levels of peroxisome proliferator-activated receptor (PPAR)-γ and thereby rescue TGF-β1-induced inflammation and mucociliary dysfunction in relevant CF models in vitro and in vivo [12]. Induction of PPAR-γ was also shown to prevent and reverse CS-induced emphysema in mouse models [7, 17]. A large clinical trial is underway to assess losartan's effects on emphysema progression ((LEEP) ClinicalTrials.gov identifier: NCT02696564). However, this trial does not focus on airway disease. Thus, we examined how CS impacts the levels of MUC5AC and inflammation markers (TGF-β1 and MMP-9) in airway epithelia in vitro and in vivo. We further determined whether losartan acts as an effective anti-inflammatory therapy to reduce airway inflammation and thereby mucin concentrations both in vitro and in a small clinical trial (ClinicalTrials.gov identifier: NCT02416102).
Methods
Lungs
Lung tissue was obtained from organ donors whose lungs were rejected for transplant and recovered for research by the Life Alliance Organ Recovery Agency at the University of Miami (Miami, FL, USA), LifeCenter Northwest (Seattle, WA, USA), and the Midwest Transplant Network (Kansas City, KS, USA). A ring of the trachea or main bronchi was cut and fixed in 10% formalin at 4°C until embedded in paraffin for sectioning and tissue staining. Cells were taken from airways of nonsmokers and smokers. The diagnosis of COPD was made by clinical criteria before the death of the patient and taken verbatim from the chart. No lung function was available confirming obstructive disease. Thus, the diagnosis was only accepted for these COPD cells if the donor had a significant smoking history and there were macropathological signs of emphysema. All COPD subjects here were still actively smoking. Airways were dissected and the tissue exposed to protease overnight as described [18–20]. Cells were harvested the following day and frozen in liquid nitrogen (considered P0 human bronchial epithelial cells (HBECs)). The University of Kansas Institutional Review Board deemed the use of these materials as nonhuman subjects research.
Cell culture and losartan treatments
Culturing of HBECs at the air–liquid interface (ALI) was performed as described (see also online supplementary methods) [18–20]. In vitro losartan (no. 61188, Millipore Sigma, St. Louis, MO, USA) treatment in the medium at 10 μM was started the day that P1 HBECs went to an ALI and were maintained throughout differentiation, except for cells from smokers (including COPD) that were treated for 24 h with losartan before CS exposure. LY2157299 (no. S2230, Selleckchem, Houston, TX, USA) at 10 µM or EXP3179 (no. 18855, Cayman Chemicals, Ann Arbor, MI, USA) at 5 µM was added to the basolateral medium of ALI cultures 24 h before CS exposure.
Immunofluorescence staining
Immunofluorescence staining of tissue sections was performed as described [21]. Tissue sections were incubated with anti-MUC5AC antibody (no. MA1-38223, Thermo Fisher Scientific, Waltham, MA, USA) at 0.4 μg·mL−1 overnight at 4°C and with Hoechst (no. H3569, Thermo Fisher Scientific) at 2 μg·mL−1 for 10 min. For staining of P1 HBECs, ALI cultures on Transwell inserts were fixed with a solution of 50%/50% methanol/acetone for 2 min at −20°C followed by three washes with PBS. A 3% BSA solution was used to block for 1 h before incubation with primary antibodies. P1 HBECs exposed to CS or room air were washed and fixed 48 h after exposure following the same immunostaining protocol. All slides were imaged with a Nikon C2+ confocal microscope (Nikon Instruments, Tokyo, Japan).
Quantitative PCR
P1 HBECs exposed to CS or room air were lysed 24 h after exposure and total RNA was isolated using the E.Z.N.A.® Total RNA kit (Omega Bio-tek, Norcross, GA, USA). Quantitative PCR (qPCR) was performed as described [8, 22] using TaqMan Gene Expression Assays (Thermo Fisher Scientific) for IL-13 (Hs00174379_m1), MMP2 (Hs01548727_m1), MMP9 (Hs00234579_m1), MMP12 (Hs00159178_m1), MUC5AC (Hs01365601_m1), TGF-β1 (Hs00998133_m1), TGF-β2 (Hs00234244_m1), CFTR (Hs00357011_m1), LRRC26 (Hs02385555_g1), and normalised to the reference gene GAPDH (4352934E).
MMP-9 activity assay
MMP-9 activity was measured in 200 µL apical PBS washes collected 24 h after room air or CS exposure using the Human Active MMP-9 Fluorokine E kit (no. F9M00, R&D Systems, Minneapolis, MN, USA), following manufacturer's instructions for nonactivated samples.
Ussing chamber
Cystic fibrosis transmembrane conductance regulator (CFTR) and large conductance, Ca2+-activated and voltage-dependent K+ channel (BK) activities were recorded in Ussing chambers as previously described (see also online supplementary methods) [23, 24]. CFTR and BK currents from P1 HBECs on Snapwell filters were measured at 4 h after the cells were exposed to CS or room air.
Airway surface liquid volume measurements
Airway surface liquid (ASL) volume estimation was performed by meniscus scanning as previously published [8, 25]. P1 HBECs exposed to CS or room air were scanned 1 h and 4 h after exposure and the ΔASL was plotted.
Ciliary beat frequency
Ciliary beat frequency (CBF) was recorded 4 h after exposure to CS or room air using a high-speed camera and analysed using the individual region-of-interest (ROI) method of SAVA software [26, 27]. Ciliary beating was recorded 1–2 mm away from the centre of the insert for 2 s and four ROIs were plotted.
Mucus concentration measurements
The percentage solids of mucin-containing fluid on top of cultures was measured according to published methods of mucus wet and dry weights using a UMX2 ultra-microbalance (Mettler Toledo, Columbus, OH, USA) [28, 29]. P1 HBECs exposed to CS or room air were tested 24 h after exposure.
Cigarette smoke exposure
CS exposure of P1 HBECs was conducted as described [8, 21, 30]. Briefly, P1 HBECs were exposed to 24 puffs from four Kentucky research cigarettes (3R4F) with a volume of 35 mL delivered every 60 s using the Vitrocell VC10 smoking robot (Vitrocell, Waldkirch, Germany) following ISO standard 3308. As controls, P1 HBECs were exposed to room air. Nicotine deposition onto the surface of HBECs after CS exposures was validated by liquid chromatography/mass spectrometry (LC-MS/MS, Florida International University, FL, USA) and showed depositions of approximately 100–120 μM of nicotine onto ALI cultures comparable to in vivo deposition of 1–2 cigarettes [31].
Human subjects and study approval
The clinical study was approved by the University of Miami Human Subject Research Office and informed consent was obtained from each participant. Clinicaltrials.gov registration can be found under NCT02416102.
The study enrolled a total of 31 participants: 16 healthy never-smokers (<100 cigarettes in a lifetime) and 15 current smokers with a smoking history of >10 pack-years and no signs of COPD by pulmonary function tests with diffusing capacity of the lung for carbon monoxide, forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC), FEV1 and FVC values from both groups in the normal range. From those 31 patients, 14 (7 smokers and 7 nonsmokers) completed the study with laboratory test results and only 5 subjects in each group had complete nasal sample datasets. The subjects were aged 35 to 70 years and not taking any ARBs prior to enrolment. Participants received 50 mg losartan for 4 weeks and then 100 mg losartan for another 4 weeks. The exclusion criteria are described in online supplementary methods.
Nasal cell collection
Nasal cells were collected from study participants using sterile cytology brushes (Medical Packaging Corporation, Camarillo, CA, USA) as described in online supplementary methods.
Statistical analyses
Data are shown as dot plots/bar graph combinations with mean±sem. Differences between two groups were compared by parametric or nonparametric tests as indicated in the figure captions depending on whether the data passed Shapiro–Wilk normality testing. The p-values for significance were accepted at <0.05. All analyses were performed using Prism (GraphPad Software, San Diego, CA, USA).
Results
Expression of MUC5AC, TGF-β1 and MMP-9 is elevated in lung tissues from smokers
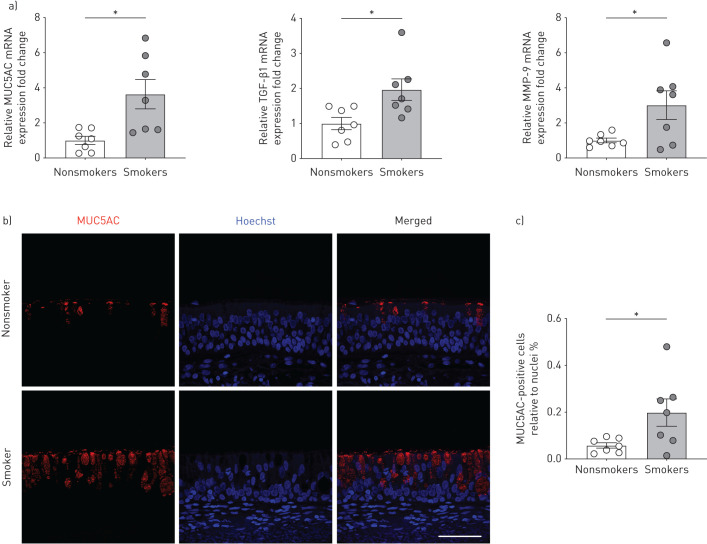
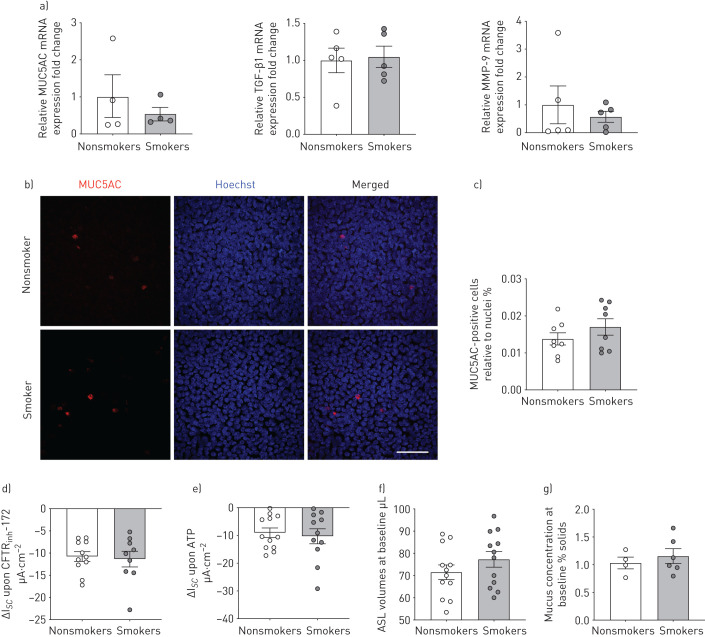
We analysed mRNA expression levels of the mucin MUC5AC and the inflammation markers TGF-β1 and MMP-9 in freshly isolated HBECs from lungs of age-matched nonsmoking and smoking donors without COPD (demographics of donors can be found in online supplementary table S1). We refer to these as passage zero (P0) cells (never expanded or cultured). mRNA expression levels of MUC5AC, TGF-β1, and MMP-9 were significantly increased in P0 HBECs of smokers compared to nonsmokers (figure 1a). Immunofluorescence staining of tracheal/bronchial tissue sections from the same donors showed that smokers displayed an increased percentage of MUC5AC-positive cells in the epithelium compared to nonsmokers (figure 1b and c). These results are largely consistent with previous reports showing increased absolute concentrations of both MUC5AC and TGF-β1 in smokers without COPD compared to nonsmokers [6, 32]. When HBECs were expanded and fully re-differentiated at the ALI, now referred to as P1 HBECs or ALI cultures, differences in mRNA expression levels of MUC5AC, TGF-β1, and MMP-9, as well as the percentage of MUC5AC-positive cells between nonsmokers and smokers were no longer apparent (figure 2a–c). Thus, P1 HBECs lose some of their in vivo characteristics during re-differentiation.
FIGURE 1.
Freshly isolated human bronchial epithelial cells (P0 HBECs) from nonsmokers and smokers. a) Quantitative mRNA expression of MUC5AC, transforming growth factor (TGF)-β1 and matrix metalloproteinase (MMP)-9 of P0 HBECs from nonsmokers and smokers. Data are shown as relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and nonsmokers (n≥6 donors for each group). b) Representative confocal images of immunofluorescent staining of bronchial tissue from a nonsmoker and a smoker. MUC5AC in red; nuclei stained with Hoechst in blue. Scale bar=50 μm. c) Quantification of MUC5AC-positive cells relative to nuclei (n=7 from ≥3 donors for each group). *: p<0.05 using t-test after passing Shapiro–Wilk normality test.
FIGURE 2.
Fully re-differentiated human bronchial epithelial cells (P1 HBECs) from nonsmokers and smokers. a) Quantitative mRNA expression of MUC5AC, transforming growth factor (TGF)-β1 and matrix metalloproteinase (MMP)-9 of fully re-differentiated P1 HBECs from nonsmokers and smokers. Data are shown as relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and nonsmokers (n≥4 donors for each group). b) Representative confocal images of immunofluorescent staining of P1 HBECs from a nonsmoker (upper panels) and a smoker (lower panels). MUC5AC is stained in red, nuclei were stained with Hoechst and represented in blue. Scale bar=50 μm. c) Quantification of MUC5AC-positive cells relative to nuclei (n=8 from ≥3 donors). d) Cystic fibrosis transmembrane conductance regulator (CFTR) currents measured by short circuit current changes upon CFTRinh172 (10 µM) application after 10 µM forskolin stimulation 4 h after exposure to room air or cigarette smoke (represented as ΔIsc upon CFTRinh172; thus, decreases indicate enhanced CFTR function) (n≥9 donors for each group). e) Voltage-dependent potassium (BK) currents measured upon ATP stimulation and represented as ΔIsc (n≥11 donors for each group) (decreases indicate enhanced BK function). f) Airway surface liquid (ASL) volumes measured 4–6 weeks after establishment of air–liquid interface (ALI) and represented as baselines (n=12 donors for each group). g) Mucus concentration depicted as percentage mucus solids measured 4–6 weeks after establishment of ALI and represented as baselines (n≥4 donors for each group). None of the comparisons were significant by t-test after passing Shapiro–Wilk normality test.
Cigarette smoke exposure induces inflammation and mucociliary dysfunction in P1 HBECs of nonsmokers
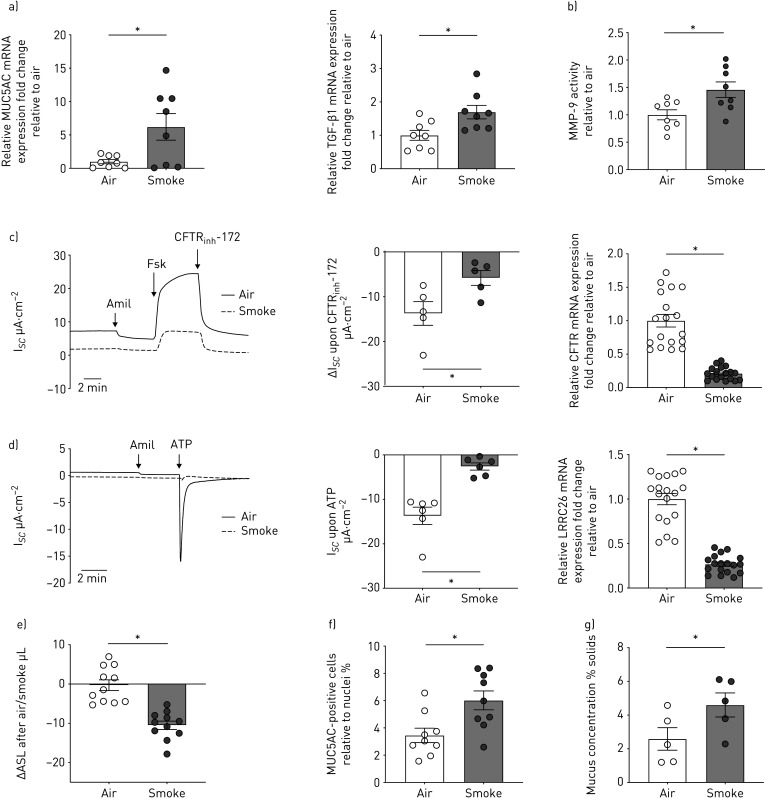
Chronic bronchitis, defined as productive cough, indicates a failure of normal mucociliary function, usually indicating a reduction in ASL volume. ASL homeostasis depends on the proper activities of ion channels such as CFTR and Ca2+-activated chloride channels as well as the apically expressed BK channel [33]. Function of the BK α subunit, KCNMA1, depends on leucine rich repeat-containing protein 26 (LRRC26), a γ subunit necessary to open BK in nonexcitable tissues [24, 34]. CFTR and BK activities, ASL volumes, and mucus concentrations (% solids) in P1 HBECs were not significantly different between nonsmokers and smokers (figure 2d–g). To recreate features of inflammation and mucus overproduction observed in P0 HBECs from smokers, we exposed P1 ALI cultures from nonsmoking subjects to CS (24 puffs from four 3R4F Kentucky research cigarettes, at one puff every minute with 8 s exhaust time via the Vitrocell VC-10) [8]. As a control, P1 HBECs were exposed to room air. CS significantly increased mRNA expression of MUC5AC and TGF-β1 (figure 3a) and activity of MMP-9 (figure 3b) 24 h after exposure. Parameters of mucociliary function were also affected: CS-exposed ALI cultures showed a significant decrease in activities of CFTR and BK (correlating with mRNA expression levels of CFTR and LRRC26, respectively) compared to air control (figure 3c and d). In addition, CS caused a concomitant loss of ASL volume (figure 3e), and a significant increase in MUC5AC-positive cells and mucus concentration (figure 3f and g). The increase in mucus solids from approximately 2% to 4% is consistent with previously published data for smokers in vivo and another study demonstrating the relationship of mucus concentrations and mucociliary clearance in vitro [28, 29].
FIGURE 3.
Fully re-differentiated human bronchial epithelial cells (P1 HBECs) from nonsmokers exposed to cigarette smoke (smoke). a) Quantitative mRNA expression of MUC5AC and transforming growth factor (TGF)-β1 in P1 HBECs 24 h after exposure to room air or smoke (24 puffs). Data are shown as relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and air control (n=8 donors for each group). b) Matrix metalloproteinase (MMP)-9 activity assay from PBS washes of P1 HBECs from nonsmokers 24 h after exposure to room air or smoke (24 puffs). Data are shown as relative to air control (n=8 from 4 donors for each group). c) Left panel: representative cystic fibrosis transmembrane conductance regulator (CFTR) trace measured by short circuit current changes upon CFTRinh172 (10 µM) application after 10 µM forskolin stimulation 4 h after exposure to room air or smoke (represented as ΔIsc upon CFTRinh172; thus, decreases indicate enhanced CFTR function) (n=5 donors for each group). Middle panel: quantification of CFTR currents upon CFTRinh172. Right panel: CFTR mRNA expression. Data are shown as relative to GAPDH and air control (n=18 from 6 donors for each group). d) Left panel: representative voltage-dependent potassium (BK) trace and quantification of currents measured upon ATP stimulation 4 h after exposure to room air or smoke (represented as ΔIsc with decreases indicating better BK function). n=6 donors for each group. Right panel: LRRC26 mRNA expression (γ subunit of BK critical for BK function). Data are shown as relative to GAPDH and air control (n=18 from 6 donors for each group). e) Airway surface liquid (ASL) volumes represented as change in volume between 1 and 4 h after air or smoke exposure (n=11 donors for each group). f) Quantification of MUC5AC-positive cells relative to nuclei 24 h after air or smoke exposure (n=9 from 3 donors for each group). g) Mucus concentration depicted as % mucus solids measured 24 h after air or smoke exposure (n=5 donors for each group). *: p<0.05, t-test after passing Shapiro–Wilk normality test for all except for the BK data (Mann–Whitney U-test).
Losartan reduces cigarette smoke-induced mucus hypersecretion and mucociliary dysfunction in vitro
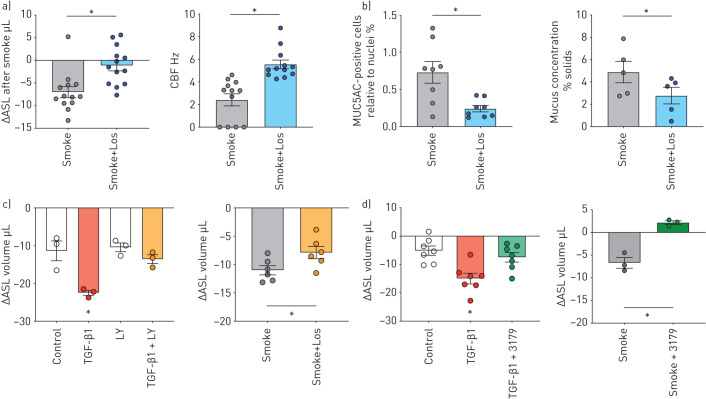
Next, we tested whether losartan, an US FDA-approved drug with a good safety track record and known anti-inflammatory properties [7, 15, 16], reduced CS-induced airway inflammation and thereby mucus concentration to levels compatible with normal mucociliary clearance. Fully re-differentiated P1 HBECs from nonsmokers were treated with 10 µM losartan throughout differentiation before exposing them to 24 puffs of CS through the VC-10 robot. We observed an improvement in ASL volumes (figure 4a), CBF (figure 4a), and a decrease in the percentage of MUC5AC-positive cells (figure 4b) and mucus concentrations (figure 4b).
FIGURE 4.
Fully re-differentiated human bronchial epithelial cells (P1 HBECs) from nonsmokers exposed to cigarette smoke (smoke) or transforming growth factor (TGF)-β1 and treated with losartan (los, 10 μM), LY2157299 (LY, 10 μM) and EXP3179 (3179, 5 μM). a) Left: airway surface liquid (ASL) volumes represented as change in volume between 1 and 4 h after smoke±losartan (Los) exposure (n=13 donors for each group). Right: ciliary beat frequency (CBF) measured 4 h after exposure to room air or smoke±Los (n=12 from 3 donors in each group). b) Left: quantification of MUC5AC-positive cells relative to nuclei 24 h after smoke±Los exposure (n=8 from 3 donors for each group). Right: mucus concentration depicted as % mucus solids measured 24 h after smoke±Los (n=5 donors for each group). c) Left: P1 HBECs from nonsmokers were treated with TGF-β1 (10 ng·mL−1) in the presence or absence of the TGF-β1 receptor inhibitor LY2157299 (10 µM) for 24 h. Control contained appropriate concentrations of dimethyl sulfoxide. LY2157299 prevents TGF-β1-induced ASL volume loss (n=3 donors). Right: P1 HBECs from nonsmokers were pre-treated with 10 µM LY2157299 before exposure to smoke (24 puffs). LY2157299 ameliorated CS-induced ASL volume loss. d) Left: P1 HBECs from nonsmokers were treated with TGF-β1 (10 ng·mL−1) in the presence or absence of EXP3179 (5 µM) for 24 h. EXP3179 prevents TGF-β1-induced ASL volume loss (n=7 donors). Right: P1 HBECs from nonsmokers were pre-treated with 5 µM EXP3179 before exposure to smoke (24 puffs). EXP3179 ameliorated CS-induced ASL volume loss (n=3 donors). *: p<0.05 either compared to all groups by one-way ANOVA followed by Holm–Sidak post hoc test or t-test; both after passing Shapiro–Wilk normality test.
TGF-β1 is probably a driver of cigarette smoke-induced inflammation and mucociliary dysfunction as pre-treatment of ALI cultures with LY2157299 (galunisertib), a selective TGF-β receptor 1 (TGFBR1) inhibitor, improved ASL volumes upon TGF-β1 and smoke exposure (figure 4c).TGF-β1- and CS-induced decreases in ASL volume were also rescued by EXP3179, the losartan metabolite with anti-inflammatory but no ARB activities, suggesting that losartan's effects are independent of AGTR1 signalling (figure 4d).
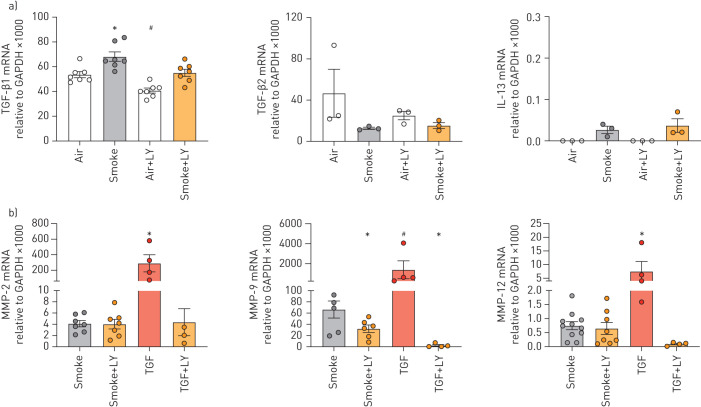
In support of the TGF-β pathway being responsible, LY2157299 decreased smoke-induced TGF-β1 and MMP-9 mRNA expression (figure 5a and b). LY2157299 had no effect on expression of TGF-β2, IL-13, MMP-2 and MMP-9 mRNA expression upon smoke exposure (figure 5a and b), while TGF-β1-induced MMP-2 and MMP-12 mRNA expressions were suppressed by LY2157299 (figure 5b).
FIGURE 5.
Fully re-differentiated human bronchial epithelial cells (P1 HBECs) from nonsmokers exposed to cigarette smoke or transforming growth factor (TGF)-β1 and treated with LY2157299 (LY). a) Expression of TGF-β1 mRNA but not of TGF-β2 mRNA is increased by smoke (two left panels). Interleukin (IL)-13 mRNA is not significantly upregulated by smoke and is not changed by LY-2157299 (10 µM). Note the low expression (right panel). b) Of matrix metalloproteinase (MMP)-2, MMP-9 and MMP-12, only MMP-9 mRNA is upregulated by smoke in a TGF-β1-dependent fashion. LY2157299 also reduced MMP-9 mRNA expression upon smoke exposure (n≥3 from ≥3 donors). Data are presented as mean±se. *: p<0.05 compared to all groups; #: p<0.05 compared to smoke only by one-way ANOVA followed by Holm–Sidak post hoc test; all after passing Shapiro–Wilk normality test. One-way ANOVA followed by Holm–Sidak after passing Shapiro–Wilk normality test or Kruskal Wallis.
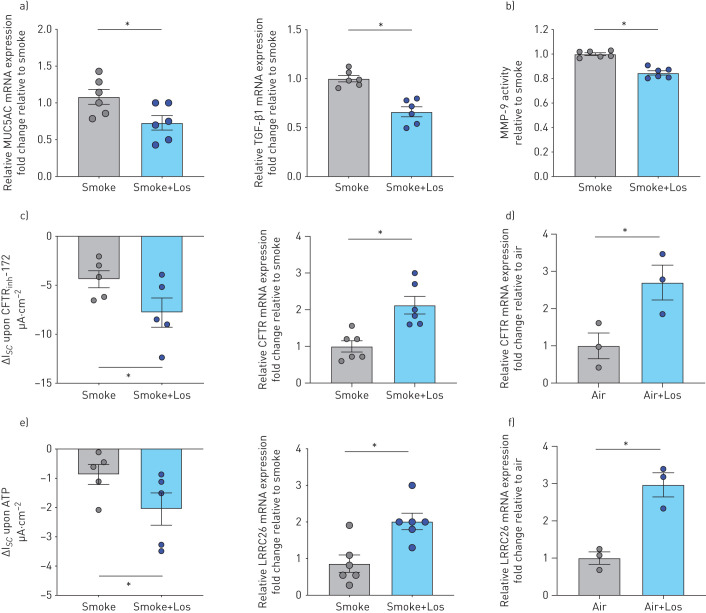
Treatment with losartan also significantly reduced MUC5AC and TGF-β1 mRNA expression as well as MMP-9 activity (figure 6a and b). Finally, losartan rescued CS-induced decreases in CFTR and BK function as well as mRNA expressions of CFTR and the functionally most relevant γ subunit of BK, LRRC26 (figure 6c–f). Interestingly, losartan also increased CFTR and LRRC26 mRNA in air controls (figure 6d–f).
FIGURE 6.
Fully re-differentiated human bronchial epithelial cells (P1 HBECs) from nonsmokers exposed to cigarette smoke (smoke) ± losartan. a) Quantitative mRNA expression of MUC5AC and transforming growth factor (TGF)-β1 in P1 HBECs 24 h after exposure to smoke (24 puffs) without and with losartan (Los) treatment (10 μM throughout differentiation). Data are shown as relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and smoke (n=5 donors for each group). b) Matrix metalloproteinase (MMP)-9 activity assay from PBS washes of P1 HBECs from nonsmokers 24 h after exposure to smoke (24 puffs) without and with Los treatment. Data are shown relative to smoke (n=6 from two donors for each group). c) Left panel: cystic fibrosis transmembrane conductance regulator (CFTR) currents represented by short circuit current changes upon CFTRinh172 (10 µM) application after 10 µM forskolin stimulation 4 h after exposure to smoke±Los (represented as ΔIsc upon CFTRinh172) (n=5 for each group). Right panel: CFTR mRNA expression. Data are shown as relative to GAPDH and smoke (n=6 donors for each group). d) CFTR mRNA expression in air control. Data shown as relative to GAPDH and air (n=3 donors for each group). e) Left panel: voltage-dependent potassium (BK) currents measured upon ATP stimulation 4 h after exposure to smoke±Los (n=5 donors for each group). Right panel: LRRC26 mRNA expression. Data are shown as relative to GAPDH and smoke (n=6 donors for each group). f) LRRC26 mRNA expression in air control. Data are shown as relative to GAPDH and air (n=3 donors for each group). *: p<0.05; all t-tests after passing Shapiro–Wilk normality test.
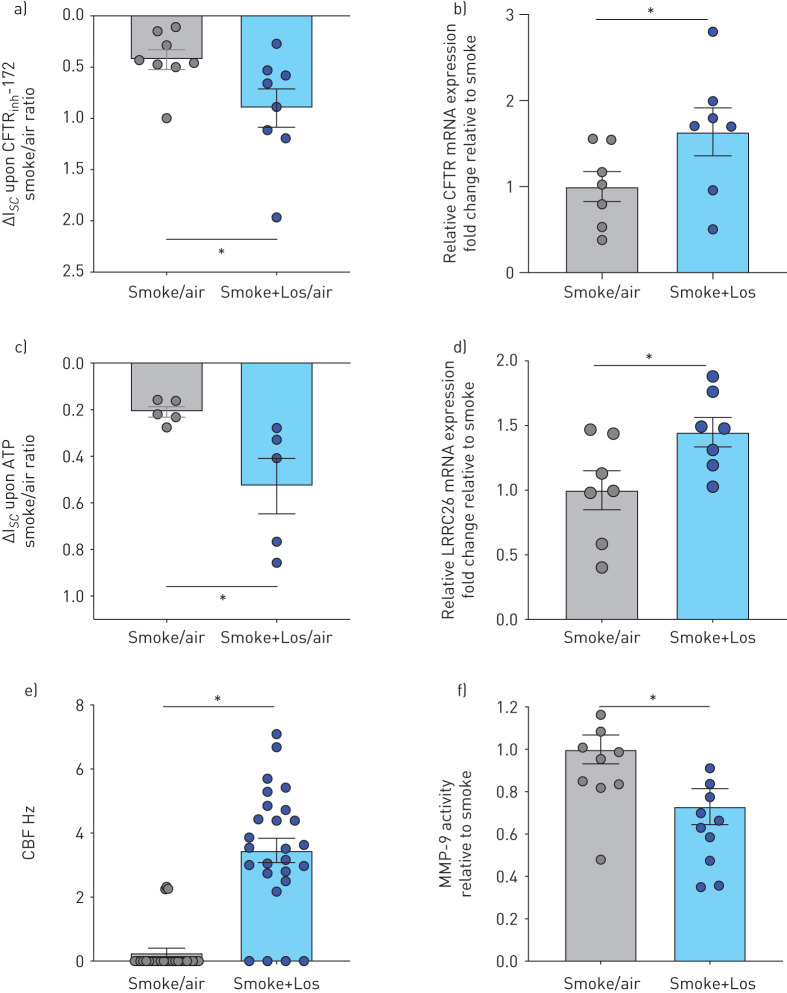
In analogy to the data obtained with airway cells from nonsmokers, cells from smokers with or without COPD also showed improvements in parameters of mucociliary function upon smoke exposure in the presence of losartan (figure 7). There were significant improvements in: 1) CFTR and BK currents as well as CFTR and LRRC26 mRNA expression (figure 7a–d); 2) CBF (figure 7e); and 3) a reduction in MMP-9 activity (figure 7f).
FIGURE 7.
Fully re-differentiated human bronchial epithelial cells (P1 HBECs) from active smokers with and without COPD exposed to cigarette smoke (smoke) and treated with losartan (Los). a) Cystic fibrosis transmembrane conductance regulator (CFTR) conductance expressed as a ratio of smoke exposure over air exposure of short circuit current changes upon CFTRinh-172 (10 µM) application after 10 µM forskolin stimulation (ΔIsc smoke/air). Note reverse y-axis similar to figure 3 (more downward=positive=improved conductance) (n=8 from 7 donors). *: p<0.05 using t-test. b) CFTR mRNA expression. Data are shown relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and smoke (n=7 from 7 donors). *: p<0.05 using t-test. c) Voltage-dependent potassium (BK) currents expressed as a ratio of smoke exposure over air exposure of short circuit current changes upon ATP stimulation (ΔIsc smoke/air). Note reverse y-axis (more downward=positive=improved conductance) (n=5 from 5 donors). *: p<0.05 using t-test. d) LRRC26 mRNA expression. Data are shown relative to GAPDH and smoke (n=7 from 7 donors). *: p<0.05 using t-test. e) Ciliary beat frequency (CBF) after exposure to smoke±Los (n=26 from 7 donors). *: p<0.05 using Mann–Whitney U-test. f) Matrix metalloproteinase (MMP)-9 activity assay from apical PBS washes obtained 24 h after exposure to smoke±Los. Data are shown as relative to smoke (≥9 from 7 donors). *: p<0.05 using t-test.
While a previous study found that losartan could ameliorate CS-induced parenchymal changes in mice [7], we provide the first evidence that losartan can restore important parameters of CS-induced mucociliary dysfunction using primary HBECs in vitro.
Losartan reduces expression levels of MUC5AC, TGF-β1 and MMP-9 in the upper airways of smokers in a clinical study
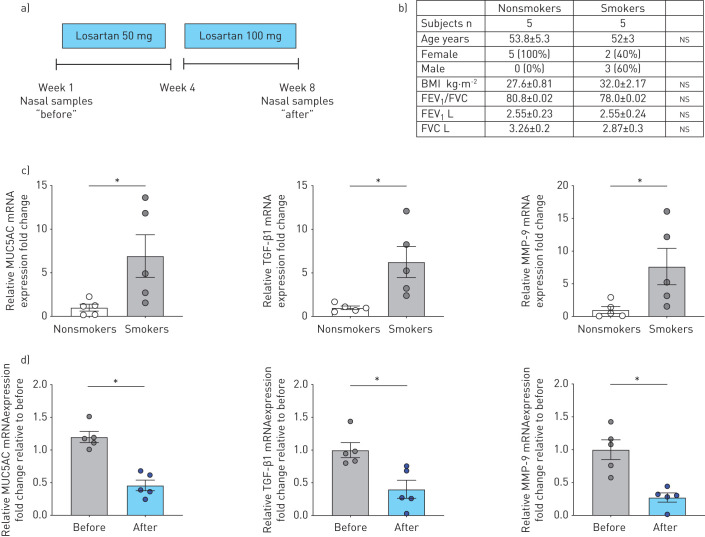
These results set the stage for a small prospective clinical study to determine whether losartan could reduce inflammation in the upper airways of currently healthy smokers. A schematic of the study can be found in figure 8a with subject demographics in figure 8b. Consistent with P0 HBECs, human nasal epithelial cells (HNECs) from smokers exhibited at baseline significantly elevated expression levels of MUC5AC, TGF-β1, and MMP-9 mRNA compared to nonsmokers (figure 8c). Losartan was administered for 4 weeks at 50 mg daily and for an additional 4 weeks at 100 mg daily before the same parameters were re-examined. mRNA levels were compared before (baseline) and after losartan treatment (week 1 and week 8) and are presented here as relative values of baselines. HNECs from smokers receiving losartan displayed significant decreases in MUC5AC, TGF-β1, and MMP-9 mRNA expression levels after 8 weeks of treatment with losartan (figure 8d), while expression levels in nonsmokers were unaffected (online supplementary figure S1). Thus, losartan effectively reduced the levels of important markers of airway inflammation as well as MUC5AC expression in the upper airways of smokers.
FIGURE 8.
Clinical trial with oral losartan and analysis of human nasal epithelial cells (HNECs) from nonsmokers and smokers. a) Study schematic (www.ClinicalTrials.gov identifier NCT02416102). b) Table of participants' demographics. ns: not significant. c) Quantitative mRNA expression of MUC5AC, transforming growth factor (TGF)-β1 and matrix metalloproteinase (MMP)-9 of HNECs from nonsmokers and smokers at baseline. Data are shown relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and nonsmokers. n=5 subjects for each group. d) Quantitative mRNA expression of MUC5AC, TGF-β1 and MMP-9 of nasal cells from smokers, before losartan treatment and 2 months after (50 mg daily for 4 weeks and 100 mg daily for an additional 4 weeks). Data are shown as relative to baseline prior to losartan administration (n=5 subjects from each group for each measurement). *: p<0.05, all t-tests after passing Shapiro–Wilk normality test. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Discussion
Airway inflammation and mucin hyperconcentration are hallmarks of cigarette smoking-related chronic airway diseases. Mucin concentrations are elevated in chronic bronchitis and absolute concentrations of MUC5AC are increased in smokers, even without COPD [6]. CS induces the expression of MUC5AC in airway epithelial cells in vitro [4], and increases levels of TGF-β1 protein and mRNA have been reported in small airway epithelial cells of smokers with and without COPD [32], Levels of MMP-9 were also significantly higher in sputum from smokers compared to nonsmokers [5]. Since inflammation can persist after smoking cessation, safe and nontoxic therapeutics that ameliorate airway inflammation and mucus hypersecretion would therefore provide a sound approach to treat smoking-related respiratory diseases.
Here, we therefore tested losartan in vitro and in vivo. We found that mRNA expressions of MUC5AC, TGF-β1, and MMP-9 are indeed elevated in lung tissue from smokers compared to nonsmokers (figure 1). Although these differences were apparent in HBECs that were never expanded, key features of CS-induced inflammation and mucus hyperconcentration were lost once HBECs were expanded and re-differentiated at the ALI (P1 HBECs). Loss of features in P1 HBECs present in the native airways could make it difficult to study airway diseases using cells. However, P1 HBECs were shown to be suitable surrogates for changes that occur in the airway epithelium after exposure to CS, airway pollutants, and other irritants [35]. Here, we exposed P1 HBECs from nonsmokers to CS generated to confirm that changes observed between smokers and nonsmokers in vivo can be consistently reproduced in vitro. We found that in vitro CS exposure of P1 HBECs from nonsmokers increased mucus concentrations from ∼2% to ∼4% solids (figure 3g), values consistent with previously published in vivo data [28] and a study demonstrating the relation of mucus concentrations and mucociliary clearance in vitro [29]. In fact, the values we obtained in CS-exposed P1 HBECs were more consistent with mucin concentrations found in chronic bronchitis subjects in vivo rather than nonsymptomatic smokers. These results suggest that in vitro exposure to CS induces relevant effects in P1 HBECs from nonsmokers and that the criteria of how many cigarettes or puffs to use should be carefully evaluated when designing studies.
CS exposure had detrimental effects on mucociliary clearance in vitro, consistent with in vivo data demonstrating a negative correlation between mucus concentration and mucociliary clearance in subjects with chronic bronchitis [29]. Exposure of P1 HBECs to CS impaired ion transport through both CFTR and BK and further led to a loss of ASL volume (figure 3). These effects are probably mediated through CS-induced increases in TGF-β1 expression as TGF-β1 signalling has been previously shown to reduce CFTR and BK activities through regulation of CFTR and LRRC26 mRNA expression, respectively [24, 36, 37]. Furthermore, we show here that the TGFBR1 inhibitor LY2157299 can ameliorate CS-induced ASL volume loss and reduce expression of MMP-9 mRNA (figure 5). There is evidence that losartan can inhibit TGF-β1 signalling and we recently showed that losartan can rescue TGF-β1-induced mucociliary dysfunction in CF airways in vitro and in a large animal model of CF-like airway disease [12]. Indeed, we found that treatment of P1 HBECs with losartan during re-differentiation could effectively decrease the expression of TGF-β1 and MMP-9 as well as MUC5AC upon CS exposure compared to non-losartan-treated and CS-exposed control P1 HBECs. Furthermore, CS-induced mucociliary dysfunction was reversed by losartan as shown by rescue of CFTR and BK channels functions, increasing ASL volume availability and reducing mucus concentration. These effects were also seen using EXP-3179, the losartan metabolite without angiotensin receptor blocking ability, indicating that these effects were not related to the ARB property of losartan.
We also showed that the results obtained in P1 cells from nonsmokers could be repeated when using cells from smokers with or without COPD (figure 7). Thus, losartan was effective even when cells were in vivo chronically exposed to smoke and possibly underwent epigenetic changes.
These in vitro findings set the stage for a small clinical study to determine whether losartan could reduce inflammation and mucus hypersecretion in the upper airways of smokers. In nasal cells of participants, we measured significant increases in TGF-β1, MMP-9 and MUC5AC mRNA expressions in smokers compared to nonsmokers, consistent with elevation of these markers observed in subjects with chronic bronchitis [6, 32]. Despite the small number of subjects completing the study and the pre/post-treatment design (suboptimal to a placebo control), we observed a significant decrease in the expression of TGF-β1, MMP-9 and MUC5AC mRNAs in those who remained on losartan for the full 8 weeks of treatment (figure 8). Although numerous studies using HNECs as a surrogate for HBECs have been described [38], their usefulness for studying airways diseases continues to be debated [39]. However, our data reveal that the relative increases in mRNA expression of TGF-β1, MMP-9 and MUC5AC in airway cells of smokers versus nonsmokers is comparable between HNECs and P0 HBECs. More importantly, losartan successfully reduced the expression of these markers in both HNECs derived from smoking subjects and CS-exposed P1 HBECs, suggesting that HNECs can serve as a surrogate for HBECs in in vitro models of CS-induced airway disease.
These studies provide support for losartan as a potential therapeutic to combat inflammation and mucus hyperconcentration in smoking-related chronic airway diseases. Losartan may also be effective in treating other airway diseases, where MUC5AC tethering impairs mucociliary transport [29, 40] or where mucus hypersecretion contributes to the pathogenesis of the disease [41].
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00394-2020.SUPPLEMENT (997.6KB, pdf)
Acknowledgments
We acknowledge Michael Myerburg (University of Pittsburgh) for providing the ASL meniscus scanning software, Brian Button (University of North Carolina at Chapel Hill) for advice on the mucus solids measurement technique, and Siddarth Rawal and Ashutosh Agarwal (University of Miami) for their assistance in using the Epilog Mini 30-W laser to cut precise tissue meshes for lifting mucus off the in vitro cultures. We also thank the Life Alliance Organ Recovery Agency at the University of Miami (Miami, FL, USA), LifeCenter Northwest (Seattle, WA, USA) and Midwest Transplant Network (Kansas City, KS, USA) for providing the lungs.
Footnotes
This article has supplementary material available from openres.ersjournals.com
This study is registered at www.clinicaltrials.gov with identifier number NCT02416102. Since it is a small clinical trial, the usefulness of data sharing is small and samples have been used. Thus, data sharing is not provided.
Author contributions: Conceived and designed the study: N. Baumlin and M. Salathe. Executed experiments and analysed the data: N. Baumlin, J.S. Dennis, M. Yoshida, A. Kis, M.D. Kim, M. Salathe. Clinical study recruitment and collection and interpretation of data: N. Baumlin, C. Aguiar, A. Schmid, E. Mendes, M. Salathe. Wrote the manuscript: N. Baumlin, M.D. Kim, and M. Salathe. Discussed the results and commented on the manuscript: all authors.
Conflict of interest: M.D. Kim reports grants from the National Institutes of Health (NIH), Cystic Fibrosis Foundation, Flight Attendant Medical Research Institute, and James and Esther King Florida Biomedical Research Program, during the conduct of the study.
Conflict of interest: N. Baumlin reports grants from the NIH, Cystic Fibrosis Foundation, Flight Attendant Medical Research Institute, and James and Esther King Florida Biomedical Research Program, during the conduct of the study.
Conflict of interest: J.S. Dennis has nothing to disclose.
Conflict of interest: M. Yoshida has nothing to disclose.
Conflict of interest: A. Kis has nothing to disclose.
Conflict of interest: C. Aguiar has nothing to disclose.
Conflict of interest: A. Schmid has nothing to disclose.
Conflict of interest: E. Mendes reports grants from the Florida Department of Health during the conduct of the study and outside the submitted work.
Conflict of interest: M. Salathe reports grants and personal fees for grant review from the NIH and the Flight Attendant Medical Research Institute; grants from the James and Esther King Florida Biomedical Research Program; grants, and personal fees for grant review and committee work from the Cystic Fibrosis Foundation; and grants from the COPD Foundation, all during the conduct of the study; and grants and personal fees from Arrowhead Pharmaceuticals outside the submitted work.
Support statement: This study was funded in part by the Flight Attendant Medical Research Institute (CIA no. 130033 and CIA no. 160011), the James and Esther King Florida Biomedical Research Program (5JK02), the CF Foundation (SALATH14G0; SALATH16G0), and the NHLBI (R01 HL-133240 and R01 HL-139365). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Collaborators GBDT. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017; 389: 1885–1906. doi: 10.1016/S0140-6736(17)30819-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 228–237. doi: 10.1164/rccm.201210-1843CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livraghi-Butrico A, Grubb BR, Wilkinson KJ, et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol 2017; 10: 395–407. doi: 10.1038/mi.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di YP, Zhao J, Harper R. Cigarette smoke induces MUC5AC protein expression through the activation of Sp1. J Biol Chem 2012; 287: 27948–27958. doi: 10.1074/jbc.M111.334375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reidel B, Radicioni G, Clapp PW, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med 2018; 197: 492–501. doi: 10.1164/rccm.201708-1590OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesimer M, Ford AA, Ceppe A, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 2017; 377: 911–922. doi: 10.1056/NEJMoa1701632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podowski M, Calvi C, Metzger S, et al. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. J Clin Invest 2012; 122: 229–240. doi: 10.1172/JCI46215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sailland J, Grosche A, Baumlin N, et al. Role of Smad3 and p38 signalling in cigarette smoke-induced CFTR and BK dysfunction in primary human bronchial airway epithelial cells. Sci Rep 2017; 7: 10506. doi: 10.1038/s41598-017-11038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 2006; 3: 413–417. doi: 10.1513/pats.200601-008AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arkwright PD, Laurie S, Super M, et al. TGF-β1 genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax 2000; 55: 459–462. doi: 10.1136/thorax.55.6.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaggar A, Hector A, Bratcher PE, et al. The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur Respir J 2011; 38: 721–727. doi: 10.1183/09031936.00173210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MD, Baumlin N, Yoshida M, et al. Losartan rescues inflammation-related mucociliary dysfunction in relevant models of cystic fibrosis. Am J Respir Crit Care Med 2020; 201: 313–324. doi: 10.1164/rccm.201905-0990OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells JM, Parker MM, Oster RA, et al. Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPDGene. JCI Insight 2018; 3: e123614. doi: 10.1172/jci.insight.123614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avci E, Avci GA. Important biomarkers that play a role in the chronic obstructive pulmonary disease process. J Med Biochem 2018; 37: 46–53. doi: 10.1515/jomb-2017-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geirsson A, Singh M, Ali R, et al. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation 2012; 126: 11 Suppl. 1, S189–S197. doi: 10.1161/CIRCULATIONAHA.111.082610 [DOI] [PubMed] [Google Scholar]
- 16.Jiao B, Wang YS, Cheng YN, et al. Valsartan attenuated oxidative stress, decreased MCP-1 and TGF-beta1 expression in glomerular mesangial and epithelial cells induced by high-glucose levels. Biosci Trends 2011; 5: 173–181. doi: 10.5582/bst.2011.v5.4.173 [DOI] [PubMed] [Google Scholar]
- 17.Shan M, You R, Yuan X, et al. Agonistic induction of PPARgamma reverses cigarette smoke-induced emphysema. J Clin Invest 2014; 124: 1371–1381. doi: 10.1172/JCI70587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 2013; 945: 109–121. doi: 10.1007/978-1-62703-125-7_8 [DOI] [PubMed] [Google Scholar]
- 19.Fulcher ML, Gabriel S, Burns KA, et al. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2005; 107: 183–206. [DOI] [PubMed] [Google Scholar]
- 20.Schmid A, Sutto Z, Schmid N, et al. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem 2010; 285: 29998–30007. doi: 10.1074/jbc.M110.113621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty DF, Nath S, Poon J, et al. Protein phosphatase 2A reduces cigarette smoke-induced cathepsin S and loss of lung function. Am J Respir Crit Care Med 2019; 200: 51–62. doi: 10.1164/rccm.201808-1518OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid A, Sailland J, Novak L, et al. Modulation of Wnt signaling is essential for the differentiation of ciliated epithelial cells in human airways. FEBS Lett 2017; 591: 3493–3506. doi: 10.1002/1873-3468.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid A, Baumlin N, Ivonnet P, et al. Roflumilast partially reverses smoke-induced mucociliary dysfunction. Respir Res 2015; 16: 135. doi: 10.1186/s12931-015-0294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzanares D, Krick S, Baumlin N, et al. Airway surface dehydration by transforming growth factor beta (TGF-beta) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. J Biol Chem 2015; 290: 25710–25716. doi: 10.1074/jbc.M115.670885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey PR, Tarran R, Garoff S, et al. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol 2011; 45: 592–599. doi: 10.1165/rcmb.2010-0484OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salathe M, Bookman RJ. Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol 1999; 520: Pt. 3, 851–865. doi: 10.1111/j.1469-7793.1999.00851.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisson JH, Stoner JA, Ammons BA, et al. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 2003; 211: Pt. 2, 103–111. doi: 10.1046/j.1365-2818.2003.01209.x [DOI] [PubMed] [Google Scholar]
- 28.Button B, Anderson WH, Boucher RC. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc 2016; 13: Suppl. 2, S156–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson WH, Coakley RD, Button B, et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med 2015; 192: 182–190. doi: 10.1164/rccm.201412-2230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krick S, Grabner A, Baumlin N, et al. Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J 2018; 52: 1800236. doi: 10.1183/13993003.00236-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clunes LA, Bridges A, Alexis N, et al. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol 2008; 32: 201–207. doi: 10.1093/jat/32.3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takizawa H, Tanaka M, Takami K, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2001; 163: 1476–1483. doi: 10.1164/ajrccm.163.6.9908135 [DOI] [PubMed] [Google Scholar]
- 33.Herschlag G, Garcia GJ, Button B, et al. A mechanochemical model for auto-regulation of lung airway surface layer volume. J Theor Biol 2013; 325: 42–51. doi: 10.1016/j.jtbi.2013.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzanares D, Gonzalez C, Ivonnet P, et al. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem 2011; 286: 19830–19839. doi: 10.1074/jbc.M110.185074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krapf M, Kunzi L, Allenbach S, et al. Wood combustion particles induce adverse effects to normal and diseased airway epithelia. Environ Sci Process Impacts 2017; 19: 538–548. doi: 10.1039/C6EM00586A [DOI] [PubMed] [Google Scholar]
- 36.Sun H, Harris WT, Kortyka S, et al. TGF-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS ONE 2014; 9: e106842. doi: 10.1371/journal.pone.0106842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snodgrass SM, Cihil KM, Cornuet PK, et al. TGF-beta1 inhibits CFTR biogenesis and prevents functional rescue of DeltaF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS ONE 2013; 8: e63167. doi: 10.1371/journal.pone.0063167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brewington JJ, Filbrandt ET, LaRosa FJ 3rd, et al. Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight 2018; 3: e99385. doi: 10.1172/jci.insight.99385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comer DM, Elborn JS, Ennis M. Comparison of nasal and bronchial epithelial cells obtained from patients with COPD. PLoS ONE 2012; 7: e32924. doi: 10.1371/journal.pone.0032924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonser LR, Zlock L, Finkbeiner W, et al. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest 2016; 126: 2367–2371. doi: 10.1172/JCI84910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson AG, Ehre C, Button B, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 2014; 124: 3047–3060. doi: 10.1172/JCI73469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00394-2020.SUPPLEMENT (997.6KB, pdf)