Abstract
Objective
We learned about the health condition of people with diabetes during the COVID-19 epidemic through a questionnaire survey. We conducted a randomized controlled study to confirm the effectiveness of remote management using the mobile phone WeChat app on comprehensive management of diabetes mellitus during the COVID-19 epidemic.
Methods
We distributed questionnaires that collected information on the health condition of people with diabetes during the COVID-19 epidemic through the WeChat app. We assigned 90 cases to the intervention group and 90 cases to the control group. The intervention group was managed remotely through the WeChat app, and the control group received traditional medical treatment. The blood glucose, blood pressure, body mass index (BMI), time in range (TIR) and incidence of hypoglycemia were compared after three months of follow-up.
Results
The BMI and postprandial blood glucose (PBG) of the control group at 3 months was significantly higher than that at baseline (P < 0.001), and TIR decreased at 3 months (P < 0.05). There was no significant difference in blood pressure compared with baseline in the control group, while blood pressure decreased in the intervention group (P < 0.05). In the intervention group, fast blood glucose(FBG) and PBG decreased compared with their baseline values, and the TIR level increased, both of which were statistically significant (P < 0.001). The FBG, PBG, and TIR of the intervention group were better than those in the control group at 3 months (P < 0.05). There was no difference in the incidence of hypoglycemia between the two groups.
Conclusion
During the COVID-19 epidemic, diabetes treatment has been facing new challenges, and the traditional treatment mode is limited. Remote management can increase TIR without increasing the risk of hypoglycemia. Remote management can prevent weight gain and improve patients’ self-management and compliance during the COVID-19 epidemic.
Keywords: Diabetes mellitus, Remote management, Blood glucose management, COVID-19, Epidemic
1. Introduction
A novel coronavirus pathogen was found through sequencing analysis of lower respiratory tract samples in December 2019. It was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the disease caused by the novel coronavirus was called COVID-19 [1,2]. On January 30, 2020, the World Health Organization declared the COVID-19 outbreak a public health emergency of international concern and a global disaster. On March 11, 2020, the outbreak escalated into a pandemic and spread across the globe [3]. Many COVID-19 patients have comorbidities, most commonly hypertension, cardiovascular and cerebrovascular diseases, and diabetes mellitus (DM). Patients with diabetes seem to develop a more severe form of the disease that requires intensive care or mechanical ventilation [4]. The mortality of COVID-19 patients with DM is also significantly higher than in those who do not have DM. The epidemiological evidence suggests that patients with DM, compared with those without DM, have a risk of fatal outcome higher than 50% from COVID-19 [5]. Patients with DM are also at higher risk of infection because of innate immune deficiency, which affects phagocytosis, neutrophil chemotaxis, and cell-mediated immunity. At the same time, the incidence of DM in severe COVID-19 cases is higher, which also reflects the high prevalence of type 2 DM (T2DM) in the elderly.
DM, a common metabolic disease, is a global pandemic and has spread from developed countries to emerging economies, especially China [6]. DM has become one of the leading cause of preventable illness and disability, as well as an extraordinary financial burden for our country. Many people with DM have concerns about going to the hospital during the epidemic, leading to delayed medication adjustment and insufficient health education. Also, during the epidemic, limited exercise, irregular diet and poor self-management ability of patients make blood glucose management more difficult. As the epidemic continues, traditional face-to-face medical treatment can no longer meet the needs of people with DM. Telemedicine, defined as medical care provided remotely through audiovisual technology, can provide services for patients with diabetes during the epidemic. Remote management, the use of mobile technology to deliver real-time health information, provide diabetes education, engages patients in their diabetes management to improve their diet, lose weight, and achieve better health outcomes. Remote management has already been demonstrated success in helping patients maintain or improve their health compared with usual care models [7]. The patients displayed a significantly greater reduction in mean body weight by remote management [8], had greater reduction in hemoglobin A1c (HbA1c) on average when compared with patients in non-telemedicine groups [9]. The patients could benefit from time and cost efficiency [10]. In addition, patients were highly satisfied with remote management, especially in patients with gestational DM (GDM) [11]and type 1DM (T1DM) [12]. With the advent of big data era and the development of artificial intelligence, the conventional management strategies of diabetes care will change [13]. During the epidemic, we use the mobile WeChat app for remote management of people with DM. The WeChat app is a free social software widely used by people of different ages in daily life, which makes DM management more convenient and efficient in a special period.
2. Methods
2.1. Study design
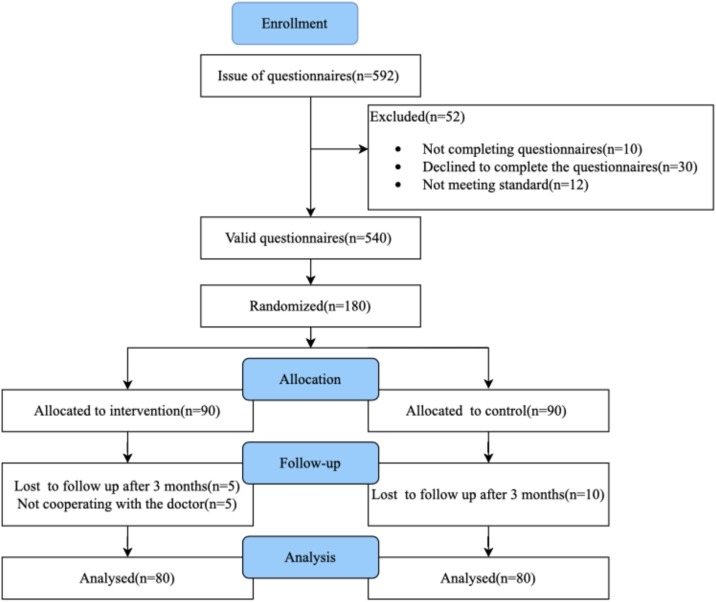
In May 2020, 592 patients who had been diagnosed with DM in the Department of Endocrinology at The Second Hospital of Jilin University and contacted the doctors with the WeChat app participated in the questionnaire survey. Through the WeChat app, we sent a self-designed electronic questionnaire on the health condition of people with DM during the COVID-19 epidemic. A total of 592 questionnaires were sent out, 52 were excluded (30 refused to complete the questionnaire, 10 did not complete all the contents of the questionnaire, and 12 did not meet the standard of questionnaires with conflicting answers to some questions). Finally, 540 valid questionnaires were completed. The results of the questionnaire survey showed that the management of diabetes during the epidemic was difficult. We designed a single center, randomized, controlled trial of diabetes management. A total of 180 patients were included in this study according to strict inclusion and exclusion criteria. The patients were numbered and randomly assigned in a 1:1 ratio to the intervention group or the control group by computer. Each group was followed up for three months. Five patients in the intervention group and ten patients in the control group were excluded who were not followed up after 3 months. Five patients in the intervention group who did not cooperate with doctors to complete the daily follow-up withdrew from study. Finally, 80 patients in the intervention group and 80 patients in the control group were analyzed (Fig. 1 ). The study protocol was approved by the Institutional Review Board of The Second Hospital of Jilin University. All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Fig. 1.
PRISMA flow diagram of the study selection process.
2.2. Sample size
We hypothesized that there would be no change in the control group and a 15% increase in TIR in the intervention group. The superiority margin is 10%. We also estimated that the standard deviation of the mean difference was 20%. At a power of 80%, type 1 error rate of 0.05 (two-tailed), randomization ratio of 1:1, dropout rate of 20%, we need 75 patients in each arm.
2.3. Inclusion and exclusion criteria
Inclusion criteria i). In possession of a smartphone and the WeChat app; ii). Fluent in spoken and written Chinese; iii). Completing valid questionnaires; iv). Signing the informed consent; v). In possession of blood glucose monitoring equipment; vi). Age 18–70 years; vii). Compliance with the diagnostic criteria for T2DM issued by the WHO in 1999 [14]; and viii). On a diet and/or oral antidiabetic agents and/or insulin, with stable doses for the past 3 months.
Exclusion criteria (i). Pregnant women or women planning pregnancy; (ii). Patients with severe complications of diabetes (acute complications, unstable diabetic retinopathy, diabetic nephropathy requiring renal replacement therapy, intolerable diabetic peripheral neuropathy, diabetic foot); (iii). Patients with other severe medical diseases; or (iv). Patients infected with COVID-19.
2.4. Intervention and follow-up
A resident made an electronic version of the questionnaire on the health condition of people with DM during the COVID-19 epidemic. Questionnaire contents included basic information (sex and age), diabetes status (type of diabetes, duration of diabetes, fasting blood glucose [FBG], postprandial blood glucose [PBG], changes of blood glucose, ketosis, frequency of hypoglycemia during the epidemic, adjustment of the treatment regimen, frequency of blood glucose monitoring, blood pressure, body mass index [BMI], diabetes complications [diabetic retinopathy, diabetic nephropathy, diabetic peripheral neuropathy]), psychological status of patients (whether suffering from anxiety or depression, whether disturbed by sleep disorder, whether having concerns about going to the hospital during the epidemic, whether going to the hospital because of diabetes, influencing factors on health during the epidemic). The resident was responsible for sending questionnaires through the WeChat app and the statistics of the questionnaire data. The resident informed of the purpose and content of the study and issued electronic informed consent. The subjects were included in the study and collected the baseline data, including age, sex, BMI, blood pressure, FBG, pre- and postprandial blood glucose and bedtime blood glucose (seven times). We calculated time in range (TIR) according to seven times self-monitoring of blood glucose (SMBG) values.
The control group received traditional medical treatment and regular DM care. Patients in the community or hospitals had face-to-face consultation on prescribing of medication, lifestyle advice, blood glucose monitoring, or learned diabetes knowledge by themselves through other ways according to their own needs. Patients could also choose to contact their doctor by telephone, but the doctor would not actively contact the patient, nor would he supervise the patient’s diet, exercise and blood glucose monitoring. The intervention group was managed remotely through the WeChat app. As an administrator, the resident set up a WeChat group. First of all, the patients received unified training on the main process of the study in the WeChat group. The residents sent remote health education knowledge twice a week, including the video; article link; and voice about the knowledge of the DM onset process, common complications and prevention; basic carbohydrate counting; the necessity of exercise; monitoring guidelines and goals; and psychological knowledge. The resident was responsible for collecting fingertip blood glucose monitoring table (FBG and 2 h PBG), diet and exercise records for two days a week, establishing health logs for each patient and summarizing them. According to the summary results, the attending would answer questions once a week, and a nurse would provide individual psychological counseling to the patients with psychological disorders. If patients suffered from hypoglycemia or hyperglycemia, they could contact the administrator at any time. Doctors would supervise patients’ exercise according to WeChat movement steps. At least, 8000 steps a day was recommended. Patients who reached the standard would be praised and encouraged in the WeChat group according to the daily exercise steps and blood glucose. The intervention group and control group were followed up at three months. BMI, blood pressure and seven times blood glucose were collected again.
2.5. Data analysis
Continuous variables with the normal distribution are expressed as the mean ± standard deviation (SD), and variables with a skewed distribution are expressed by median and quartile. Student’s t-test and Wilcoxon signed-rank test were used for comparison between two groups in variables, which were normally and not normally distributed, respectively. Categorical variables were expressed as frequencies (percentages), and comparisons were assessed using the Chi-square test. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 21.0 for Windows.
3. Results
3.1. Questionnaire results
The general characteristics of DM are present in Table 1 . The percentage of males was 63.3% and that of females was 36.7%. The majority of patients were 40–60 years old, accounting for 53.3%. Patients with T2DM were the most, accounting for 90.6%. The duration of diabetes was 1–5 years in 45.6% of patients. Since the outbreak of COVID-19, 42.8% of the patients had higher blood glucose than before, 6.7% of the patients developed ketosis, and only 30% of the patients adjusted their treatment regimen. Hypertension was found in 41.7% of patients. The results of questionnaire survey showed that the patients with diabetic retinopathy, diabetic nephropathy and diabetic peripheral neuropathy were 21.8%, 21.1% and 23.9% respectively. A total of 26.7% of patients experienced hypoglycemia of different degrees during the epidemic period, the most frequent occurrence was once a month (11.7%). 37.8% of the patients were able to monitor their blood glucose once every 1–3 days. At the same time, in 37.8% of the patients, the interval of blood glucose monitoring was more than one week, including once every two weeks (15%), once a month (8.3%), and once more than one month (14.4%).
Table 1.
General characteristics of participants with DM.
| (n) | (%) | |
|---|---|---|
| Sex | ||
| Male | 242 | 63.3 |
| Female | 198 | 36.7 |
| Age | ||
| <18 | 9 | 1.7 |
| 18–30 | 54 | 10 |
| 31–40 | 99 | 18.3 |
| 41–50 | 159 | 29.4 |
| 51–60 | 129 | 23.9 |
| 61–70 | 66 | 12.2 |
| >70 | 24 | 4.4 |
| Type of diabetes | ||
| Type 1 diabetes | 36 | 6.6 |
| Type 2 diabetes | 489 | 90.6 |
| Gestational diabetes | 9 | 1.7 |
| Special type diabetes | 6 | 1.1 |
| Duration of diabetes | ||
| <1 y | 75 | 13.9 |
| 1–5 y | 246 | 45.6 |
| 6–10 y | 90 | 16.7 |
| 11–15 y | 72 | 13.3 |
| 15–20 y | 39 | 7.2 |
| >20 y | 18 | 3.3 |
| Diabetic complications | ||
| Diabetic retinopathy | 69 | 12.8 |
| Diabetic nephropathy | 114 | 21.1 |
| Diabetic peripheral neuropathy | 129 | 23.9 |
| Changes of blood glucose | ||
| Increased | 231 | 42.8 |
| Decreased | 57 | 10.5 |
| No change | 252 | 46.7 |
| Ketosis | ||
| Yes | 36 | 6.7 |
| No | 504 | 93.3 |
| Adjusting treatment regimen | ||
| Yes | 162 | 30 |
| No | 378 | 70 |
| Hypertension | ||
| Yes | 225 | 41.7 |
| No | 315 | 58.3 |
| Hypoglycemia | ||
| Once a day | 9 | 1.7 |
| Once every 3 days | 21 | 3.9 |
| Once a week | 24 | 4.4 |
| Once every 10 days | 18 | 3.3 |
| Once every half a month | 9 | 1.7 |
| Once a month | 63 | 11.7 |
| None | 396 | 73.3 |
| Blood glucose monitoring | ||
| Once every 1–3 days | 204 | 37.8 |
| Once a week | 132 | 24.4 |
| Once every two weeks | 81 | 15.0 |
| Once a month | 45 | 8.3 |
| Once more than one month | 78 | 14.4 |
As shown in Table 2 , 8.9% of the patients had anxiety or depression disorders; 22.8% of the patients had sleep disorders; and 71.7% of the patients had concerns about going to the hospital due to the epidemic. Only 11.1% of patients went to hospital because of poor blood glucose control. The investigation of the factors affecting health during the epidemic showed that 65.6% of the patients felt it was inconvenient to go to the hospital, 47.2% of the patients were restricted from exercising because of the outbreak, 21.6% of the patients suffered from psychological pressure, and 13.9% of patients faced difficulties in prescribing drugs.
Table 2.
Psychological status of participants during the epidemic.
| (n) | (%) | |
|---|---|---|
| Anxiety or depression | ||
| Yes | 48 | 8.9 |
| No | 492 | 91.1 |
| Sleep disorders | ||
| Yes | 123 | 22.8 |
| No | 417 | 77.2 |
| Concerns about going to hospital | ||
| Yes | 387 | 71.7 |
| No | 153 | 28.3 |
| Going to hospital for diabetes | ||
| Yes | 60 | 11.1 |
| No | 480 | 88.9 |
| Influencing factors on health | ||
| Inconvenient to go to hospital | 354 | 65.6 |
| Prescribing difficulty | 75 | 13.9 |
| Psychological pressure | 117 | 21.6 |
| Restricted exercise | 255 | 47.2 |
| Others | 0 | 0 |
3.2. Follow-up results
There were no significant differences in age, sex ratio, BMI, blood glucose, TIR and blood pressure between the two groups at baseline, as shown in Table 3 .
Table 3.
Clinical characteristics of the control group and intervention group at baseline.
| Control group | Intervention group | t/χ2/Z | P | |
|---|---|---|---|---|
| Age | 47.65 ± 12.41 | 48.83 ± 13.38 | −0.576 | 0.566 |
| Male/Female | 48/32 | 49/31 | 0.026 | 0.871 |
| BMI(kg/m2) | 24.75 (23.10,26.68) | 25.35 (23.56,27.62) | −1.662 | 0.097 |
| FBG(mmol/l) | 8.9 (7.6,11.6) | 8.7 (7.8,10.1) | −0.521 | 0.603 |
| PBG(mmol/l) | 14.88 (11.82,17.90) | 14.5 (12.3,16.2) | −1.198 | 0.231 |
| TIR(%) | 33 (12,52) | 43 (24,56) | −1.455 | 0.146 |
| SBP(mmHg) | 130 (120,125) | 130 (120,140) | −1.354 | 0.176 |
| DBP(mmHg) | 80 (79,90) | 80 (70,90) | −0.048 | 0.962 |
BMI: body mass index; FBG: fasting blood glucose; PBG: postprandial blood glucose; TIR: time in range; SBP: systolic blood pressure; DBP: diastolic blood pressure.
The BMI of the control group, as shown in Table 4 , was significantly higher than that of the baseline at 3 months (P < 0.001). There was no significant difference in blood pressure compared with baseline in the control group, while blood pressure decreased in the intervention group (P < 0.05). In the control group, PBG was significantly higher (P < 0.001), and TIR decreased at 3 months (P < 0.05). In the intervention group, FBG and PBG decreased compared with baseline, and TIR level increased, both of which were statistically significant (P < 0.001). Three months later, the FBG, PBG, and TIR of the intervention group were better than those in the control group (P < 0.05).
Table 4.
Follow up results at 3 months.
| BMI (kg/m2) |
SBP (mmHg) |
DBP (mmHg) |
FBG (mmol/l) |
PBG (mmol/l) |
TIR (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group |
| Baseline | 24.75 (23.10,26.68) | 25.35 (23.56,27.62) | 130 (120,125) | 130 (120,140) | 80 (79,90) | 80 (70,90) | 8.9 (7.6,11.6) | 8.7 (7.8,10.1) | 14.88 (11.8,17.9) | 14.5 (12.3,16.2) | 33 (12,52) | 43 (24,56) |
| 3-months | 25.17 (23.23,27.80) | 25.52 (23.51,27.79) | 128 (121,134) | 130 (120,140) | 80 (78,90) | 80 (70,85) | 8.9 (8.0.10.5) | 8.3 (7.4,8.9)* | 15.7 (13.9,18.8) | 11.0 (8.7,12.4)* | 28 (25,31) | 63 (48,74)* |
| Z | −5.028 | −0.353 | −1.495 | −3.27 | −0.605 | −2.791 | −0.670 | −5.033 | −5.677 | −7.771 | −2.586 | −7.777 |
| P | <0.001 | 0.724 | 0.135 | 0.001 | 0.545 | 0.005 | 0.503 | <0.001 | <0.001 | <0.001 | 0.01 | <0.001 |
P < 0.05 control group VS intervention group.
In the intervention group, five patients withdrew from the study who could not complete the regular blood glucose monitoring, and five patients did not complete the final follow-up. The lost rate of follow-up was 11.1% both in the control group and in the intervention group at 3 months. In the intervention group, 80% of the patients were satisfied with remote management. Hypoglycemia occurred in three patients in the intervention group during the whole process. One patient had hypoglycemia caused by inadequate carbohydrate, and two patients had hypoglycemia caused by excessive exercise. In the control group, three patients experienced hypoglycemia. One patient injected too much insulin, and the other two patients did not have meat in time. There was no difference in the incidence of hypoglycemia between the two groups.
4. Discussion
The outbreak of COVID-19 has become the most serious challenge facing the world in recent years. It is rampant all over the world, forcing people's life and work style to change. As a global disease, the ninth edition of the International Diabetes Federation Diabetes Atlas shows that DM affects 463 million people worldwide. In China, the number of individuals with DM has reached 116 million, accounting for one-third of the total number of DM patients in the world [15]. The epidemic has severely affected people with DM, as they were isolated at home, with limited physical activity, irregular diet and sleep, and weight gain. Because of the concentration of medical resources in epidemic areas and patients’ fear of going to hospital, the treatment of DM has been greatly limited. According to our questionnaire survey results, the traditional face-to-face medical treatment mode could not meet the needs of patients under the epidemic situation, and a new medical treatment mode was urgently needed. With the development of the Internet and the widespread application of smart phones, mobile medicine is emerging to provide health services and improve health conditions through mobile and wireless devices. Mobile medicine is very suitable for remote management of DM, which can break the communication barrier between doctors and patients. Doctors can communicate with patients frequently and conduct health education in time, which can promote blood glucose control and guide self-management [16].
At present, much research on the remote management of DM has been carried out. The common forms of diabetes remote management include follow-up through mobile phone, sending text messages, sending emails, combining information network platforms with intelligent monitoring devices, and applying mobile apps. The main contents of diabetes remote management include diet and exercise guidance [17], blood glucose monitoring [18], health education and psychological support [19]. People with DM can benefit from different ways of remote management. In this study, the WeChat app was used for diabetes remote management. The follow-up results of our study showed that BMI was significantly increased in the control group, while in the intervention group, there was no statistically significant difference in BMI. In our study, the main contribution to BMI were diet and exercise guidance. According to the questionnaire survey, exercise limitation was present in 47.2% of patients during the epidemic. We formulated personalized exercise prescriptions according to the patients’ conditions and distributed knowledge about exercise. At the same time, we used the WeChat movement steps to monitor physical activity. The goal was to achieve 150 min of effective exercise per week, which played an important role in controlling body weight. We also focused on dietary education to make the dietary structure more rational and diversified. Numerous applications on physical activity, diet management and intensive lifestyle intervention had shown success in weight loss [20]. Physical activity under the supervision and guidance of doctors also indicated more effective in reducing weight than on their own [21]. A recent study on remote lifestyle coaching improved Glucose and weight loss for people with T2DM [22].
Use of mobile phone interventions for diabetes self-management can significantly improve glycemic control in individuals with diabetes by promoting improvement in diabetes self-management activities [23]. In this study, because HbA1c examination could not be carried out during the epidemic, we compared blood glucose changes through TIR which were calculated through blood glucose monitoring values at enrollment and 3 months. TIR refers to the time or percentage of 24-h glucose in the target range (usually 3.9–10 mmol/L, or 3.9–7.8 mmol/L) [24]. In our study, TIR is the percentage of 24-h glucose in the range of 3.9–10 mmol/L. TIR can be obtained by continuous blood glucose monitoring or calculated by SMBG of at least 7 points. Studies show that the results are similar [25]. Previous studies found that TIR was associated with the risk of diabetic microvascular and macrovascular complications [26,27]. At 3 months, the FBG and PBG improved significantly, TIR increased by 22% on average through remote management without increasing the frequency of hypoglycemia. In the traditional treatment group, TIR decreased by 6% on average during the outbreak. The FBG, PBG, and TIR of the intervention group were better than those in the control group, indicating the effect of remote management during the epidemic. A meta-analysis of 13 studies on mobile phone apps for diabetes showed that HbA1c in the intervention group decreased by 0.44% on average compared with that in the control group. Meanwhile, it also significantly improved the self-care awareness of people with DM, which proved the potential value of mobile medicine in the management of diabetes [28]. Liang et al. [29] conducted a meta-analysis on 1657 people with T1DM or T2DM who used text messages to send SMBG values and receive self-management information. The results showed that, compared with the control group, the HbA1c in the mobile medical intervention group decreased by 0.5% after 6 months, and the effect in people with T2DM was more significant than that in people with type T1DM.
The supervision of patients’ SMBG is one of the main contents of remote management, and it is also an important factor to promote patients’ blood glucose reaching the standard. SMBG is a powerful tool to prevent hypoglycemia and improve the quality of life in people with DM [30]. Our study found that only 37.8% of patients had SMBG more than once a week. In this study, we sent out knowledge of the necessity and precautions of SMBG, which alleviated patients' anxiety about blood glucose monitoring. Additionally, we required patients to monitor blood glucose for two consecutive days a week. Our result was consistent with previous study, which found a 0.7% reduction in HbA1c in patients undergoing SMBG more than once a day, confirming that SMBG contributes to glycemic control [31]. Odom et al. reported the effect of SMBG combined with the internet on blood glucose. They used wireless glucose meters to connect to a secure portal, through which health care workers could check blood glucose. At least every 2–4 weeks, they contacted each patient by telephone, message, e-mail, instrument information and sent diabetes education knowledge. After two years of follow-up, mean HbA1C was reduced by 1.8%, and patients’ diabetes knowledge, self-management and self-monitoring were improved [32].
People with DM have a certain degree of psychological disorders and sleep disorders. The questionnaire survey in our study showed that 8.9% of the patients had anxiety or depression disorder, 22.8% had a sleep disorder, and 21.6% experienced health impairment due to psychological stress. A meta-analysis of 11 epidemiological studies concluded that people with T2DM who were not depressed at baseline had a 1.24-fold increased risk of developing or relapsing depression in subsequent years [33]. Depression and diabetes often occur at the same time. In the intervention group, the patients could have psychological consultation. The patients were encouraged by the care of the doctors, which alleviated their anxiety. Patients’ confidence in treatment was increased by doctor’s praise and encouragement in the WeChat group. These potential effects enhanced the effectiveness of remote administration. The personal diabetes management study indicated that using a remotely connected diabetes management system was associated with increased treatment satisfaction, reduced diabetes distress, and improved glycemic control in individuals with insulin-treated Diabetes [34]. A study about telecommunications in the management of T2DM also suggested that the application of electronic technology to provide a virtual environment for psychological care had overcome the difficulties of traditional psychological treatment, including face-to-face mode, set number and duration of sessions, and significant personnel resources, providing beneficial psychological services for patients with DM [18].
There are some limitations in our study. Our department did not have a mature remote management system before the epidemic. During the epidemic, in view of feedback on the questionnaire results of patients, we formed a temporary team to conduct the remote management of people with DM using the WeChat app. However, because of the limited doctors and considering the cost, we could not carry out large-scale remote management. Next, we will establish a more complete remote management system and a mature team to help more patients with DM.
5. Conclusion
During the COVID-19 epidemic, diabetes treatment is facing new challenges and the traditional treatment mode is limited. Remote management can manage people with diabetes outside the hospital. Remote management can improve TIR without increasing the risk of hypoglycemia. At the same time, through the supervision and encouragement of doctors, patients can avoid weight gain and improve self-management ability and compliance during the COVID-19 epidemic.
Funding
None.
Conflict of interest
None.
Acknowledgments
None.
References
- 1.Huang C.L., Wang Y.M., Li Y.X.W. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it 2020 [31/03/2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 3.World Health Organization. Rolling updates on coronavirus disease (COVID-19) 2020 [31/03/2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 4.Li B., Yang J., Zhao F., Zhao F.M. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T. The effects of economic development and built environment on diabetes in CHINA. Popul. Health Metr. 2017;15:35. doi: 10.1186/s12963-017-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.W.H., Chan C.K.Y., Chua S.S., Chaiyakunapruk N. Comparative effectiveness of telemedicine strategies on type 2 diabetes management: a systematic review and network meta-analysis. Sci. Rep. 2017;7:12680. doi: 10.1038/s41598-017-12987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leichter S.B., Bowman K., Adkins R.A., Jelsovsky Z. Impact of remote management of diabetes via computer: the 360 study–a proof-of- concept randomized trial. Diabetes Technol. Ther. 2013;15:434–438. doi: 10.1089/dia.2012.0323. [DOI] [PubMed] [Google Scholar]
- 9.Heitkemper E.M., Mamykina L., Travers J., Smaldone S. Do health information technology self-management interventions improve glycemic control in medically underserved adults with diabetes? A systematic review and meta-analysis. J. Am. Med. Inform. Assoc. 2017;24:1024–1035. doi: 10.1093/jamia/ocx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.Y., Lee S.W.H. Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol. Ther. 2018;20:492–500. doi: 10.1089/dia.2018.0098. [DOI] [PubMed] [Google Scholar]
- 11.Alqudah A., McMullan P., Todd A. Service evaluation of diabetes management during pregnancy in a regional maternity hospital: potential scope for increased self-management and remote patient monitoring through mHealth solutions. BMC Health Serv. Res. 2019;19:662. doi: 10.1186/s12913-019-4471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu T., Pujara S., Sutton S., Rhee M. Telemedicine in the management of type 1 diabetes. Prev. Chronic Dis. 2018;15:E13. doi: 10.5888/pcd15.170168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellahham S. Artificial intelligence: the future for diabetes care. Am. J. Med. 2020;133:895–900. doi: 10.1016/j.amjmed.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 14.World Health Orgnization . WHO Document Production Services; Geneva: 2006. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. [Google Scholar]
- 15.Saeedi P., Salpea P., Karuranga S. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020;2:80–86. doi: 10.1016/j.diabres.2020.108086. [DOI] [PubMed] [Google Scholar]
- 16.Shan R., Sarkar S., Martin S.S. Digital health technology and mobile devices for the management of diabetes mellitus: state of the art. Diabetologia. 2019:877–887. doi: 10.1007/s00125-019-4864-7. [DOI] [PubMed] [Google Scholar]
- 17.Martin S.S., Feldman D.I., Blumenthal R.S. mActive: a randomized clinical trial of an automated mHealth intervention for physical activity promotion. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanzola G., Losiouk E., Favero S.D. Remote blood glucose monitoring in mHealth scenarios: a review. Sensors Basel (Basel) 2016;16:1983. doi: 10.3390/s16121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Ozairi E., Ridge K., Taghadom E. Diabetes and TelecommunicationS (DATES) study to support self-management for people with type 2 diabetes: a randomized controlled trial. BMC Public Health. 2018;18:1249. doi: 10.1186/s12889-018-6136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonn S.E., Alexandrou C., Steiner K.H. App-technology to increase physical activity among patients with diabetes type 2-the DiaCert-study, a randomized controlled trial. BMC Public Health. 2018;18:119. doi: 10.1186/s12889-018-5026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colberg S.R., Sigal R.J., Yardley J.E. Physical activity/exercise and diabetes: a position statement of the American diabetes association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollyky J.B., Bravata D., Yang J. Remote lifestyle coaching plus a connected glucose meter with certified diabetes educator support improves glucose and weight loss for people with type 2 diabetes. J. Diabetes Res. 2018;16 doi: 10.1155/2018/3961730. 2018:3961730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmen H., Torbjornsen A., Wahl A.K. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian Randomized Controlled Trial RENEWING HEALTH. JMIR Mhealth Uhealth. 2014;2:e57. doi: 10.2196/mhealth.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battelino T., Danne T., Bergenstal R.M. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck R.W., Calhoun P., Kollman C. Use of continuous glucose monitoring as an outcome measure in clinical trials. Diabetes Technol. Ther. 2012;14:877–882. doi: 10.1089/dia.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J.Y., Ma X.J., Zhou J. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 27.Lu J.Y., Ma X.J., Shen Y. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol. Ther. 2020;22:72–78. doi: 10.1089/dia.2019.0251. [DOI] [PubMed] [Google Scholar]
- 28.Bonoto B.C., de Araújo V.E., Godói I.P. Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth. 2017;5:e4. doi: 10.2196/mhealth.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang X., Wang Q., Yang X., Cao J. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet. Med. 2011;28:455–463. doi: 10.1111/j.1464-5491.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 30.Schnell O., Alawi H., Battelino T. The role of self-monitoring of blood glucose in glucagon-like peptide-1-based treatment approaches: a European expert recommendation. J. Diabetes Sci. Technol. 2012;6:665–673. doi: 10.1177/193229681200600323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans J.M., Newton R.W., Ruta D.A. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999;319:83–86. doi: 10.1136/bmj.319.7202.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odom J.M., Stancil M., Nelson B. Improving diabetes control through remote glucose monitoring in a diabetes self-management program for employees of a health system. Clin. Diabetes. 2019;37:203–210. doi: 10.2337/cd18-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouwen A., Winkley K., Twisk J. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora P., Buskirk A., Lyden M. Use of a novel, remotely connected diabetes management system is associated with increased treatment satisfaction, reduced diabetes distress, and improved glycemic control in individuals with insulin-treated diabetes: first results from the personal diabetes management study. Diabetes Technol. Ther. 2017;19:715–722. doi: 10.1089/dia.2017.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]