Dear editors,
As the COVID-19 pandemic slowly recedes, the number of clinically recovered subjects increases steadily. To minimize the risk of viral transmission in the community, several countries worldwide are currently endorsing a “test-based” strategy for hospital discharge and discontinuation of home-isolation, which requires 2 negative results of RT-PCR for SARS-CoV-2 RNA on nasopharyngeal swabs collected ≥24 h apart.1 Emerging evidences are indicating that RT-PCR positivity may persist for several weeks after the resolution of symptoms,2, 3, 4, 5 while the decline in viral infectivity occurs rather quickly (i.e. within one or two weeks since symptoms onset).5, 6, 7 Whether the persistently-positive recovered patients might still shed infectious virus is thus uncertain, and this implies the risk for many of them to remain hospitalized, or in shelter-in-place, for a much longer time than necessary, with significant social distress and economic commitment.
In order to help in estimating the burden, and the temporal extent, of persistent RT-PCR positivity, and thus support the design of sustainable follow-up protocols, we retrospectively analyzed 13,475 longitudinal SARS-CoV-2 molecular tests (performed from March 3 to June 4, 2020), of 7608 laboratory-confirmed and clinically recovered COVID-19 patients. Patients were tested at symptoms resolution while still hospitalized (N = 501; range of follow-up: 1–53 days), and/or after hospital discharge (N = 7127; range of follow-up post-discharge: 14–74 days); 50.6% of them were male, and median (IQR) age was 51 (41–59) years.
At symptoms resolution, after a median (IQR) of 21 (15–28) days since their onset, nearly half of the 501 hospitalized patients tested (46.9%, N = 235) had detectable virus in nasopharyngeal swabs, in all cases with cycle-thresholds (Cts) values ≥24 (viral-load <1 × 106 by quantitative droplet-PCR). These patients had higher nasopharyngeal viral-load at hospital-admission, compared to those who tested negative (median [IQR] Cts: 27.2 [23.8–32.0] vs. 31.3 [25.2–42.1], respectively; p = 0.016 by Mann-Whitney test), and a shorter duration of disease, calculated from symptoms onset to clinical recovery (median [IQR]= 21 [15–28] vs. 28 [20–36] days; p<0.001).
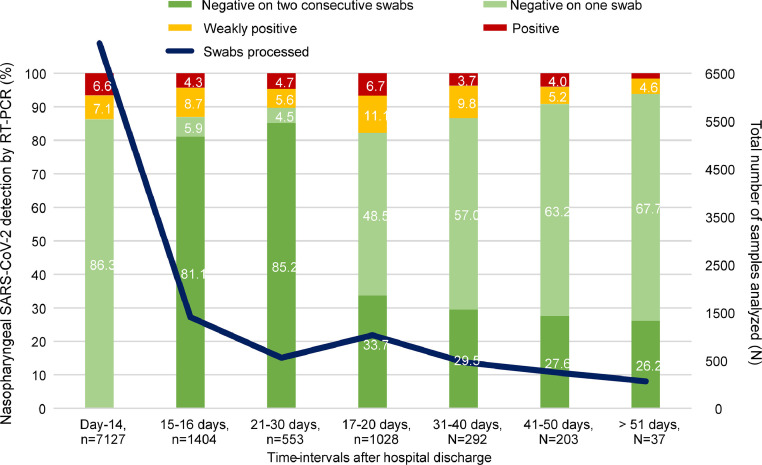
Fig. 1 reports the snapshots of RT-PCR results after hospital discharge.
Fig. 1.
Snapshots of nasopharyngeal SARS-CoV-2 RNA detection by RT-PCR after hospital discharge. The results of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) real-time PCR (RT-PCR) on nasopharyngeal swabs are reported at different time-intervals after hospital discharge, for patients who recovered from Coronavirus Disease 2019 (COVID-19). The total number of nasopharyngeal swabs processed are also reported (blue line). RT-PCR was performed d by AllplexTM 2019-nCoV assay (Seegene) and CobasⓇ SARS-CoV-2 assay (Roche Diagnostics). Positive RT-PCR results are defined by the detection of at least two of three target genes (N, E and RdRP genes), while weakly positive RT-PCR results are defined by the detection of one of them.
At day-14 post-discharge, the positivity rate was still 13.7% (976/7127). Between day-41 and day-60, 28/191 patients (14.7%) were persistently positive, even though with low viral-loads (range Cts: 39.2–43.6; viral-load below 1 × 103). According to current Italian Health Authority and ECDC indications,1 all of them remained in shelter-in-place restriction, yet without proved public health risk. No positive RT-PCR results were observed beyond day-60, in the few patients who reached this time-point (N = 8).
Negative-to-positive RT-PCR fluctuations occurred in 264/2521 patients retested after a first negativity (10.5%), in 90.5% of cases within the first month post-discharge (196/264 [74.2%] within day-20, and 43/264 [12.3%] between day-20 and day-30).
During follow-up, 2182/2655 patients (82.2%) had 2 consecutive RT-PCR negative results, within a median (IQR) of 16 (16–19) days after hospital discharge, and thus discontinued molecular monitoring.
To the best of our knowledge, none of the patients monitored after hospital discharge have ever shown a resurgence of COVID-19 symptoms, regardless of RT-PCR results.
Overall, our results confirm the prolonged RT-PCR positivity in a significant proportion of recovered COVID-19 patients (46.9% at clinical recovery, 13.7% at day-14 after hospital discharge, and 14.7% between day-41 and day-60 after hospital discharge). By taking into account the non-negligible risk for negative-to-positive fluctuations, as also previously reported,2, 3, 4 these long-term positivity rates could be underestimated.
While considering public-health orders, we should underline that RT-PCR positivity after recovery does not necessarily implies the presence of a viable or transmissible virus. Viral culture attempts from respiratory specimens failed after the first 8–18 days since symptoms onset,5, 6, 7 and/or from respiratory specimens characterized by RT-PCR Ct values >24.7 All patients included in our analysis complied with one or both these criteria, ever since their first control after clinical recovery. The data published so far lead us to consider the contagiousness of our persistently positive (and persistently asymptomatic) patients rather unlikely, even if the risk of viral transmission should not be definitely rule out (especially at of few days after the symptoms resolution).
Based on our data, the optimal post-recovery follow-up strategy should include an integrated approach between the exclusively ``symptom based'' one (as recommended by the CDC8) and the one based only on the evaluation of the negativity of the RT-PCR (as recommended by the ECDC1), possibly by integrating the latter with a quantitative evaluation of the viral load. A pressing clinical and social need to define a maximum time and viral load limit beyond which the positivity of the RT-PCR loses significance, is thus felt now more than ever, as this is the only way to allow their safe return to the community, and not to prolong their isolation beyond clinical and public-health utility.
Funding
This work was supported by the Italian Ministry of Education, University and Research [PRIN grant: 20179JKAMZ].
Acknowledgments
We thank Dr. Silvia Nerini and all the staff of the Microbiology and Virology Laboratory of ASST Grande Ospedale Metropolitano Niguarda for outstanding technical support in processing swab samples, performing laboratory analyses and data management.
References
- 1.ECDC . ECDC. 2020. European Centre for Disease Prevention and Control. Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19, 8 April 2020. Stockholm.https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-first%20update.pdf Available from. [Google Scholar]
- 2.Peng J., Wang M., Zhang G., Lu E. Seven discharged patients turning positive again for SARS-CoV-2 on quantitative RT-PCR. Am J Infect Control. 2020 Apr 10 doi: 10.1016/j.ajic.2020.03.017. PubMed PMID: 32317126. Pubmed Central PMCID: 7151314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu W., Chen Q., Wang T. Letter to the Editor: three cases of re-detectable positive SARS-CoV-2 RNA in recovered COVID-19 patients with antibodies. J Med Virol. 2020 May 5 doi: 10.1002/jmv.25968. PubMed PMID: 32369214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff A. COVID-19 follow up testing. J Infect. 2020 May 11 doi: 10.1016/j.jinf.2020.05.012. PubMed PMID: 32407758. Pubmed Central PMCID: 7212964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W.D., Chang S.Y., Wang J.T., Tsai M.J., Hung C.C., Hsu C.L. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020 Apr 10 doi: 10.1016/j.jinf.2020.03.063. PubMed PMID: 32283147. Pubmed Central PMCID: 7151379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 Apr 1 doi: 10.1038/s41586-020-2196-x. PubMed PMID: 32235945. [DOI] [PubMed] [Google Scholar]
- 7.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2020 May 22 doi: 10.1093/cid/ciaa638. PubMed PMID: 32442256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Symptom-based strategy to discontinue isolation for persons with COVID-19. Accessed May 24, 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/strategy-discontinue-isolation.html.