Persistent or fixed airflow obstruction (FAO) is prevalent in up to 60% of patients with severe asthma [1] and is associated with older age, more rapid decline in lung function and increased symptoms [1–3]. The underlying mechanisms of FAO in asthma are unknown, but growing evidence suggests that parenchymal changes resulting in loss of elastic recoil and decreased lung stiffness (i.e. increased lung compliance) contribute to FAO [2, 4]. In a recent study of older asthma patients with FAO, decreased lung stiffness was the sole predictor of more severe airflow obstruction, as measured by reduced forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio [2].
Short abstract
Higher body mass index (BMI) is associated with less severe airway obstruction in older asthma patients with fixed airflow obstruction. This is potentially mediated through BMI-related mechanisms that increase lung stiffness (i.e. reduce lung compliance). https://bit.ly/3jBwCNy
To the Editor:
Persistent or fixed airflow obstruction (FAO) is prevalent in up to 60% of patients with severe asthma [1] and is associated with older age, more rapid decline in lung function and increased symptoms [1–3]. The underlying mechanisms of FAO in asthma are unknown, but growing evidence suggests that parenchymal changes resulting in loss of elastic recoil and decreased lung stiffness (i.e. increased lung compliance) contribute to FAO [2, 4]. In a recent study of older asthma patients with FAO, decreased lung stiffness was the sole predictor of more severe airflow obstruction, as measured by reduced forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio [2].
Obesity is a common comorbidity of asthma, including those with FAO [3], and is associated with increased symptoms and reduced response to standard treatments, but not with more severe airflow obstruction [5, 6]. The relationship between obesity and asthma is complex and potentially involves an interaction of inflammatory, mechanical and genetic factors. The classic abnormality in obesity is reduced operating lung volume, which decreases the lung's recoil on the airways thus reducing airway calibre; this could explain asthma-like symptoms such as dyspnoea and wheeze, even in patients without asthma, and may contribute to airway hyperresponsiveness [7]. In addition, obesity changes the mechanics of the lungs such that lungs are stiffer and elastic recoil is increased (pressure at any given lung volume) [8]. Thus, changes in the lung's elastic properties would theoretically counteract any effects of low lung volume and asthmatic bronchoconstriction on airway narrowing, i.e. obesity might counteract airflow obstruction in asthma. This has not been tested in asthma with no published data on the relationship between body mass index (BMI) versus lung stiffness or elastic recoil in asthmatic participants. Therefore, the aim of this study was to determine the relationship between BMI versus lung stiffness and elastic recoil pressures in asthmatic participants with FAO and a range of impairments of FEV1/FVC. We hypothesised that increasing BMI would increase lung stiffness and increase elastic recoil pressures. Some data from this cohort have been previously published [2]; however, the relationships with BMI examined in this study are novel.
Individuals with a physician diagnosis of asthma who were ≥40 years old and were current nonsmokers with a smoking history ≤5 pack-years were recruited from hospital clinics [2]. All patients were treated with high-dose inhaled fluticasone/eformoterol combination treatment of 1000/40 µg·day−1 for 2 months to control any steroid-responsive inflammation. Participants performed standard pulmonary function tests according to American Thoracic Society/European Respiratory Society (ATS/ERS) standards after 2 months of treatment. Post-bronchodilator spirometry was performed to confirm FAO, defined as an insignificant bronchodilator response post inhaled salbutamol 400 µg with reduced baseline FEV1 [2]. Lung elastic recoil pressure was measured using an oesophageal balloon as previously described [2]. Briefly, the pressure–volume (P–V) curve was constructed from pooled datapoints from five interrupted deflation manoeuvres from total lung capacity (TLC) to functional residual capacity (FRC). At least 30 acceptable datapoints were plotted and an exponential function, V=A−Be−KP, was fitted to the P–V curve between 50% and 100% of TLC using a least-squares fit, where V is volume, A is the horizontal asymptote, and B is the distance between A and the extrapolated y-axis intercept. The ratio B/A, expressed as a percentage, is an index of lung elastic recoil pressure; a high ratio indicates increased recoil pressure (rightward shift of the P–V curve). K is an index of the curvature of the exponential relationship between P and V as a measure of lung stiffness. Decreased K indicates a reduced slope of the P–V curve at lower lung volumes near FRC, hence increased stiffness. Predicted values were calculated using published equations [9]. Correlations between BMI and lung function measurements were assessed using Spearman's rank test.
Eighteen asthmatic participants with a mean age±sd of 64.1±8.0 years were enrolled. Median (IQR) BMI was 27.8 (24.6–31.0) kg·m−2; 5 out of 18 patients were obese (BMI >30 kg·m−2), 8 out of 18 were overweight (BMI 25–30 kg·m−2) and 5 out of 18 were of normal weight (BMI <18.5–24.9 kg·m−2). All participants were taking inhaled corticosteroids with or without a long-acting β-agonist. Mean post-bronchodilator FEV1/FVC ratio was 0.57±0.08 (mean z-score: −2.6±0.7) and was within normal range in two participants despite having reduced FEV1. Both participants had a clear history of asthma with a positive methacholine challenge test, history of positive bronchodilator response and normal TLC measured by plethysmography. Gas trapping, defined as residual volume (RV)/TLC z-score >1.64, was present in 6 out of 18 (33%) participants. Median (IQR) lung elastic recoil (B/A%) in this cohort was 52 (44–77) and was reduced in 5 out of 18 (28%) participants, and median (IQR) lung stiffness (K) was 0.197 (0.131–0.267) cmH2O−1 and was reduced in 9 out of 18 (50%) participants. B/A and K were correlated to each other (rs=−0.53, p=0.02) and 4 out of the 5 participants with low elastic recoil also had reduced lung stiffness.
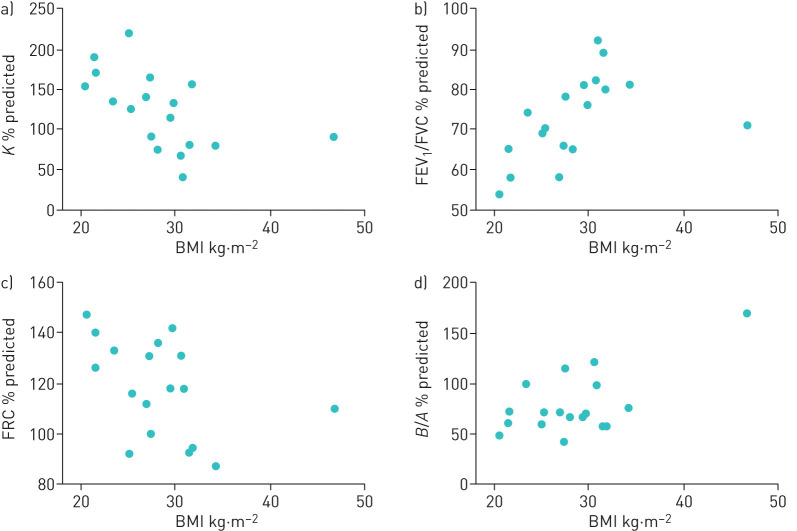
Higher BMI was associated with increased lung stiffness (lower K % predicted) (rs=−0.65, p=0.003) and higher FEV1/FVC % predicted (rs=0.74, p=0.0005) (figure 1a and b). Higher BMI was also related to lower FRC (rs=−0.50, p=0.04) (figure 1c), but not to measurements of lung elastic recoil (B/A%) (figure 1d) (rs=0.28, p=0.26), gas trapping (RV/TLC) (rs=0.10, p=0.7), FVC (rs=−0.30, p=0.23) or TLC (rs=−0.4, p=0.1) (all % predicted).
FIGURE 1.
Univariate correlations between body mass index and a) lung stiffness (K), b) airway obstruction (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC)), c) functional residual capacity (FRC) and d) lung elastic recoil index (B/A) using Spearman's rank correlation. Higher BMI was correlated with lower K (increased stiffness) (rs= −0.65, p=0.003), higher FEV1/FVC ratio (rs=0.74, p=0.0005) and lower FRC (rs= −0.50 p=0.04), but was unrelated to B/A (rs=0.28, p=0.26).
In summary, in this small study of asthma patients over the age of 40 years with FAO and negligible smoking history, higher BMI was associated with less airflow obstruction and with stiffer lungs but was unrelated to lung elastic recoil pressure, FVC or TLC. These results suggest that higher BMI might be protective of airflow obstruction in asthma by opposing the effects of asthma on lung stiffness.
The association between increased lung stiffness (reduced compliance) and increased BMI is consistent with previous findings in healthy individuals [10, 11]; however, these measurements were made in supine position and under general anaesthesia, which may have affected the results. The novel finding in our study is that higher BMI is similarly associated with increased lung stiffness in asthmatic participants with FAO. Although the mechanisms by which obesity increases lung stiffness in asthma are unknown, several mechanisms may contribute. In obesity, pleural pressure is increased (i.e. less negative) and transpulmonary pressure is reduced (i.e. less positive), that is, the lung parenchyma experiences less distending pressure [12]. This may promote peripheral airway closure and atelectasis in the dependent lung zones, which has been observed to be greater in obese participants during bronchoconstriction [13]. Basal airway closure and atelectasis may, in turn, increase lung stiffness. A recent ventilation imaging study in obese but non-asthmatic participants showed increased ventilation to the upper, non-dependent lung regions and altered distributions of airway narrowing during bronchoconstriction [14]. The authors argued that increased lung stiffness may have contributed to the altered ventilation distribution.
Another possible contributing factor to the reduced lung compliance is surfactant dysfunction related to obesity and/or asthma. Pulmonary surfactant reduces the surface tension at the air–liquid interface, preventing airway collapse at end-expiration. Adipose tissue releases systemic inflammatory adipokines and cytokines that may deactivate surfactant, thus increasing surface tension and reducing lung compliance. This is consistent with a previous study that demonstrated an association between alterations in surfactant proteins and increased lung stiffness in an animal model of obesity [15]. The increase in airway closure during bronchial challenge in non-asthmatic obese participants is also in keeping with surfactant dysfunction [13, 16].
Adipose tissue might also affect lung stiffness by its presence in the airways. Transverse airway sections from post mortem lungs of asthmatic and non-asthmatic individuals demonstrated adipose tissue within the airway wall, which was related to BMI [17]. This might result in thickening of the airway wall and increased airway wall stiffness. This in turn may contribute to increased overall lung stiffness since during lung inflation, airways must also distend because of parenchymal tethering [18], thus contributing to the pressure required to inflate the lungs to the same extent. It is, however, difficult to disentangle between effects on lung versus airway wall stiffness.
Lungs become less stiff with age in healthy participants. In the present study, BMI was unrelated to age, and, therefore, the relationships we report are not due to younger participants having a higher BMI. Although lung stiffness was inversely associated with increasing age (rs=0.5, p=0.035), this relationship was marginal and driven by one data point and, therefore, is not likely to be physiologically important.
In summary, we found that higher BMI was associated with less airflow obstruction, which was potentially mediated through BMI-related mechanisms that increase lung stiffness. This study confirms that obesity interacts with asthma in a complex manner and affects the lung elastic properties. Thus, higher BMI may be protective of airflow obstruction in this asthma phenotype but could also alter airway behaviour during airway smooth muscle constriction, thereby modifying the clinical manifestations of asthma in obesity.
Footnotes
This study is registered at www.anzctr.org.au with identifier number ACTRN12615000985583. The data sets generated and/or analysed during the study are available from the corresponding author on reasonable request.
Conflict of interest: S. Rutting has nothing to disclose.
Conflict of interest: D.G. Chapman has nothing to disclose.
Conflict of interest: T. Badal has nothing to disclose.
Conflict of interest: F. Sanai has nothing to disclose.
Conflict of interest: S.C. Zimmermann reports nonfinancial support for travel and accommodation for educational respiratory symposia attendance from GlaxoSmithKline, Boehringer Ingelheim, Menarini, AstraZeneca and Novartis, outside the submitted work.
Conflict of interest: C. Thamrin has nothing to disclose.
Conflict of interest: G.G. King reports unrestricted research grants to support research projects, fees for consultancy services (which include lectures and advisory board services) and conference attendance support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Menarini and MundiPharma, and research grants from the National Health & Medical Research Council, professional societies, The University of Sydney, and philanthropic individuals and societies, outside the submitted work.
Conflict of interest: K.O. Tonga has nothing to disclose.
Support statement: This study was supported by a bridging grant from the University of Sydney, the Lung Foundation Australia Ludwig Engel Grant-in-Aid and a philanthropic grant from the Berg Family Foundation. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Brown T, Jones T, Gove K, et al. . Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J 2018; 52: 1801444. doi: 10.1183/13993003.01444-2018 [DOI] [PubMed] [Google Scholar]
- 2.Tonga KO, Chapman DG, Farah CS, et al. . Reduced lung elastic recoil and fixed airflow obstruction in asthma. Respirology 2019; 25: 613–619. doi: 10.1111/resp.13688 [DOI] [PubMed] [Google Scholar]
- 3.Bennett GH, Carpenter L, Hao W, et al. . Risk factors and clinical outcomes associated with fixed airflow obstruction in older adults with asthma. Ann Allergy Asthma Immunol 2018; 120: 164–168. doi: 10.1016/j.anai.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelb AF, Yamamoto A, Mauad T, et al. . Unsuspected mild emphysema in nonsmoking patients with chronic asthma with persistent airway obstruction. J Allergy Clin Immunol 2014; 133: 263–265. doi: 10.1016/j.jaci.2013.09.045 [DOI] [PubMed] [Google Scholar]
- 5.Schatz M, Hsu JW, Zeiger RS, et al. . Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 2014; 133: 1549–1556. doi: 10.1016/j.jaci.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med 2002; 162: 1477–1481. doi: 10.1001/archinte.162.13.1477 [DOI] [PubMed] [Google Scholar]
- 7.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018; 12: 755–767. doi: 10.1080/17476348.2018.1506331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behazin N, Jones SB, Cohen RI, et al. . Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010; 108: 212–218. doi: 10.1152/japplphysiol.91356.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colebatch H, Greaves I, Ng C. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol Respir Environ Exerc Physiol 1979; 47: 683–691. [DOI] [PubMed] [Google Scholar]
- 10.Tomescu DR, Popescu M, Dima SO, et al. . Obesity is associated with decreased lung compliance and hypercapnia during robotic assisted surgery. J Clin Monit Comput 2017; 31: 85–92. doi: 10.1007/s10877-016-9831-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelosi P, Croci M, Ravagnan I, et al. . The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 1998; 87: 654–660. [DOI] [PubMed] [Google Scholar]
- 12.Loring SH, Behazin N, Novero A, et al. . Respiratory mechanical effects of surgical pneumoperitoneum in humans. J Appl Physiol (1985) 2014; 117: 1074–1079. doi: 10.1152/japplphysiol.00552.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman DG, Berend N, King GG, et al. . Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J 2008; 32: 1563–1569. doi: 10.1183/09031936.00114007 [DOI] [PubMed] [Google Scholar]
- 14.Rutting S, Mahadev S, Tonga KO, et al. . Obesity alters the topographical distribution of ventilation and the regional response to bronchoconstriction. J Appl Physiol (1985) 2020; 128: 168–177. doi: 10.1152/japplphysiol.00482.2019 [DOI] [PubMed] [Google Scholar]
- 15.Inselman LS, Chander A, Spitzer AR. Diminished lung compliance and elevated surfactant lipids and proteins in nutritionally obese young rats. Lung 2004; 182: 101–117. doi: 10.1007/s00408-003-1048-4 [DOI] [PubMed] [Google Scholar]
- 16.Peters U, Subramanian M, Chapman DG, et al. . BMI but not central obesity predisposes to airway closure during bronchoconstriction. Respirology 2019; 24: 543–550. doi: 10.1111/resp.13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliot JG, Donovan GM, Wang KCW, et al. . Fatty airways: implications for obstructive disease. Eur Respir J 2019; 54: 1900857. doi: 10.1183/13993003.00857-2019 [DOI] [PubMed] [Google Scholar]
- 18.Mitzner W, Blosser S, Yager D, et al. . Effect of bronchial smooth muscle contraction on lung compliance. J Appl Physiol (1985) 1992; 72: 158–167. doi: 10.1152/jappl.1992.72.1.158 [DOI] [PubMed] [Google Scholar]