Abstract
The natural history of COVID-19 infection in children is still evolving as the pandemic unfolds. Few cases of severe and often fatal COVID-19 have been reported although the infection is mild in the large majority. Children with cancers are recognised as a high risk group for all infections. Since there aren’t any definite treatment guidelines established in children with severe COVID, treatment is guided by adult recommendations which too are often not evidence based. We report the case of a 4-year-old girl with severe COVID-19 associated pneumonia who presented to us as febrile neutropenia. The use of convalescent plasma along with steroids and IVIG showed dramatic results in this child and she recovered without the need for any specific treatment. This is highlighted as one of the earliest cases that is reporting the use of convalescent plasma in a child; the first ever in a child with underlying malignancy.
Keywords: COVID-19, COVID-19 convalescent plasma, COVID-19 in paediatrics, COVID-19 pneumonia, COVID-19 with acute lymphoblastic leukemia
1. Introduction
The COVID-19 pandemic has been seen as a threat to adults especially those with co-morbidities and the elderly. Data so far on children have shown that they are at low-risk for serious infections [1]. Children with cancers have always been a high risk-group for infections due their underlying immunocompromised state. With particular reference to COVID-19, they have been shielded as much as possible by reducing hospital visits to minimize risk to themselves, their family and healthcare workers. Multiple reports have documented more severe disease in adult oncology patients. In the literature on pediatric cancer patients with COVID, majority have had mild disease or were picked up on mandatory screening. Although there have been few reports of severe disease in pediatric cancer patients, there are still no established guidelines for treating these children [2]. We report a child with severe COVID-19 disease requiring high-flow nasal oxygen therapy with raised inflammatory markers who eventually responded to timely intervention.
2. Case
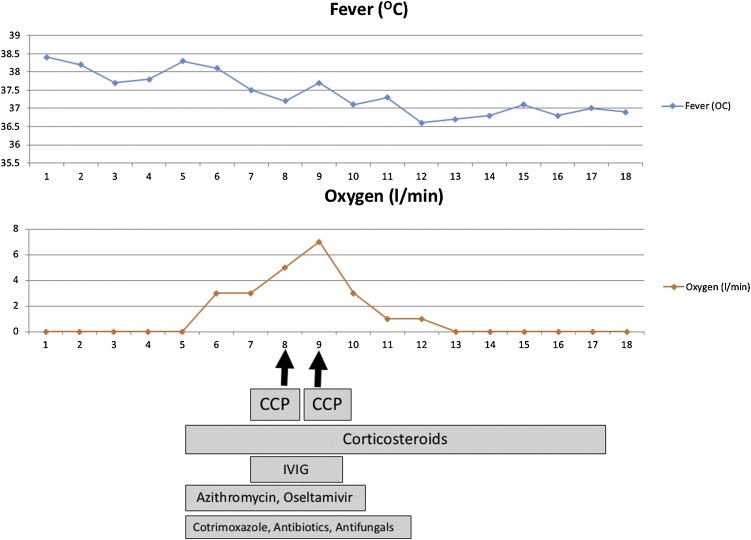
4-year-old girl, with standard-risk B Lineage Acute Lymphoblastic Leukemia in remission and on interim maintenance therapy (6 Mercaptopurine and Methotrexate), presented to us with fever and neutropenia. She had high-grade fever with mild tachypnea (fever associated) and no localizing signs. There was no history of contact with COVID-19 suspect or confirmed patients either at home or in the hospital. All standard precautions such as facemask, hand hygiene and social distancing were being maintained by the family. As per the department protocol, complete blood count was done which showed neutropenia (absolute neutrophil count of 223 cells/μl) and broad spectrum antibiotic (Ceftriaxone) was started after blood culture. She remained stable except for continued fever. She did not have cough, sore-throat, diarrhea, vomiting or difficulty in breathing in the first 3 days of illness and saturation was maintained >98 % in room air. On day 5 of illness, she was found to have increase in respiratory rate with bilateral wheeze. Respiratory rate was 55/min and severe hypoxia was documented (oxygen saturation in room air was 60 %). She was otherwise stable with normal hemodynamic parameters and no intercostal/ subcostal retractions. Although fever had started improving, in view of the hypoxia she was shifted to the COVID-suspect ward and evaluated. The initial COVID rapid antigen and Tru-Nat were reported negative. Oxygen was started at 2–3 litres/min by face mask on which she maintained oxygen saturation >95 %. X-ray chest was suggestive of bilateral fluffy opacities with no evidence of effusion. Antibiotics were escalated to Meropenem, Azithromycin and Amikacin and antifungal (Voriconazole), antiviral (Oseltamivir) and Cotrimoxazole at therapeutic doses (for suspected Pneumocystis jiroveci) were introduced in view of severe hypoxia. The possibilities considered were viral pneumonia (COVID-19, influenza or other viruses) and Pneumocystis pneumonia. Bronchoalveolar lavage was considered; however, in view of suspected COVID it was deferred. Intravenous Immunoglobulin (IVIG) infusion was given at a total dose of 1 g/ kg over the first 3 days of admission in view of low Ig G level (Table1 ). Biochemical investigations revealed raised C-reactive protein, d-Dimer, Ferritin and Procalcitonin which also suggested the strong possibility of COVID-19 infection. Her hypoxia worsened meanwhile, with increasing oxygen requirement to 7 L/min with face mask. RT PCR for SARS−COV-2 RNA from nasopharyngeal swab (which tested for ORF genes and RNA dependent polymerase gene) sent on Day 3 of admission was reported negative and her serology work up for COVID antibodies (IgM) was also negative. In view of a high index of suspicion, RT-PCR was repeated using a different kit (tested for nucleocapsid N-gene and envelop E-gene) which were reported as positive. Steroids were introduced as hydrocortisone initially and was later switched to dexamethasone @0.2 mg/kg once a day. She received COVID convalescent plasma (CCP) @15 mL per kg on Days 8 and 9 of illness. The transfusion episodes were uneventful and there was no transfusion reaction. Both units were derived from different COVID recovered donors with demonstrable IgG antibodies. She showed remarkable improvement by day 10 with reduction of respiratory rate, work of breathing and oxygen requirement. Over the next 4 days, oxygen was tapered and stopped. Antiviral agents Lopinavir-Ritonavir combination and Remdesivir although considered were not given as she improved following introduction of steroids and CCP. Repeat D-Dimer showed reducing trend (Table 1) and hence anticoagulation and Tocilizumab was also was deferred. She was discharged after remaining asymptomatic for 5 days. She remains well on follow up with no symptoms, normal physical examination and vital signs in the 4th week from onset of illness. The timeline of clinical course and treatment is depicted in Fig. 1 .
Table 1.
Lab parameters of the patient.
| Lab Parameters | Normal | Day 2 | Day 3 | Day 5 | Day 7 | Day8 | Day 10 | Day 12 | Day 18 |
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/dl) | 7.9 | 7.4 | 6.6 | 7.1 | 11.4 | ||||
| TLC (/μl) | 1500 | 2120 | 1810 | 7350 | 5880 | ||||
| Neutrophils (/μl) | 223 | 301 | 349 | 2260 | 2160 | ||||
| Lymphocytes(/μl) | 1070 | 1580 | 743 | 2670 | 1860 | ||||
| Platelets (/μl) | 19,000 | 15,400 | 39,500 | 64,900 | 120,000 | ||||
| CRP (mg/L) | 0−6 | 21.7 | 19.8 | ||||||
| Procalcitonin (ng/mL) | <0.5 | 0.86 | |||||||
| LDH (U/dl) | 0−248 | 1127 | |||||||
| D-Dimer (ng/mL) | <500 | 3352 | 877 | ||||||
| S. Ferritin (ng/mL) | 7−140 | 1443 | |||||||
| IgG (mg/L) | 500−1564 | 253 | |||||||
| SARS-CoV-2RNA | RAT: Neg TruNat: Neg | RTPCR ORF Gene: Neg N gene and E gene: Positive | RTPCR ORF Gene: Neg N gene and E gene: Positive |
Fig. 1.
Chronological history of the presentations and treatment of the patient.
3. Discussion
The incidence and severity of COVID-19 infection is remarkably less in children compared to adults. However, severe and fatal forms of the disease have been reported even in children especially in those with underlying illnesses and those who present with hyper-inflammatory state [3]. Children with cancer are a high-risk population; but unlike in adults there has been no report so far on increased prevalence or severity or mortality from this infection. Since the onset, recommendations for care of patients undergoing cancer regarding prevention of COVID-19 and cancer treatment during COVID-19 pandemic have been published [2]. Most treating centers have adhered to a policy of continuation of standard care for curable cancers, with emphasis on reducing hospital visits as much as possible. In suspected and diagnosed patients of COVID-19 with underlying cancer, regional or institutional protocols are adhered to [4].
In the setting of this pandemic, any febrile neutropenic episode is to be considered COVID-19 especially if it occurs in association with suggestive features such as breathlessness, sore-throat or cough [5]. In the absence of a known contact or residence in a containment area, our patient was managed as febrile neutropenia with broad spectrum antibiotic and a close watch on symptoms pertaining to COVID-19. Among the clinical presentation, high grade fever followed by onset of dyspnea and “happy hypoxia” was classically observed in our patient in the first week. However dry cough, sore-throat or gastrointestinal symptoms were absent [6].
Regarding managing the patient, in the absence of solid evidence for any therapeutic agent so far, supportive therapy with early institution of corticosteroids and IV immunoglobulin helped stabilize the patient. The benefit of convalescent plasma in still being debated even in adults. The first living update of the systematic review by Cochrane database on this topic has reported an uncertain benefit [7]. However, the conduct of a randomized trial in this setting has ethical challenges especially in those with severe disease. There has been one published case so far on the use of CCP in a child who incidentally also had an underlying hematological disease [8]. We observed dramatic improvement in supplemental oxygen requirement following infusion of CCP and reduction in viral load within a week without the use of any specific antivirals.
The case is being presented to highlight 3 aspects of this infection. Any patient especially a cancer patient presenting with fever should be evaluated for COVID-19 in today’s scenario. Early diagnosis can help in keeping a close watch on the development of hypoxia and other complications of the disease. Secondly, despite 4 negative prior reports (rapid antigen, Tru-Nat, IgM COVID-19 and RT-PCR ORF gene), we repeated PCR for other genes which came positive. In our current setting, COVID-19 is the foremost cause of severe hypoxia and the clinical index for suspicion and treatment should be very high. Finally, as the disease evolves, prognostic markers and treatment guidelines for severe COVID-19 needs to be well delineated for this subset of population. The positive outcome following use of IVIG, steroids and convalescent plasma alone is being reported here without the need for more specific therapy that is suggested such as Remdesivir, Tocilizumab or other similar agents.
Author contribution
NR, RS, MR were involved in patient care. SA and SD were involved in collecting and issuing convalescent plasma. DS and SR were involved in the microbiological diagnosis. DKG mentored the clinical management. All authors contributed to manuscript preparation and revisions.
Funding sources
This research did not receive any specific graft from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
References
- 1.Kaspers G.J.L. COVID-19: how will this impact children with cancer, now and in the future? Expert Rev Anticancer Ther. 2020;20(7):527–529. doi: 10.1080/14737140.2020.1781621. [DOI] [PubMed] [Google Scholar]
- 2.Stokes C.L., Patel P.A., Sabnis H.S., Mitchell S.G., Yildirim I.B., Pauly M.G. Severe COVID-19 disease in two pediatric oncology patients. Pediatr Blood Cancer. 2020;67(9) doi: 10.1002/pbc.28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oualha M., Bendavid M., Berteloot L., et al. Severe and fatal forms of COVID-19 in children. Arch Pediatr. 2020;27(5):235–238. doi: 10.1016/j.arcped.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari A., Zecca M., Rizzari C., et al. Children with cancer in the time of COVID-19: An 8-week report from the six pediatric onco-hematology centers in Lombardia, Italy. Pediatr Blood Cancer. 2020;67(8) doi: 10.1002/pbc.28410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores V., Miranda R., Merino L., et al. SARS-CoV-2 infection in children with febrile neutropenia. Ann Hematol. 2020;99(8):1941–1942. doi: 10.1007/s00277-020-04115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y., Liu F., Fang Y., et al. A report of clustered COVID-19 in a hematology ward. Blood Adv. 2020;4(12):2736–2738. doi: 10.1182/bloodadvances.2020001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piechotta V., Chai K.L., Valk S.J., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7(7) doi: 10.1002/14651858.CD013600.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figlerowicz M., Mania A., Lubarski K., et al. First case of convalescent plasma transfusion in a child with COVID-19-associated severe aplastic anemia [published online ahead of print, 2020 Jul 1] Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102866. [DOI] [PMC free article] [PubMed] [Google Scholar]