Graphical abstract
Keywords: Mass screening, Seroprevalence, SARS-CoV-2, COVID-19, Italy
Dear Editor,
We read with interest the article by C. Dimeglio and colleagues, in which the authors apply a mathematical model to conclude that a SARS-CoV-2 (Severe Acute Respiratory Sindrome Coronavirus 2) seroprevalence of at least 50% is required to avoid an infection rebound after removal of containment measures1. In our study we found that SARS-CoV-2 seroprevalence was dramatically lower than this threshold, even in an area of initially unrestricted viral circulation.
Italy was the first European country that suffered a wide spread of Coronavirus Disease 2019 (COVID-19), caused by a novel betacoronavirus which was first identified in China and denominated SARS-CoV-2 (Severe Acute Respiratory Sindrome Coronavirus 2), which caused hundreds of thousands of cases2. During the epidemic, testing was restricted to severely symptomatic cases. Consequently, the true extent of the SARS-CoV-2 infection remains unknown.
We estimated SARS-CoV-2 seroprevalence in the municipality of Castiglione d'Adda, a rural town of about 4550 inhabitants located South-East of Milan, which has been heavily affected by SARS-CoV-2 infection since the earliest stages of the epidemic. As of June 21, 2020, 184 confirmed cases of COVID-19 were reported, the large majority of which requiring hospitalization, accounting for about 4% of the total population. At the same time, 47 deaths were officially attributed to COVID-19.
In this study, the entire population of Castiglione D'Adda was invited to perform a lateral-flow immunocromatographic tests on capillary blood (Prima Lab, Switzerland) from the 18th of May to the 7th of June. News about the mass screening was disseminated by the town municipality. A random sample of 562 subjects (stratified per sex and age) was invited to undergo confirmatory tests by chemiluminescent method on venipuncture drawn blood (CLIA, IgG anti-SARS-CoV-2, Abbott, USA) and SARS-CoV-2 PCR on NPS, regardless of RICT results. More detailed information about the randomization procedure and the study design are available on the complete protocol, published on medrXiv pre-print server3.
The analysis of IgG prevalence in the different age groups was performed by logistic regression models with response variable equal to 1 for positive IgG results, and 0 for negative IgG results. Age and gender were included as independent variables. Results were reported in terms of estimated probabilities of being positive to IgG test as a function of age, with respective 95% confidence intervals.
Results presented in this paper are based on 509 people selected in the random sample who agreed to undergo venipuncture to perform CLIA serologies. Characteristics of the selected population are reported in Table 1 .
Table 1.
Characteristics of 509 subjects in the random sample.
| IgG negative (n = 394) | IgG positive (n = 115) | |
|---|---|---|
| Gender (Female) | 200 (50•8%) | 49 (42•6%) |
| Age (years; median, SD) | 46•0, 20•6 | 55•4, 19•5 |
| Age groups: | ||
| 0–19 | 56 (91•8%) | 5 (8•2%) |
| 20–39 | 92 (82•9%) | 19 (17•1%) |
| 40–59 | 142 (78•0%) | 40 (22•0%) |
| ≥ 60 | 104 (67•1%) | 51 (32•9%) |
| Contact with verified case | 93 (23•6%) | 61 (53•0%) |
| Smoker | 92 (23•4%) | 10 (8•7%) |
| Cardiovascular diseases | ||
| CAD/MI | 10 (2•5%) | 3 (4•3%) |
| Arrhythmias | 14 (3•6%) | 5 (4•3%) |
| Hypertension | 68 (17•3%) | 32 (27•8%) |
| Other | 14 (3•6%) | 14 (12•2%) |
| At least one of the above: | 84 (21•3%) | 47 (40•9%) |
| Rheumatic diseases | 19 (4•8%) | 11 (9•6%) |
| Diabetes mellitus | 12 (3•0%) | 6 (6•2%) |
| Chronic Lung diseases | ||
| Asthma | 20 (5•1%) | 2 (1•7%) |
| COPD | 1 (0•3%) | 1 (0•9%) |
| Other | 9 (2•3%) | 4 (3•5%) |
| At least one of the above: | 29 (7•4%) | 7 (6•1%) |
| Oncological pathologies | ||
| Solid Tumors | 20 (5•1%) | 6 (5•2%) |
| Oncochematological | 2 (0•5%) | 2 (1•7%) |
| At least one of the above: | 22 (5•6%) | 8 (7•0%) |
| Symptomatic | ||
| Fever | 65 (16•5%) | 66 (57•4%) |
| Cough | 57 (14•5%) | 31 (27•0%) |
| Anosmia | 23 (5•8%) | 37 (32•2%) |
| Dysgeusia | 27 (6•9%) | 46 (40•0%) |
| Dispnea | 23 (5•8%) | 13 (11•3%) |
| Rush: | 11 (2•8%) | 4 (3•5%) |
| Arthromyalgia | 34 (8•6%) | 36 (31•3%) |
| At least one of the above: | 124 (31•5%) | 89 (77•4%) |
| Other symptoms | 54 (13•7%) | 23 (20•0%) |
Numerical variables are presented as means.
CAD: Coronary Artery Disease; MI: Miocardial Infarction; COPD: Chronic Obstructive Pulmonary Disease.
The overall seroprevalence found in the tested sample was 22.6% (95% confidence interval 17.2%- 29.1%). Interestingly, seroprevalence increases with increasing age (as shown in Table 1).
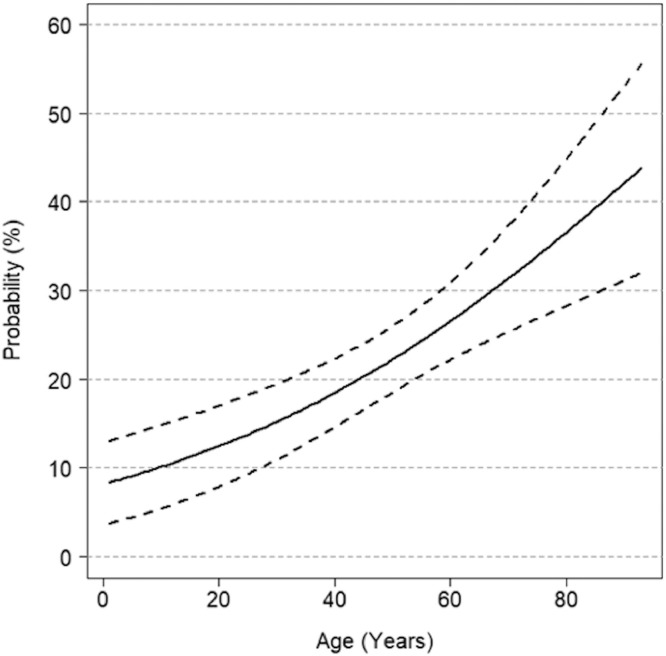
In multivariate analyses, a significant effect of age was found (p<0.0001) while no significant association between IgG positivity and gender emerged (p = 0.2560). The possible existence of a non-linear effect of age was tested by including spline polynomials, without significant results (p = 0.9078). Furthermore, an age/gender interaction effect did not result significant (p = 0.5199). Estimates of probabilities of being positive to IgG test, from a model including only age as independent variable, are reported in Fig. 1 .
Fig. 1.
Estimated probability of IgG positivity as a function of age
Solid line: estimates, dashed lines: 95% confidence intervals.
Since the early phases of the pandemic, advanced age was identified as an independent predictor for severe disease and worse outcomes4. Beside this, it remains unclear if the limited number of cases reported in children5 is due to a milder course of disease, with a larger percentage of asymptomatic cases, or to a lower susceptibility to infection, as our results seem to suggest.
Different ACE2 expression according to age have been postulated to explain clinical expression and susceptibility to the infection. In particular, a higher expression of ACE2 in lung tissues in advanced age groups had been speculated6 , 7. Moreover, a variable susceptibility to other coronavirus such as HCoV-NL63, which also use ACE2 as cell receptor in humans, in different age groups, has been also reported in different age groups8.
Another possible explanation may be that an asymptomatic/pauci-symptomatic infection, more common in younger subjects, could elicit a less marked, or transient, antibody response, as already found in the closely related Middle East Respiratory Syndrome Coronavirus (MERS-CoV)9.
A possible confounding factor in our findings could be related to social distancing measures: schools of any grade were among the first institutions to be closed in Italy, starting from the 5th of March. This could have led to a lower exposure to the infection in children in pre-scholar and scholar age groups.
In conclusion, our findings suggest that SARS-CoV-2 IgG seroprevalence increases with increasing age and these data suggest a lower susceptibility to infection in the lower age groups. These findings have important implications in epidemiology and public health, particularly in designing future population screenings, and could be an important contribution in the re-opening process, especially considered that more than three-fourths of the population could be still susceptible to SARS-CoV-2 infection, even in an area of initially unrestricted viral circulation.
Declaration of Competing Interests
The authors declare no conflicts of interest. All authors have seen and approved the final manuscript.
Acknowledgments
Acknowledgments
MG, GP, FC, DB, AG, RR and EB defined the study protocol. RR, AP, FC, DB and GP cooperated in the practical execution of the study and data gathering. GP drafted a first version of the manuscript, which was then revised and integrated by MG, EB, AG, CEG and SC. CO was responsible of serologies and NPSs execution. EB, PB and GM analyzed the data. All authors approved the final version of the manuscript.
Funding
The study was funded thanks to the non-conditioning economical support from CISOM (Corpo Italiano di Soccorso dell'Ordine di Malta), Banca Mediolanum, Fondazione Rava, Mylan Italia and FC Internazionale Milano.
Ethical approval
The study was approved by University of Milan's Ethical Committee.
References
- 1.Dimeglio C., Loubes J.-.M., Deporte B. The SARS-CoV-2 seroprevalence is the key factor for deconfinement in France. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EpiCentro. Coronavirus | Istituto Superiore di Sanità, https://www.epicentro.iss.it/coronavirus/ (accessed 25th of June, 2020).
- 3.Pagani G., Bernacchia D., Conti F. Dynamics of the SARS-CoV-2 epidemic in the earliest-affected areas in Italy: mass screening for SARS-CoV-2 serological positivity (SARS-2-SCREEN) medRxiv. 2020 2020.06.06.20124081. [Google Scholar]
- 4.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P.-.I., Hu Y.-.L., Chen P.-.Y. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Zhou W., Yang L. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol., 2020. DOI: 10.1002/path.5471. [DOI] [PMC free article] [PubMed]
- 8.Huang S.-.H., Su M.-.C., Tien N. Epidemiology of human coronavirus NL63 infection among hospitalized patients with pneumonia in Taiwan. J Microbiol Immunol Infect. 2017;50:763–770. doi: 10.1016/j.jmii.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe P.G., Perera R.A.P.M., Park W.B. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis. 2017;23:1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]