Abstract
Objective
To determine the risk of dementia after the development of late-onset epilepsy.
Methods
We used data from the Atherosclerosis Risk in Communities (ARIC) cohort study, which started in 1987 to 1989 with 15,792 mostly Black and White men and women from 4 US communities. We identified late-onset epilepsy (LOE; seizures starting at age 67 or later) from linked Medicare claims data. We used a Cox proportional hazards regression model to evaluate associations between LOE and dementia through 2017 as ascertained from neuropsychological testing, interviews, and hospital discharge surveillance, and we used multinomial logistic regression to assess the risk of dementia and mild cognitive impairment in the subset with full neuropsychological assessments available. We adjusted for demographics and vascular and Alzheimer disease risk factors.
Results
Of 9,033 ARIC participants with sufficient Medicare coverage data (4,980 [55.1%] female, 1993 [22.1%] Black), 671 met the definition of LOE. Two hundred seventy-nine (41.6%) participants with and 1,408 (16.8%) without LOE developed dementia (p < 0.001). After a diagnosis of LOE, the adjusted hazard ratio for developing subsequent dementia was 3.05 (95% confidence interval 2.65–3.51). The median time to dementia ascertainment after the onset of LOE was 3.66 years (quartile 1–3, 1.28–8.28 years).
Interpretation
The risk of incident dementia is substantially elevated in individuals with LOE. Further work is needed to explore causes for the increased risk of dementia in this growing population.
New-onset epilepsy affects 90 to 150 per 100,000 older adults,1–3 including >700,000 persons in the US Medicare population alone.3,4 Diagnosed dementia is a known risk factor for epilepsy, with an elevated risk of epilepsy 5 to 10 times that in age-matched adults without dementia5; neuropathologic studies show that 17% of patients with autopsy-confirmed Alzheimer disease (AD), which is the most common neurodegenerative dementia, also have epilepsy.6,7 Conversely, recent studies have also shown an increased risk of incident dementia in patients with adult-onset epilepsy,8–11 and 1 retrospective study showed an increased risk of epilepsy in the preclinical AD stage.12
Possible mechanisms for this co-occurrence include the association of vascular risk factors such as diabetes and hypertension with late-onset epilepsy (LOE),13 which could lead to vascular dementia,14 and β-amyloid and tau, disease proteins that begin to aggregate in the brain before cognitive decline in AD15 and that have been hypothesized as causes of seizures in AD16–19 due to altered synaptic transmission and increased network hyperexcitability.19 We previously showed that cognitive scores decline over time faster in those with LOE than in those without20 and that the APOE4 genotype, a major genetic risk factor for AD21 and for β-amyloid accumulation,22 is itself associated with risk of LOE.13 However, because many risk factors for LOE are also risk factors for dementia,13 the additional effect of LOE on dementia risk in the context of these comorbid conditions is unknown.
We hypothesized an elevated risk of developing dementia after LOE, adjusting for comorbid conditions such as diabetes, hypertension, and APOE4 that are associated with vascular and Alzheimer dementia as well as with LOE. We analyzed prospectively collected longitudinal cohort study data to examine the development of dementia after LOE in individuals without dementia at the time of LOE.
Methods
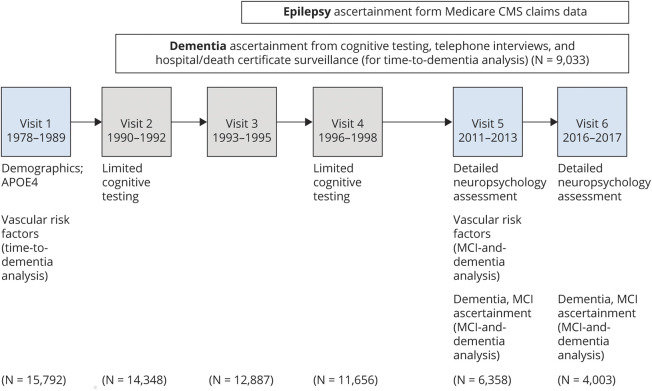
The Atherosclerosis Risk in Communities (ARIC) study is a community-based, longitudinal cohort study that started with baseline visits in 1987 to 1989 among 15,792 mostly Black and White men and women in Jackson, MS; Forsyth County, North Carolina; Washington County, Maryland; and suburbs of Minneapolis, MN23 (figure 1). Participants have attended 7 in-person visits between 1987 and 2019 (visit 7 completed just before this analysis) and have been followed up annually and semiannually (since 2012) with telephone calls. In addition, hospitalization discharge records are collected on all ARIC participants, and Medicare claims data have been linked with participant data.24
Figure 1. Timeline of visits and assessments in ARIC.
Dementia ascertainment for time to incident dementia analysis used surveillance data from hospitalizations and death certificates as well as study cognitive testing. Cognitive status ascertainment for mild cognitive impairment (MCI) and dementia analysis took place after detailed neuropsychological assessments at visits 5 and 6. ARIC = Atherosclerosis Risk in Communities; CMS = Centers for Medicare and Medicaid Services; N = total ARIC participants at each visit.
Identification of LOE
To identify LOE, we used Centers for Medicare & Medicaid Services (CMS) fee-for-service (FFS) outpatient, inpatient, and Carrier claims from 1991 to 2015, linked with ARIC cohort participant data. We defined LOE using ≥2 seizure- or epilepsy-related ICD-9 or -10 primary diagnostic codes (345.00–345.91, epilepsy; 780.39, seizure/convulsion; G40.0–G40.919, epilepsy; and R56.9, seizure/convulsion) from separate visits (1 outpatient and 1 inpatient claim, 2 separate inpatient claims, or 2 claims for separate outpatient visits from the Carrier and outpatient claims). We included only participants with at least 2 years of claims data before the first seizure-related code in order to identify incident epilepsy. We used age at onset of 67 years or later to allow for the 2 years of seizure-free codes (following Medicare eligibility at age 65) before the diagnosis of LOE; thus, age 67 was the earliest at which participants could qualify for a diagnosis of LOE. We included Black participants in Mississippi and North Carolina and White participants in Maryland, Minnesota, and North Carolina and excluded those of other races, as is standard in ARIC due to small numbers.13 To focus on the risk of dementia in persons with LOE without a preexisting condition such as brain tumor, we excluded those with a history of brain tumor, brain surgery, brain radiation, or multiple sclerosis; those with claims-identified seizures prior to age 67; and those who did not give consent for DNA to be used. We also excluded participants without at least 2 years of FFS coverage, those with gaps in FFS coverage, and those whose date of the first seizure-related code was after the date of dementia ascertainment.
Cognitive assessments and identification of dementia and mild cognitive impairment
All ARIC participants who attended in-person visits at visits 2 (1990–1992), 4 (1996–1998), 5 (2011–2013), and 6 (2016–2017) had cognitive testing with the Delayed Word Recall Test, Digit Symbol Substitution Test, and Word Fluency Test. At visits 5 and 6, testing also included the Mini-Mental State Examination, Boston Naming Test, Wechsler Memory Scale-III, Trail Making Test Parts A and B, animal naming, and incidental learning. Those who had predefined deficits and a sample with normal cognition also received additional testing and questionnaires.25 Because visit 7 was completed late in 2019, without completion of adjudicated dementia diagnoses by the time this manuscript was prepared, these data were not included in this analysis.
Dementia in ARIC was classified using assessments obtained at visits 5 and 6, and the time of dementia onset was determined before visit 5 and between visits 5 and 6 from prior interviews (with participants and informants), telephone calls, and hospital discharge surveillance data. For those who did not attend visits 5 and 6 (due to either visit nonparticipation or death), dementia diagnosis and date of onset were also identified from prior interviews, telephone calls, and hospital discharge and surveillance data as previously described.14,25 We used these dementia diagnoses in an analysis of time to dementia.
Mild cognitive impairment (MCI) was also ascertained at visits 5 and 6 using neurocognitive assessments and informant interviews, as previously described.14,25 Because diagnostic criteria rely on standardized cognitive testing, MCI diagnoses could be determined only for individuals seen in-person at visit 5 or 6. We used these dementia and MCI diagnoses in an analysis of MCI and dementia.
Covariates
Age, sex, and race were collected at visit 1. Information on self-reported smoking status and alcohol use was collected at each visit. Blood pressure was measured 2 or 3 times at each visit, averaged, and recorded; hypertension was defined as mean systolic blood pressure ≥140 mm Hg, mean diastolic blood pressure ≥90 mm Hg, or use of an antihypertensive medication. Diabetes was defined as fasting blood glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, use of diabetic medications or insulin, or self-report of physician-diagnosed diabetes. Body mass index (BMI) was calculated from height and weight at each visit. The APOE4 genotype (0, 1, or 2 APOE ε4 alleles) was determined at visit 1 (TaqMan assay; Applied Biosystems, Foster City, CA).26 Prevalent strokes were self-reported at visit 1, and incident strokes during ARIC follow-up are determined in ARIC using surveillance hospital discharge records adjudicated by computer algorithm and independent physician reviewers.27 We used covariates from visit 1 to adjust in the time to incident dementia analysis and covariates from visit 5 to adjust in the MCI and dementia analysis.
We included Black participants in Mississippi and North Carolina and White participants in Maryland, Minnesota, and North Carolina, and we excluded those of other races, as is standard in ARIC due to small numbers.13 We also excluded those with a history of brain tumor, brain surgery, brain radiation, or multiple sclerosis; those with claims-identified seizures before age 67; and those who did not give consent for DNA to be used.
Data analysis
We used Stata 15.0 (StatCorp, College Station, TX) for statistical analysis. A 2-sided value of p=0.05 was considered significant.
We assessed the association of LOE with dementia in 2 ways, first analyzing all participants for whom dementia ascertainment was available to minimize the influence of differential attrition at later visits (time to dementia analysis) and then analyzing data for only those participants who had in-person neuropsychological testing at visits 5 and 6 (MCI and dementia analysis).
Time to incident dementia analysis
To assess the risk of dementia (ascertained from neuropsychological testing, telephone interviews, or surveillance data) after LOE, we used a Cox proportional hazards model with the 67th birthday (the earliest time at which ARIC participants could be eligible for the definition of LOE) as the origin and the date of dementia onset (as ascertained from neurocognitive assessments, surveillance codes, or telephone interviews with participants and informants) as the event time. Censoring occurred at death or date of last contact. We adjusted for baseline (visit 1) age, sex, combined center-race variable, education level, and APOE4 genotype, as well as covariates obtained at visit 1: hypertension, diabetes, smoking history, BMI, alcohol use, and stroke. We used time-varying variables for LOE and stroke (0 before the date of the first event, 1 after). For this analysis, we excluded participants whose date of the first seizure-related code was after the date of dementia ascertainment.
MCI and dementia analysis
To assess the relationship between LOE and MCI or dementia as determined by detailed in-person neurocognitive assessment, we used multinomial logistic regression. Because MCI was ascertained only at visits 5 and 6, we restricted this to participants who attended both visits 5 and 6. We adjusted for demographics and for covariates as measured at visit 5 (hypertension, diabetes, BMI, smoking history, BMI, and alcohol use). To determine the effect of LOE on incident MCI or dementia, we excluded those who had a diagnosis of dementia at visit 5, those with MCI at both visits 5 and 6, and those who had a first seizure date after visit 5.
Interactions
We checked for interactions between LOE and sex, LOE and race, and LOE and APOE4 genotype.
Sensitivity analyses
To investigate the possibility that undiagnosed dementia preceded LOE, we performed a sensitivity analysis in the analysis of time to dementia excluding participants who developed dementia within 2 years of the first seizure-related code. In addition, because of the recently described relationship between some anticholinergic medications such as carbamazepine and dementia,28 we also performed a sensitivity analysis adjusting the analysis of MCI and dementia for use of carbamazepine or oxcarbazepine at visit 5.
Standardized protocol approvals, registrations, and patient consents
All participants provided written informed consent at each study visit (consent was obtained from designated surrogates along with the participant's assent in those with dementia, impaired mental status, or diminished capacity). The institutional review boards at each participating institution approved the study.
Data availability
A public-use ARIC dataset is available through BioLINCC. Additional data are available to researchers who submit a manuscript proposal to the ARIC publications committee; due to participant consent and privacy, the full data are not publically available.
Results
Participants
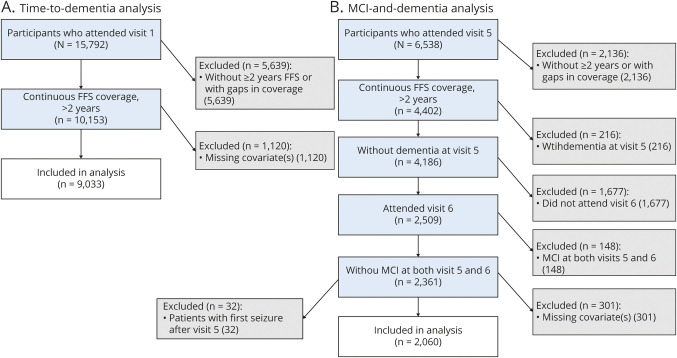
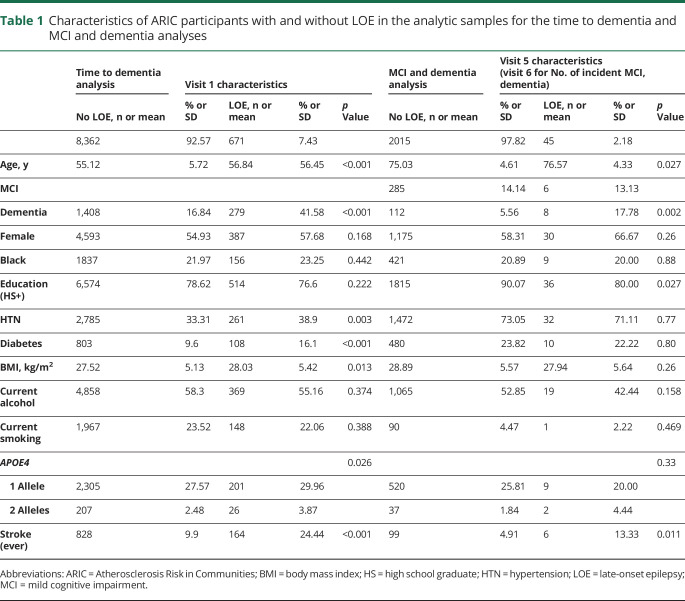
Of 9,033 eligible participants with at least 2 years of continuous FFS coverage (figure 2A), 671 met the definition of LOE with ≥2 seizure-related CMS codes. Participants who developed LOE were slightly older at baseline (56.5 vs 55.1 years, p < 0.001) and more likely to have hypertension (38.9% vs 33.3%), diabetes (16.1% vs 9.6%), and 2 alleles of the APOE4 genotype (3.9% vs 2.5%) than were those without LOE (table 1). Participants with LOE were also more likely to have a stroke at any time than were those without LOE (24.4% compared to 9.9% p < 0.001). These findings are consistent with our previous work showing increased risk of LOE in participants with these characteristics.13
Figure 2. Inclusions for each analysis.
(A) Time to incident-dementia analysis. The analysis of incident dementia uses dementia ascertainment from neuropsychology tests, participant and informant interviews, hospital codes, and death certificates. (B) Mild cognitive impairment (MCI) and dementia analysis. The analysis of visit 5 to 6 incident dementia or MCI uses only participants who attended both visits 5 and 6 and had cognitive status diagnosed after full neuropsychology assessment. FFS = fee-for-service.
Table 1.
Characteristics of ARIC participants with and without LOE in the analytic samples for the time to dementia and MCI and dementia analyses
Dementia
A total of 1,687 individuals developed dementia at some point during follow-up (18.6%). The incidence of dementia was higher in Black participants than in White participants: 21.4 (20.0–22.9) vs 13.7 (13.1–14.4) per 1,000 person-years. Dementia was diagnosed in 279 of the 671 participants with LOE (41.6%) compared to 1,408 cases of dementia among the 8,362 participants without LOE (16.8%; figure 3). The incidence of dementia was 14.8 (14.2–15.4) per 1,000 person-years in participants without LOE and 32.7 (29.2–36.7) per 1,000 person-years in participants with LOE.
Figure 3. Cumulative Incidence of dementia in ARIC participants with and without LOE.
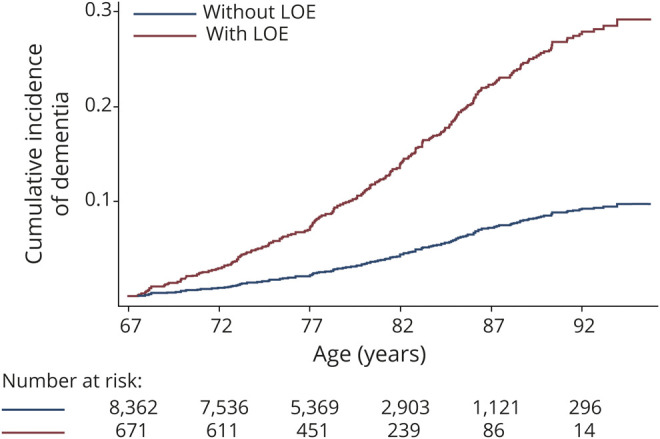
Dementia is ascertained after age 67, the earliest age at which participants could be eligible for the diagnosis of late-onset epilepsy (LOE) in this study. ARIC = Atherosclerosis Risk in Communities.
Time to incident dementia analysis
Fifty participants had a diagnosis of dementia before LOE, and 219 had a diagnosis of dementia after the onset of LOE (2 had their first diagnoses at the same time). Of those diagnosed with dementia after LOE, the median time to dementia ascertainment after development of seizures was 3.66 years (quartile 1–3, 1.28–8.28 years).
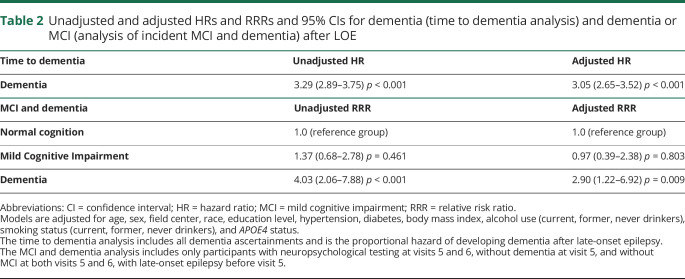
Participants with LOE had a hazard ratio (HR) of 3.29 (95% confidence interval [CI] 2.87–3.75) for developing dementia compared to those without LOE (excluding those who were diagnosed with dementia before the first seizure-related code; table 2). After adjustment for demographics and other vascular risk factors, including stroke, as above, the HR for subsequent dementia associated with LOE was 3.05 (95% CI 2.65–3.51).
Table 2.
Unadjusted and adjusted HRs and RRRs and 95% CIs for dementia (time to dementia analysis) and dementia or MCI (analysis of incident MCI and dementia) after LOE
In this analysis, there was an interaction between LOE and stroke (p for interaction = 0.002), with a stronger relationship with dementia risk in those without stroke. In those without a history of stroke, the HR for dementia after LOE was 3.39 (95% CI 2.89–3.97). In those with a history of stroke, the HR for dementia after LOE was 2.16 (95% CI 1.59–2.94). There were no interactions between race, sex, or the APOE4 genotype and the effect of LOE on the risk of dementia.
MCI and dementia analysis
When we restricted the analysis to only the participants who attended both visits 5 and 6 and had LOE ascertainment available (figure 2B), LOE was again associated with an increased risk of incident dementia; 2,509 participants without dementia at visit 5 attended visits 5 and 6 and had sufficient FFS Medicare information available. Of those, 148 had MCI at both visits 5 and 6 and were excluded. Of the remaining 2,361 participants, 32 with LOE had a first seizure after visit 5 and were excluded to assess the effect of prevalent LOE on incident cognitive impairment. Of the remaining participants, 2,060 had all covariate information available and were included in analysis.
Of these 2,060 participants without dementia at visit 5 and without MCI at both visits 5 and 6, 45 had LOE preceding visit 5. Two hundred ninety-one participants developed MCI (of whom 6 had LOE) and 120 developed dementia (of whom 8 had LOE) by visit 6. After adjustment for demographics and comorbid conditions as above, the relative risk ratio (RRR) for dementia at visit 6 for those with LOE was 2.90 (95% CI 1.22–6.92), while the RRR for MCI was 0.97 (95% CI 0.40–2.38).
There was no interaction between LOE and race, APOE4 genotype, sex, or stroke in the MCI and dementia analysis.
Sensitivity analyses
From the time to incident dementia analysis, we excluded participants who had a diagnosis of dementia within 2 years of the first seizure to exclude individuals in whom early undiagnosed dementia may have actually preceded LOE onset. In this model, the adjusted HR for developing dementia at least 2 years after LOE was 2.11 (95% CI 1.78–2.50). We performed an additional sensitivity analysis excluding 178 participants with LOE who had first diagnosis of dementia within 5 years of the first seizure. In this group, the elevated risk of dementia in persons with LOE persisted, with an adjusted HR of 1.43 (95% CI 1.17–1.76).
Because a recent study associated antiseizure drugs with anticholinergic effect with later dementia,28 we additionally adjusted the MCI and dementia analysis for the use of carbamazepine or oxcarbazepine at visit 5 in the analysis of those participants who attended visits 5 and 6 (for whom detailed medication data were available). In this analysis, results were similar; the RRR of dementia was 2.89 (95% CI 1.21–6.89), and the RRR of MCI was 0.97 (95% CI 0.40–2.38).
Discussion
LOE was associated with an increased risk of subsequent dementia in older adults in both of our models after adjustment for comorbid conditions and history of stroke. Compared to those without LOE, participants with LOE had an ≈3-fold elevated risk of subsequently developing dementia. This increased risk persisted after the exclusion of those with dementia diagnosis within 2 years or 5 years of a first seizure and after adjustment for the use of anticholinergic antiseizure medications associated with dementia.
We previously found that the incidence of LOE is slightly higher in ARIC (3.33 [95% CI 3.08–3.61] per 1,000 person-years after age 60) than in other claims-identified reports, which may be due in part to a high percentage of Black participants in ARIC (who have a higher incidence of LOE, 4.71 [95% CI 4.12–5.40] vs 2.88 [95% CI 2.60–3.18] per 1,000 person-years).13
Prior studies have shown that LOE is associated with an increased risk of later stroke,29,30 and recent studies have similarly suggested an increased risk for incident dementia in those with LOE.8–11 Other retrospective studies have suggested an increased risk of dementia in those with epilepsy at any age,31 with a stronger association in those with epilepsy diagnosed later in life.32 Our study provides further evidence of this increased risk and furthermore adjusts for the possibly confounding effects of risk factors associated with both LOE and dementia (including hypertension, diabetes, and the APOE4 genotype13,14,33,34).
Of all ARIC participants with available ascertainment of dementia, 17% of those with dementia had LOE. This finding is in agreement with the 17% of those with autopsy-confirmed AD who had seizures in 2 previous neuropathologic studies.6,7
Dementia is a known risk factor for LOE,5 with an elevated risk of seizures in patients with AD. The importance of seizures (both clinical and subclinical) in AD is beginning to be recognized, and patients with AD with seizures may have a more rapid cognitive decline than those without seizures.35,36 Seizures can also impair cognition and memory in the absence of dementia; for example, older adults with temporal lobe epilepsy can exhibit transient epileptic amnesia.37 Indeed, there is likely a bidirectional relationship between epilepsy and dementia, with common causative neuropathology, as well as cognitive effects of seizures themselves. The preclinical phase of dementia may be years long, with pathologic changes decades before cognitive impairment is recognized. In particular, amyloid deposition is thought to begin in cognitively normal individuals decades before evidence of cognitive impairment is present.38 Transgenic mouse models of AD demonstrate that animals with pathologic amyloid39 or tau40 have epileptic spiking and seizures. Preexisting amyloid and/or tau pathology may similarly cause both seizures and later cognitive impairment in persons with LOE and subsequent dementia; multiple potential mechanisms for AD pathology to cause seizures have been proposed,19 including altered synaptic transmission, increased network hyperexcitability, reduced GABAergic interneuron activity, and alterations in voltage-gated ion channels.19,41,42
We observed an interaction between LOE and stroke, with a lower (but still substantial) association between LOE and dementia in those with a history of stroke. This may be due to the known strong association between stroke and dementia,43 which may wash out the contributions of LOE to cognitive impairment. There also may be undercapturing of dementia diagnoses among participants with stroke in the ascertainment from CMS codes because physicians may be reluctant to make a separate code for dementia in those with cognitive impairment after stroke.
We did not observe a relationship between LOE and MCI, despite the strong relationship between LOE and dementia. This may be due to greater functional impairment in participants with LOE, making them more likely to be diagnosed with dementia.
Among participants with LOE, 24.4% had a stroke at any time during study follow-up, of whom 139 persons (18.7% of participants with LOE) had a stroke before the development of LOE and therefore likely have poststroke epilepsy. The participants with LOE also have higher rates of hypertension and diabetes than participants without LOE, suggesting that microvascular disease may also be an etiology for LOE in this population.13 This cohort is likely similar to other populations of LOE in whom cerebrovascular disease, neurodegenerative diseases, traumatic brain injury, and brain tumors are major causes.3,44,45 Preclinical, unrecognized neurodegenerative disease is a likely etiology in a subset, even though our findings persisted after the exclusion of participants diagnosed with dementia up to 5 years after the first seizure. The preclinical phase of neurodegenerative diseases lasts for many years; thus, seizures likely occur in a subset of persons due to early dementia-related neuropathologic changes19 in the absence of clinical symptoms in some individuals. MRI was not available for all participants, which precluded identification of some other etiologies such as mesial temporal sclerosis.
The strengths of this study include the detailed, validated neuropsychological assessments used to diagnose dementia and MCI and the high-quality longitudinal data on participant health status and comorbid conditions. Our study also has several limitations. The diagnosis of LOE using ICD codes is a potential limitation due to the risk of misclassification; however, previous studies using this method have shown that the use of ≥2 ICD codes has 94.4% sensitivity and 91.7% specificity for the identification of epilepsy, validated with chart review.46 Similar claims-based definitions are used and generally corroborated and accepted in research on epilepsy in the elderly population.4,47 We were able to adjust for the use of carbamazepine and oxcarbazepine in the analysis of incident MCI and dementia, which have been associated with a moderate increased risk of dementia due to moderate anticholinergic effects (adjusted odds ratio 1.39 [95% CI 1.22–1.57]).28 However, we were not able to adjust for antiseizure medication use in the analysis of time to incident dementia because specific medication use was not available for all participants. While ARIC has clinical record review and adjudication of all clinically recognized strokes, MRI is not available for all participants, so some silent infarcts may have been missed, which could be associated with both epilepsy and cognitive decline. In addition, we do not have information about the type and number of seizures experienced by individual participants, which prevents us from examining the effect of seizures themselves vs underlying conditions that lead to epilepsy. We were also unable to observe a relationship between LOE and incident MCI, which may be due to the relatively small number of participants with seizures occurring before visit 5 who met our inclusion criteria. Finally, while AD is thought to be the primary cause of dementia in this cohort,25 other neurodegenerative dementias are also associated with higher seizure risk,48 and we did not distinguish between Alzheimer and non-Alzheimer dementias.
While there was a risk of bias from differential attrition in the number of participants with LOE who attended visits 5 and 6 for the detailed neuropsychological testing for determination of MCI or dementia (in the MCI and dementia analysis), we also examined the diagnosis of dementia ascertained from telephone interviews (with participants and informants), hospitalizations, and death records, which included 9,033 participants, and results were similar (the analysis of time to dementia).
This study demonstrates an increased risk of dementia in older adults with a previous diagnosis of LOE; however, verification in a clinical population is needed. Further work is also needed to determine whether seizures directly contribute to this risk (and the effects of their types and frequencies) or whether shared neuropathology such as vascular pathology, β-amyloid, or tau explains the risk. Clinicians should be aware of the risk of cognitive impairment and dementia in patients with LOE, who may need assistance with medication management and other activities and who may wish to take this risk into account when planning for future healthcare needs.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- FFS
fee-for-service
- HR
hazard ratio
- ICD
International Classification of Diseases
- LOE
late-onset epilepsy
- MCI
mild cognitive impairment
- RRR
relative risk ratio
Appendix. Authors
Footnotes
Editorial, page 1074
Study funding
The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services, under contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. Neurocognitive data are obtained by grants U01 HL096812, U01 HL096814, U01 HL096899, U01 HL096902, and U01 HL096917 from the National Heart, Lung, and Blood Institute, with funding also provided by the National Institute of Neurological Disorders and Stroke. This study was also supported by contract K24 AG052573 (Dr. Gottesman) from the National Institute on Aging.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology 2004;62(suppl 2):S24–S29. [DOI] [PubMed] [Google Scholar]
- 2.Cloyd J, Hauser W, Towne A, et al. Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res 2006;68(suppl 1):S39–S48. [DOI] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993;34:453–468. [DOI] [PubMed] [Google Scholar]
- 4.Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology 2012;78:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology 1996;46:727–730. [DOI] [PubMed] [Google Scholar]
- 6.Rauramaa T, Saxlin A, Lohvansuu K, Alafuzoff I, Pitkänen A, Soininen H. Epilepsy in neuropathologically verified Alzheimer's disease. Seizure 2018;58:9–12. [DOI] [PubMed] [Google Scholar]
- 7.Mendez MF, Catanzaro P, Doss RC, Arguello R, Frey WH. Seizures in Alzheimer's disease: clinicopathologic study. J Geriatr Psychiatry Neurol 1994;7:230–233. [DOI] [PubMed] [Google Scholar]
- 8.Costa C, Romoli M, Liguori C, et al. Alzheimer's disease and late-onset epilepsy of unknown origin: two faces of beta amyloid pathology. Neurobiol Aging 2019;73:61–67. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami O, Koike Y, Ando T, et al. Incidence of dementia in patients with adult-onset epilepsy of unknown causes. J Neurol Sci 2018;395:71–76. [DOI] [PubMed] [Google Scholar]
- 10.Keret O, Hoang TD, Xia F, Rosen HJ, Yaffe K. Association of late-onset unprovoked seizures of unknown etiology with the risk of developing dementia in older veterans. JAMA Neurol 2020;94158:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keret O, Brauner R, Beninger F, Steiner I, Glik A. New unprovoked idiopathic seizures and later development of dementia, a case series. Alzheimer Dement 2019;15:P709. [Google Scholar]
- 12.Difrancesco JC, Tremolizzo L, Polonia V, et al. Adult-onset epilepsy in presymptomatic Alzheimer's disease: a retrospective study. J Alzheimers Dis 2017;60:1267–1274. [DOI] [PubMed] [Google Scholar]
- 13.Johnson EL, Krauss GL, Lee AK, et al. Association between midlife risk factors and late-onset epilepsy. JAMA Neurol 2018;75:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minkeviciene R, Rheims S, Dobszay MB, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci 2009;29:3453–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paudel YN, Angelopoulou E, Jones NC, et al. Tau related pathways as a connecting link between epilepsy and Alzheimer's disease. ACS Chem Neurosci 2019;10:4199–4212. [DOI] [PubMed] [Google Scholar]
- 18.Sen A, Capelli V, Husain M. Cognition and dementia in older patients with epilepsy. Brain 2018;141:1592–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer's disease: causes and clinical relevance. Lancet Neurol 2017;16:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson EL, Krauss GL, Walker KA, et al. Late-onset epilepsy and cognitive trajectories. AES Annu Meet Abstr Database 2019;AESnet.org:A.385. [Google Scholar]
- 21.Corder E, Saunders A, Strittmatter W, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ARIC Investigators. The Atherosclerosis Risk in Communities study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 24.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of heart failure events in Medicare claims: the Atherosclerosis Risk in Communities (ARIC) study. J Card Fail 2016;22:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimer Dement (Amst) 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Schneider ALC, Zhou Y, et al. The ARIC-PET amyloid imaging study brain amyloid differences by age, race, sex, and APOE. Neurology 2016;87:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke. Stroke 2014;45:3219–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia. JAMA Intern Med 2019;179:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wannamaker BB, Wilson DA, Malek AM, Selassie AW. Stroke after adult-onset epilepsy: a population-based retrospective cohort study. Epilepsy Behav 2015;43:93–99. [DOI] [PubMed] [Google Scholar]
- 30.Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet 2004;363:1184–1186. [DOI] [PubMed] [Google Scholar]
- 31.Breteler MM, de Groot RR, van Romunde LK, Hofman A. Risk of dementia in patients with Parkinson's disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol 1995;142:1300–1305. [DOI] [PubMed] [Google Scholar]
- 32.Breteler MMB, Van Duijn CM, Chandra V, et al. Medical history and the risk of Alzheimer's disease : a collaborative re-analysis of case-control studies. Int J Epidemiol 1991;20:S36–S42. [DOI] [PubMed] [Google Scholar]
- 33.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years. Ann Intern Med 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers RH, Schaefer EJ, Wilson PW, et al. Apolipoprotein E epsilon4 association with dementia in a population-based study: the Framingham study. Neurology 1996;46:673–677. [DOI] [PubMed] [Google Scholar]
- 35.Vossel KA, Ranasinghe KG, Beagle AJ, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer's disease. Ann Neurol 2016;80:858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker J, Libretto T, Henley W, Zeman A. A longitudinal study of epileptic seizures in Alzheimer's disease. Front Neurol 2019;10:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler CR, Graham KS, Hodges JR, Kapur N, Wardlaw JM, Zeman AZJ. The syndrome of transient epileptic amnesia. Ann Neurol 2007;61:587–598. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gureviciene I, Ishchenko I, Ziyatdinova S, et al. Characterization of epileptic spiking associated with brain amyloidosis in APP/PS1 mice. Front Neurol 2019;10;1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sánchez M, García-Cabrero A, Sánchez-Elexpuru G, Burgos D, Serratosa J. Tau-induced pathology in epilepsy and dementia: notions from patients and animal models. Int J Mol Sci 2018;19:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng A, Wang J, Ghena N, et al. SIRT3 haploinsufficiency aggravates loss of GABAergic interneurons and neuronal network hyperexcitability in an Alzheimer's disease model. J Neurosci 2020;40:694–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin J, Reiman EM, Beach TG, et al. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology 2020;94:e2404–e2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mijajlović MD, Pavlović A, Brainin M, et al. Post-stroke dementia: a comprehensive review. BMC Med 2017;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsay RE, Macias FM, Rowan AJ. Diagnosing epilepsy in the elderly. Int Rev Neurobiol 2007;81:129–151. [DOI] [PubMed] [Google Scholar]
- 45.Lühdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia 1986;27:458–463. [DOI] [PubMed] [Google Scholar]
- 46.Reid AY, St Germaine-Smith C, Liu M, et al. Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res 2012;102:173–179. [DOI] [PubMed] [Google Scholar]
- 47.Choi H, Pack A, Elkind MSV, Longstreth WT, Ton TGN, Onchiri F. Predictors of incident epilepsy in older adults: the Cardiovascular Health Study. Neurology 2017;88:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beagle AJ, Darwish SM, Ranasinghe KG, La AL, Karageorgiou E, Vossel KA. Relative incidence of seizures and myoclonus in Alzheimer's disease, dementia with Lewy bodies, and frontotemporal dementia. J Alzheimers Dis 2017;60:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A public-use ARIC dataset is available through BioLINCC. Additional data are available to researchers who submit a manuscript proposal to the ARIC publications committee; due to participant consent and privacy, the full data are not publically available.