Abstract
Objective
To test the hypothesis that patients with deep intracerebral hemorrhage (ICH) would encounter hematoma expansion (HE) more frequently compared to patients with lobar ICH.
Methods
Patients with ICH with neuroimaging to calculate HE were analyzed from the multicenter Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) cohort. Patients with laboratory coagulopathy or preceding anticoagulant use were excluded to assess relationships of ICH location alone (deep vs lobar) with HE, defined as >33% relative growth. Odds ratios (ORs) and 95% confidence intervals (CIs) for these relationships were estimated with logistic regression. Sensitivity and specificity determined HE thresholds best associated with poor 3-month outcomes (modified Rankin score 4-6) stratified by location.
Results
There were 1,049 patients with deep and 408 patients with lobar ICH analyzed. Deep ICH locations were more likely to have HE (adjusted OR 1.57, 95% CI 1.08–2.29) after adjustment for age, sex, race, baseline hematoma size, and intraventricular hemorrhage. However, this difference was nonsignificant (adjusted OR 1.35, 95% CI 0.81–2.24) after controlling for time from symptom onset to admission CT in a subgroup analysis of 729 patients with these data. Yet, the threshold of HE best associated with poor outcomes was smaller in deep (30%) compared to lobar (50%) ICH.
Conclusions
While HE was more frequent in deep than lobar ICH, this could be due to differences in symptom onset to admission CT times in our cohort. However, patients with deep ICH appear particularly vulnerable to the deleterious effects of small volumes of HE. Further studies should clarify whether ICH location needs to be considered in HE treatment paradigms.
Current spontaneous intracerebral hemorrhage (ICH) treatment approaches largely focus on acute blood pressure control and reversal of coagulopathy in efforts to prevent hematoma expansion (HE), a well-identified, potentially modifiable cause of worse outcome after ICH.1 Commonly identified factors associated with HE include larger baseline hematoma size, preceding anticoagulant medication use, and more rapid times from symptom onset to initial neuroimaging.2 However, recent ICH trials focused on rapid procoagulant treatments to prevent HE have not yielded improved clinical outcomes.3–5 It is unclear whether this is due to shortcomings of the treatments themselves or limitations of a homogeneous treatment approach for a heterogeneous disease.
The heterogeneity of ICH is apparent in comparisons of the most common spontaneous, primary ICH locations seen in clinical trials and clinical practice: deep and lobar ICHs. Specifically, deep ICH is known to have worse outcomes compared to lobar ICH.6–8 This is thought to be driven primarily by differences in anatomic structural injury and mass effect compromise of midline structures rather than HE differences between these locations. A commonly used threshold of HE in clinical trials and observational studies is >33% relative or >6 mL absolute growth from baseline admission scan. However, it is unclear whether deep and lobar ICHs differ in radiographic HE outcomes given potential limitations of including absolute growth (>6 mL) thresholds to define HE in prior studies.2,9–12 Relative HE thresholds (i.e., >33%) may be better for defining HE when groups with largely different baseline hematoma volumes are compared.12
Current clinical trials and observational studies often analyze deep and lobar ICHs together despite the aforementioned heterogeneity between these locations. A large, multicenter dataset confirming HE differences between deep and lobar ICHs, not related to medication effect, would provide a rationale to account for the radiographic and clinical outcome heterogeneity of these ICH locations and could change clinical practice and trial enrollment. We hypothesized that in patients with spontaneous, primary ICH without preceding anticoagulant medication use, deep locations would be more likely to encounter relative HE (primarily defined as >33% relative growth, the most commonly used relative HE threshold) compared to lobar locations. We in addition explored the effects of HE on the relationship between ICH location and outcome and sought to identify HE thresholds best associated with poor outcome, stratified by deep and lobar locations, using a large, multicenter ICH cohort.
Methods
The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study is a large, prospective, multiethnic, multicenter ICH cohort study. ICHs secondary to vascular malformation/aneurysm, venous sinus thrombosis, malignancies, and ischemic stroke with hemorrhagic transformation are excluded from ERICH. For patients enrolled, baseline demographic, race/ethnicity (self-reported as non-Hispanic Black, non-Hispanic White, Hispanic), medical history, medication use, clinical data, radiographic data, date of symptom onset, admission laboratory values, and 3-month outcomes were collected by trained study staff with methodology described previously.13 Enrollment was conducted from 2010 to 2016.
Standard protocol approvals, registrations, and patient consents
This observational study was approved by the Institutional Review Board at each enrolling site with informed consent obtained from all patients or family when appropriate.13
Patient selection
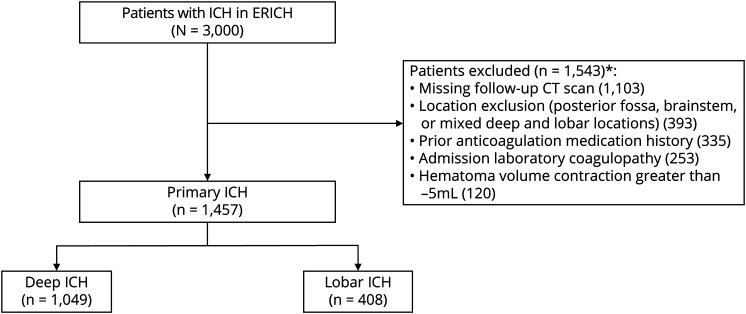
Deep or lobar ICHs with both baseline and follow-up CT imaging were included. ICH location was adjudicated by a blinded neuroimaging core analysis review according to suspected origin of hemorrhage. ICH location was defined as deep if the origin of the hemorrhage territory was a proximal branch of the middle, anterior, or posterior cerebral arteries (i.e., lenticulostriate and thalamostriate vessels). Lobar ICH was defined if the origin of hemorrhage was a distal cortical branch of the middle, anterior, or posterior cerebral arteries. Patients with brainstem/infratentorial ICH, ICH in mixed/multiple locations, primary intraventricular hemorrhage (IVH), and symptom onset to admission CT of >1 day were excluded. To best assess the association of ICH location alone on HE, patients with preceding anticoagulant use (oral or IV) or coagulopathy on admission laboratory results6 (spontaneously elevated international normalized ratio >1.7, platelet count <50 × 103/µL) were also excluded (figure).
Figure. Patient inclusion and exclusion.
ERICH = Ethnic/Racial Variations of Intracerebral Hemorrhage; ICH = intracerebral hemorrhage. *Each category is not mutually exclusive.
Neuroimaging
Semiautomated hematoma size measurements (Alice software; Parexel Corp, Waltham, MA) were estimated for all CT scans with the use of a centralized, blinded neuroimaging core and previously described protocols.13,14 The initial admission head CT scan was measured as the baseline scan. A follow-up head CT within 2 days of admission was chosen for final hematoma size measurements for HE calculations. If multiple scans were obtained within these 2 days, the final head CT was chosen for hematoma size measurement to best assess HE over that time period. The exact time from symptom onset to admission CT (hours) was calculated when available. The primary radiographic outcome was HE defined with a well-validated threshold of >33% relative HE9; this approach accounts for expected baseline hematoma size differences between deep and lobar ICHs. HE was secondarily assessed as a continuous variable of absolute expansion (milliliters) and other validated definitions of HE: (1) >33% or >6 mL and (2) >6 mL growth. Although continuous measurements of hematoma change can occasionally yield negative values (i.e., hematoma contraction), we excluded patients who were found to have large negative hematoma volume changes >−5 mL. This was performed to best account for confounders such as acute surgical treatment before follow-up CT scan creating nonphysiologic negative changes in hematoma size (figure).
Outcome assessment
The modified Rankin Scale score (dichotomized as good 0–3, poor 4–6) at discharge and 3-month outcomes were used as the clinical outcome measures. Three-month outcomes were obtained via standardized phone interviews by trained research staff, as described previously.13
Statistical analysis
Intergroup differences were determined by applying the Mann-Whitney U or t test for continuous variables and the χ2 or Fisher exact test for categorical variables. Logistic regression (or linear regression for models with HE defined as a continuous variable of absolute growth in milliliters) was used to assess the a priori hypothesis that there would be an association of deep ICH location with increased odds of HE after adjustment for expected demographic/clinical differences in ICH location and previously identified covariates of HE: baseline ICH volume,2 age, sex, race, and presence of IVH. In case of missing data, complete case analysis was performed if these made up <15% of the total cohort. A separate subgroup analysis was performed for patients with ICH with available data on symptom onset to admission CT time, which was incorporated as an adjusted covariate in the HE outcome models.
We also used logistic regression models to assess the association of ICH location with poor discharge and 3-month outcomes after adjusting for significant intergroup differences and covariates of ICH outcome chosen a priori: baseline ICH volume, age, and presence of IVH,15 along with sex and race. A mediation analysis was performed to estimate the effect of HE (as the mediator) on the relationship between ICH location (independent variable) and poor 3-month outcomes (dependent variable). We in addition assessed the sensitivities and specificities of relative HE (percent) thresholds best associated with poor 3-month outcomes stratified by location: deep vs lobar. Receiver operating characteristic curves were generated separately for deep and lobar ICH locations, and area under the curve analyses were performed to assess the ability of HE (as relative continuous measurement of percent growth) to predict poor outcome for each ICH location. Statistical significance was assessed at p < 0.05. Analyses were performed with SAS (SAS Institute, Cary, NC).
Data availability
The data on which this article is based can be obtained on reasonable request to the corresponding author.
Results
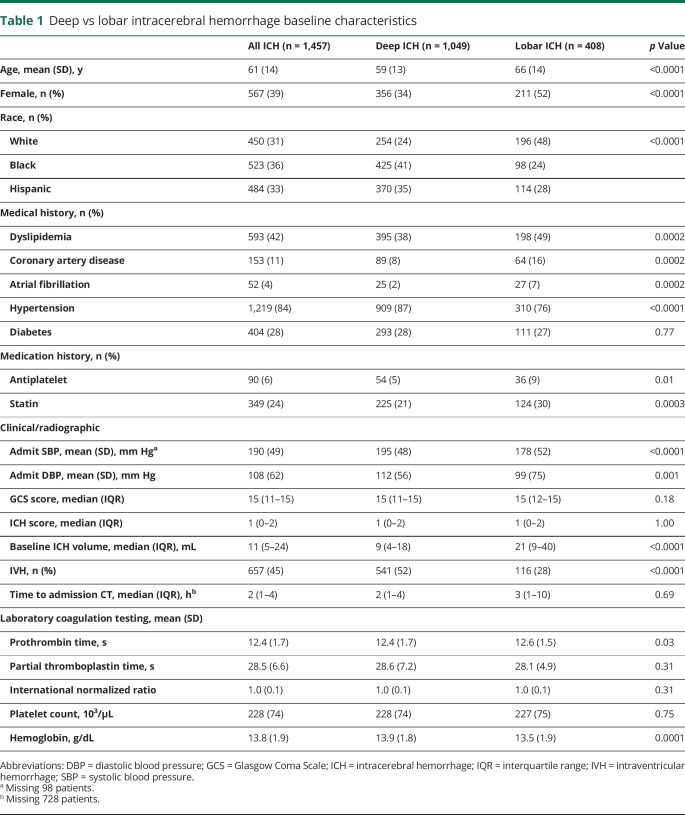
Of 3,000 patients with spontaneous ICHs enrolled in ERICH, 1,457 patients met inclusion criteria for this analysis (figure). Of these ICHs, 1,049 were deep (72%) and 408 were lobar (28%) in location. Notable differences were seen between patients with deep and lobar ICHs. Patients with deep ICHs were significantly younger, were predominantly male, and had a higher prevalence of hypertension but less dyslipidemia and cardiac comorbid conditions compared to patients with lobar ICH. There were also notable racial differences seen between ICH locations, specifically with more Black and Hispanic patients having deep ICH. Patients with deep ICH had worse admission Glasgow Coma Scale scores, higher admission systolic blood pressure (SBP), smaller baseline hematoma volumes, and more IVH compared to patients with lobar ICH. No clinically relevant differences in admission plasma–based coagulation testing were noted. Of patients with available symptom onset to admission CT times, there were no significant time differences between groups (table 1).
Table 1.
Deep vs lobar intracerebral hemorrhage baseline characteristics
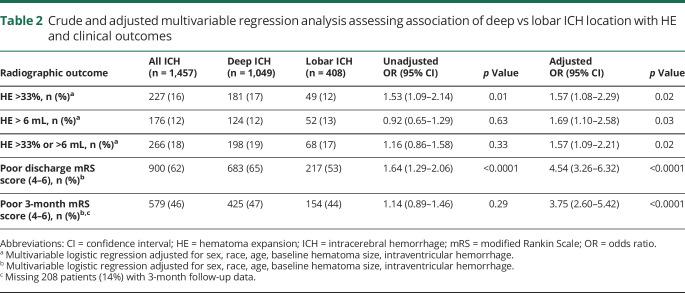
Deep ICH was associated with increased odds of HE (adjusted odds ratio [OR] 1.57, 95% confidence interval [CI] 1.08–2.29) compared to lobar ICH. There was also an association of deep ICH with increased odds of HE defined as >6 mL absolute growth (adjusted OR 1.69, 95% CI: 1.10–2.58) and with the use of traditional HE definitions that combine both relative and absolute growth: >33% or >6 mL (adjusted OR 1.57, 95% CI 1.09–2.21) (table 2). In analyses evaluating HE as a continuous variable of absolute growth (milliliters), we conversely identified an association of deep ICH with less absolute HE growth compared to lobar ICH (unadjusted β = −1.09, 95% CI −2.09 to −0.08). However, there was no significant association after adjustment for baseline ICH volume, age, sex, race, and IVH (adjusted β = 0.97, 95% CI −0.16 to 2.10). In a subgroup analysis of patients with data available on time from symptom onset to admission CT (n = 729), the association of deep ICH with increased odds of HE was nonsignificant (adjusted OR 1.35, 95% CI 0.81–2.24). Adding sensitivity analysis to the primary model, adjusting for differences in admission SBP, medical history of hypertension, and prior antiplatelet medication use as a confounders in addition to the other covariates, did not influence the effect of deep ICH with increased odds of HE (adjusted OR 1.62, 95% CI 1.11–2.36).
Table 2.
Crude and adjusted multivariable regression analysis assessing association of deep vs lobar ICH location with HE and clinical outcomes
Deep ICH location was associated with increased odds of poor outcomes at both discharge (adjusted OR 4.54, 95% CI 3.26–6.32) and 3-month follow-up (adjusted OR 3.75, 95% CI 2.60–5.42) (table 2). Mediation analysis revealed that the relationship of deep ICH with poor 3-month outcomes was mediated in part by HE (Sobel test p = 0.02). Specifically, HE accounted for ≈15% of the relationship of deep ICH and poor outcome.
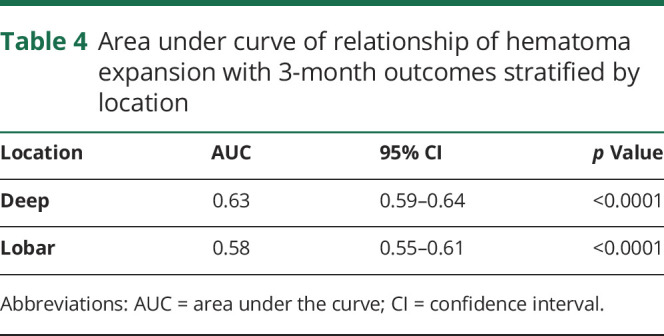
When using sensitivity and specificity to determine optimal HE thresholds associated with poor 3-month outcomes, we identified that the relative HE (percent) growth associated with poor 3-month outcomes was 30% for deep and 50% for lobar ICH. This translated to a threshold of 2.5 mL of absolute growth in deep ICH compared to 10.5 mL for lobar ICH. While these thresholds showed the highest sensitivity and specificities compared with these clinical outcomes, the overall sensitivity and specificities were low, and there was poor separation between these thresholds (table 3). In addition, the area under the receiver operating characteristic curve for relative HE (continuous percent) growth predicting poor outcome was 0.63 for patients with deep ICH and 0.58 for patients with lobar ICH (table 4).
Table 3.
HE thresholds best associated with 3-month outcomes in deep and lobar ICH
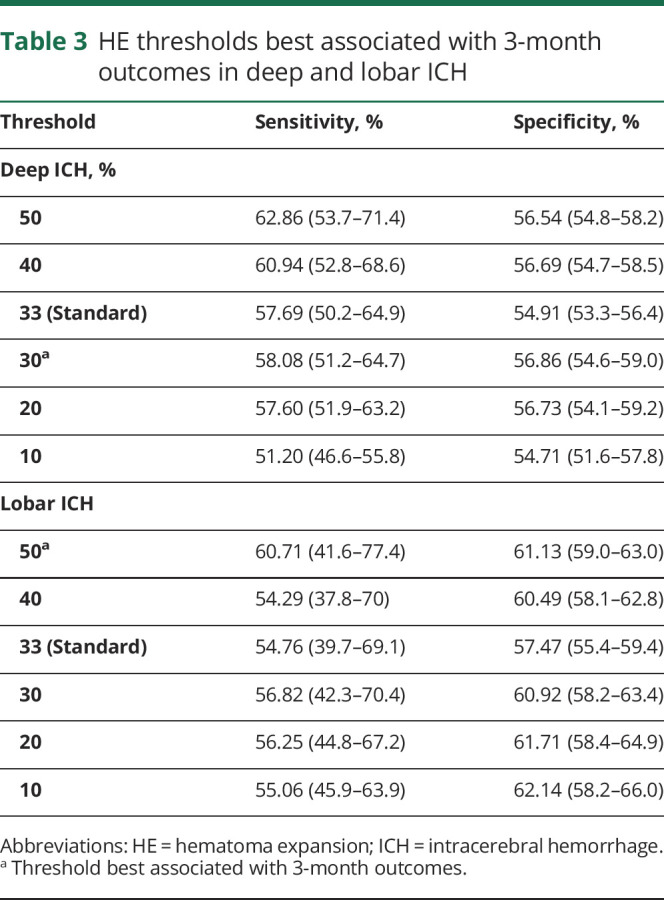
Table 4.
Area under curve of relationship of hematoma expansion with 3-month outcomes stratified by location
Discussion
Current clinical ICH coagulopathy treatment paradigms are largely uniform across patients with all types of ICH. These paradigms rely on identifying patients with recent anticoagulation medication use or evidence of coagulopathy using admission plasma–based coagulation tests. This allows the ability to target and initiate coagulopathy treatments in efforts to minimize HE and to improve clinical outcomes. In our multicenter, multiethnic ICH cohort with such patients excluded, we identified HE in ≈16% of the overall cohort, reflecting a patient population at risk for undertreatment of HE.
However, in addition to identifying significant demographic and baseline clinical heterogeneity between patients with deep and those with lobar ICHs, we identified that patients experiencing deep ICH had a higher likelihood of HE compared to those with lobar ICH. Although this finding may suggest that ICH location could be viewed as a novel risk factor associated with HE, it is necessary to highlight that we noted an attenuation of the association of deep ICH location with increased odds of HE after adjusting for time from symptom onset to admission CT in a subgroup of patients with these available data. This time covariate is well known to be associated with HE. While there were no significant intergroup differences in symptom onset to admission CT times, it is possible that this important time variable played a role in our cohort's findings. Conversely, it is also possible that we identified this attenuated relationship in this subgroup analysis due to limitations of the smaller subgroup sample size given that only 50% of the original cohort had available data on this time covariate.
There have not been consistently identified HE differences between deep and lobar ICHs in prior studies,2,9,10,12 and it is uncertain whether these studies have been limited by aforementioned sample size issues or the use of absolute HE thresholds (>6 mL) in comparisons of groups with largely different baseline hematoma volumes.11,12 In our primary analysis, which did not incorporate data on times from symptom onset to admission CT as a covariate, we identified increased odds of HE regardless of the definition of HE used. Yet, it is worth noting that the effect of deep ICH location on HE seemed to vary when absolute HE thresholds (>6 mL) were used. This variation was seen in our unadjusted and adjusted models evaluating the relationship of ICH location on HE defined as (1) >6 mL and (2) >33% or >6 mL. This may suggest the limitations of the use of >6 mL HE thresholds in analyses of groups with different baseline hematoma volumes. Finally, when looking at HE as a continuous measure of absolute growth rather than a binary threshold, it appeared that deep ICH had less absolute growth (in milliliters) compared to lobar ICH. With deep ICH typically being smaller than lobar ICH, it is not surprising that the absolute HE growth measurements would be less in deep locations. It could be posited from our data that while deep ICHs encountered HE (defined as a binary variable) more often, the extent of absolute expansion (in milliliters) was less than in lobar ICH, but its effect is still substantial on outcome.
When investigating relative percent HE thresholds best associated with poor 3-month outcomes, we identified that patients with deep ICH required smaller relative HE growth compared to lobar ICH. Specifically, 30%, or 2.7 mL, of HE was most strongly associated with poor 3-month outcomes in deep ICH compared to the 50%, or 10.5 mL, of HE required for lobar ICH. It is possible that because of the high density of corticospinal tracts in deep locations, less HE here will create greater impacts on clinical outcome compared to lobar locations where corticospinal tracts are further dispersed. However, it is important to highlight that while we attempted to weigh sensitivities and specificities to create the most optimal and smallest threshold of HE associated with poor outcomes, the overall sensitivities and specificities of HE predicting poor outcomes in our cohort were low, and the separation between thresholds was poor. This necessitates external validation of our findings to assess whether HE thresholds stratified by location will be a more appropriate outcome measure in future studies.
Similar to prior data, we identified associations of deep ICH location with poor 3-month outcomes.7 Although prior literature has attributed the association of deep ICH location and poor outcome primarily to regions of anatomic injury, we discovered that this relationship was mediated in part by HE. This suggests the need to consider that location-based outcome heterogeneity may be driven in part by radiographic HE heterogeneity. Whether the mechanism behind these HE differences is simply driven by patients with deep ICH presenting more rapidly to hospital settings or true hemostatic differences between deep and lobar locations requires further clarification. External investigation using a post hoc analysis of a clinical trial cohort in which clear, rapid symptom onset times are required for inclusion would clarify our findings.
However, our nontrial cohort may be a better representation of general patients with ICH seen in clinical practice. Deep ICH has consistently been shown to have faster presentation times given the dramatic clinical symptom presentation in these patients compared to the more nonspecific symptoms resulting from lobar ICH. With patients with lobar ICH presenting in a more delayed fashion, their risk of HE may have passed by the time of their initial ICH diagnosis, and our data may suggest that a much larger amount of HE is required to affect their outcomes. With smaller HE required to create worse outcomes in deep ICH, patients with deep ICH appear to be a particularly vulnerable patient population who may benefit from more aggressive treatments in both clinical practice and research studies.
If our location-based HE differences are not merely due to time-based differences in symptom onset to presentation, the underlying mechanism behind our findings is unclear. It is plausible that there may be inherent coagulation differences between ICH locations (or etiologies) not identifiable with traditional plasma-based coagulation tests. Recent literature suggests that patients with deep ICH have slower, suboptimal clotting kinetics compared to patients with lobar ICH seen using whole-blood coagulation testing.16,17 Separately, while we adjusted for SBP differences in our study, it is still possible that blood pressure may play a role. It can be hypothesized that deep lenticulostriate arteriolar rupture is under higher pressure than more lobar arteries given dilution of arterial pressure burden over longitudinal segments of distal lobar arterioles. This would result in more HE in deep compared to lobar locations due to blood pressure gradient differences across the cerebral circulation. Although the mechanism behind our findings is still unknown, at the very least, our data confirm that deep ICHs and lobar ICHs are very different, heterogeneous disease processes that require acknowledgment in future studies.
Our study has several strengths, including a large multicenter, multiethnic cohort; prospective collection of data; and a blinded, centralized imaging adjudication. Several limitations also require acknowledgment. As stated, there were significant missing data on symptom onset to admission CT times. There was also a 14% loss to 3-month follow-up, and it is worth noting that these patients were more likely older patients with lobar ICH locations and more severe ICH (greater baseline ICH volume and worse ICH scores), potentially subjecting the inclusion sample to bias. However, there were no striking differences in the relationship of ICH location with outcomes when the more complete dataset was used in terms of discharge outcomes. In addition, there was a large exclusion group due to the absence of follow-up CT scanning, which subjected our cohort to selection bias. Patients with ICH at the extremes of clinical presentation who either are too sick or have minimal clinical symptoms will often not receive follow-up imaging, and this may prevent our analyzed cohort from being a truly representative sample of patients with ICH seen in the general population. In our cohort, it appeared that our missing data were driven by the latter, with more patients with lobar ICH and subsequently less IVH missing follow-up CT (data not shown). We also recognize the limitation that our cohort was subject to unmeasured confounding and did not include data on CT angiographic spot-sign testing or patients with cerebellar/brainstem ICH. Furthermore, we did not investigate the role of blood pressure treatment effect, ICH etiology (cerebral amyloid vs hypertension or others), or APOE on HE. While the diagnostic workup required to obtain optimal ICH etiology assessment may not be feasible for hyperacute HE management strategies, a look into ICH etiologies, locations (while including cerebellar location) using Boston criteria, and genetics data may be beneficial to clarify potential mechanistic explanations for our findings.
We identified significant differences in baseline characteristics, radiographic HE outcomes, and clinical outcomes between deep and lobar ICHs. While our findings of HE being more frequent in patients with deep ICH may be driven by differences in symptom onset to admission times, we in addition identified that less HE may be required to create worse clinical outcomes in deep compared to lobar ICH. This suggests that patients encountering deep ICH may be particularly vulnerable to the deleterious effects of HE. Further work is required to investigate mechanistic drivers for our findings and whether future HE treatment strategies need to account for ICH location moving forward.
Glossary
- CI
confidence interval
- ERICH
Ethnic/Racial Variations of Intracerebral Hemorrhage
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- HE
hematoma expansion
- OR
odds ratio
- SBP
systolic blood pressure
Appendix 1. Authors
Study funding
This study was supported by a grant from the National Institute of Neurologic Disorders and Stroke (U-01-NS069763; D. Woo). A. Boehme is supported by NIH National Institute of Neurological Disorders and Stroke R03 NS101417 and NIH National Institute on Minority Health and Health Disparities R21 MD012451. D. Roh is supported by the National Blood Foundation. This report does not represent the official view of National Institute of Neurological Disorders and Stroke, the NIH, the National Blood Foundation, or any part of the US federal government. No official support or endorsement of this article by National Institute of Neurological Disorders and Stroke or NIH is intended or should be inferred.
Disclosure
D. Roh receives consulting fees from Portola Pharmaceuticals. All other authors report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 2.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 2014;71:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137. [DOI] [PubMed] [Google Scholar]
- 4.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016;387:2605–2613. [DOI] [PubMed] [Google Scholar]
- 5.Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018;391:2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 2012;43:2592–2597. [DOI] [PubMed] [Google Scholar]
- 7.Delcourt C, Sato S, Zhang S, et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 2017;88:1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sreekrishnan A, Dearborn JL, Greer DM, et al. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care 2016;25:384–391. [DOI] [PubMed] [Google Scholar]
- 9.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 2011;76:1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcone GJ, Biffi A, Brouwers HB, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol 2013;70:988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yogendrakumar V, Demchuk AM, Aviv RI, et al. Location of intracerebral haemorrhage predicts haematoma expansion. Eur Stroke J 2017;2:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roh D, Sun CH, Murthy S, et al. Hematoma expansion differences in lobar and deep primary intracerebral hemorrhage. Neurocrit Care 2019;31:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013;44:e120–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 15.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke J Cereb Circ 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 16.Roh D, Chang T, Zammit C, et al. Functional coagulation differences between lobar and deep intracerebral hemorrhage detected by rotational thromboelastometry: a pilot study. Neurocrit Care 2019;31:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roh D, Torres GL, Cai C, et al. Coagulation differences detectable in deep and lobar primary intracerebral hemorrhage using thromboelastography. Neurosurgery Epub 202 Mar 13. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on which this article is based can be obtained on reasonable request to the corresponding author.