Abstract
Objective
To collect information on frequency of pregnancy and delivery complications in Charcot-Marie-Tooth (CMT) disease and on CMT course during pregnancy.
Methods
Through an ad hoc online questionnaire, we investigated pregnancy and neuropathy course in women with CMT adhering to the Italian CMT Registry. Data were compared to those of controls (recruited among friends and unaffected relatives) and the Italian (or other reference) population.
Results
We collected data on 193 pregnancies from 86 women with CMT (age 20–73 years) with 157 deliveries (81.4%) after a mean of 38.6 gestational weeks. In women with CMT, there were no differences compared to controls (59 pregnancies and 46 deliveries from 24 controls) and the reference population for miscarriages (11.4%) and planned (21.0%) and emergency (14.0%) cesarean sections. We found a significantly higher frequency of placenta previa (1.6% vs 0.4%), abnormal fetal presentations (8.4% vs 4.5%), and preterm deliveries (20.3% vs 6.9%; most in week 34–36 of gestation) compared to reference populations. Excluding twins, newborn weight did not differ from the reference population. Postpartum bleeding rate in patients with CMT (2.1%) was similar to that of the general population (2.4%). CMT status worsened during 18 of 193 pregnancies (9.3%) with no recovery in 16 of them and with similar figures in the CMT1A and non-CMT1A subtypes.
Conclusions
We observed higher rates of placenta previa, abnormal presentations, and preterm deliveries in CMT, but pregnancy outcome and newborn weight and health were similar to those of the reference populations. Worsening of CMT is not infrequent and occurs not only in CMT1A. Pregnant women with CMT should be monitored with particular care.
Charcot-Marie-Tooth (CMT) disease is the most common inherited neuromuscular disorder, with an estimated prevalence of 9.4 to 20.1:100,000.1 It is a hereditary neuropathy that affects motor and sensory peripheral nerves to a variable extent.2 Increased rates of pregnancy complications and instances of worsening of CMT during pregnancy have been described.3–6 However, there are no large systematic studies of this issue, and recommendations for management of CMT during pregnancy are not available.7 Moreover, the few literature data are somehow discrepant. In a relatively large Norwegian series of 49 women with CMT with 108 births from the Medical Birth Registry of Norway, the authors reported a higher rate of postpartum bleeding and abnormal presentations compared with the reference group.3 Operative deliveries were twice as frequent in women with CMT.3 In the general population, uterine atony is the most common cause for postpartum bleeding8; consequently, the authors were tempted to assume that CMT can influence uterine function. On the contrary, in a retrospective study by questionnaire administration in Germany and Australia, the authors found no increase in pregnancy and delivery complications in a smaller series of 21 women (45 gestations) affected by demyelinating CMT (type 1, CMT1).4 However, 38% of them reported exacerbation of CMT during at least 1 pregnancy, with persistence of such disability in two-thirds of the cases after delivery, especially for weakness and walking ability. A very recent study by the same group, having identical study design but no overlap in the patient cohorts, yielded similar results.5 In detail, 37.8% and 37.5% of cases (82 gestations) reported a deterioration during pregnancy and after delivery, respectively, with subsequent improvement in only 3.7% and 5%.
In another retrospective cohort of 178 patients with different hereditary neuromuscular diseases, obstetric complications were not increased in 33 patients with CMT (68 pregnancies, 63 births), but a deterioration of the CMT disease was reported in 32% of pregnancies and was persistent in 22%.6
We performed a large case-control study among women with CMT registered in the Italian CMT Registry with the aim of collecting information on the occurrence of pregnancy and delivery complications, the newborn conditions, and the CMT course during pregnancy. The last issue might be particularly important in patients with CMT1A (the most common CMT type, associated with the duplication of the PMP22 gene) because progesterone, the levels of which increase during pregnancy, is known to increase PMP22 expression.9
Methods
We developed a National CMT Registry (registronmd.it) in collaboration with the Associazione del Registro (alliance between Italian patient associations, including ACMT-Rete, and Telethon-Italy Foundation). It is a dual registry in which the patient registers herself/himself, chooses a reference center among 9 spread all over Italy (Fondazione IRCCS Istituto Carlo Besta of Milan; IRCCS Ospedale San Raffaele of Milan; universities of Genoa, Verona, Parma, Naples, Catanzaro, and Messina; Cattolica Sacro Cuore University of Rome) where the attending clinician, in an ad hoc visit, collects a minimal dataset of information and administers clinical scales (CMT Examination/Neuropathy Scores, CMTES/CMTNS).10 Registered patients had the chance to participate in the present study, which required completion of online self-reported questionnaires related to 5 important issues. One of them regarded disease course and complications during pregnancy. Controls also completed the online questionnaires and were recruited among friends and unaffected relatives of participants with CMT, matched as much as possible for age. The recruitment lasted 3 years (2015–2017). The questionnaire (available in Italian language on request) was developed through 2 focus groups with patients and a psychologist with the supervision of a gynecologist. It was written using understandable terms, and the questions were direct and simple. For those answers for which we were in doubt, we interviewed the patient by phone. The questionnaire investigated the number and course of pregnancies and deliveries (with 108 main questions) and the course of CMT during pregnancy and puerperium (with 54 questions). Information about pregnancy outcome was specifically collected considering miscarriage, voluntary abortion, induction of labor, vaginal delivery (natural or instrumental), and cesarean section (both planned and in emergency). For the delivery period, we considered fetal presentation, complications during labor/delivery, and occurrence of fetal distress. Information about the newborns included weight at birth and occurrence of icterus, infections, hypothermia, and prematurity. Newborns were considered small for gestational age when below the fifth percentile for weight at birth. We also calculated the percentage of newborns whose weight was <2.5 kg.
We compared the data from patients with CMT with those of controls and, because controls were a small sample, also with data from the Italian population or other reference populations available from National Health System data and literature publications. The National Health System data are based on the Certificato Di Assistenza al Parto questionnaire. This questionnaire is completed by law by the midwife for all deliveries. All records are reviewed and data are confirmed by local medical officers.
As far as the CMT disease course is concerned, both occurrence of new symptoms and worsening of already existing ones were considered to investigate CMT status during pregnancy and puerperium. In detail, we asked about weakness and sensory symptoms (in hands, feet, and other areas), cramps, pain different from cramps, fatigue, and the need for new devices for walking. When disease worsening was reported, we also investigated degree of recovery if any. We then analyzed rate of pregnancy complications and CMT course during pregnancy in patients with CMT1A compared to patients without CMT1A. We grouped all the other CMT types because the numbers of patients for each of them were too low for a separate analysis.
Standard protocol approvals, registrations, and patient consents
The Institutional Ethics Committee at each center approved the study. Written informed consent was obtained from all participants in the Registry, and online informed consent was obtained from all those completing the questionnaires.
Statistical analysis
A description of participant characteristics at baseline was provided in terms of absolute numbers and percentages for categorical data and means with SDs for continuous data. The 95% confidence intervals were computed with the exact binomial method.
Data availability
Data that support the findings of this study are available from the corresponding author (D.P.) and will be shared anonymously by request from any qualified investigator.
Results
Population
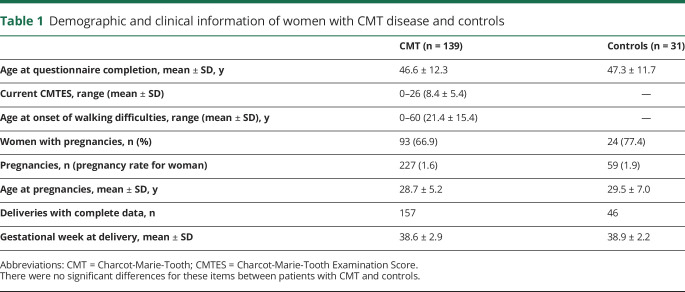
We collected data on 139 patients with CMT and 31 age-matched controls who participated in the study. Their characteristics are reported in table 1. There were no differences between women with CMT and healthy controls as far as current age, age at pregnancies, and gestational week at delivery are concerned. Ninety-three patients and 24 controls became pregnant during the period from 1960 to 2015. Among the 46 patients who did not have pregnancies (one-third of women with CMT compared to less than one-fourth of controls), CMT contributed to the decision not to have children in 17 women (12.2% of the total, 37% of those who did not have pregnancies). However, the overall rate of pregnancy per woman (1.6) did not differ from that of controls (1.9) and the reference population (1.7 in the 1962–2015 period, data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=IT).
Table 1.
Demographic and clinical information of women with CMT disease and controls
Detailed information about pregnancy course was collected from the questionnaires completed by 86 women with CMT (193 gestations and 157 deliveries with 163 newborns; there were 6 sets of twins) and 24 controls (59 gestations and 46 deliveries with 1 set of twins). Among patients with CMT, 44 of 86 patients (≈50%) had a diagnosis of CMT1A with 109 of 193 pregnancies; the other most frequent CMT types were CMT1B (7 patients, 19 pregnancies), CMT2A (6 patients, 12 pregnancies), CMTX1 (5 patients, 10 pregnancies), CMT2I/J (4 patients, 7 pregnancies), and CMT2F (2 patients, 4 pregnancies).
Pregnancy course and complications
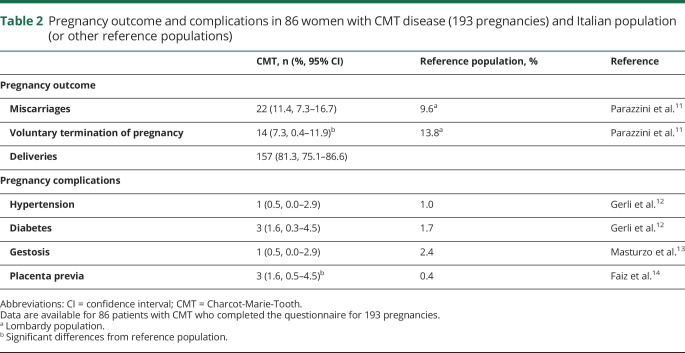
Course, outcome, and complications of pregnancies are summarized in table 2.
Table 2.
Pregnancy outcome and complications in 86 women with CMT disease (193 pregnancies) and Italian population (or other reference populations)
In brief, 193 pregnancies for patients ended up in 157 deliveries (with detailed information available in 143) vs 59 pregnancies and 46 deliveries (with detailed information in 43) for controls. Miscarriages occurred in 22 of 193 (11.4%) patients and 9 of 59 (15.3%) controls; the reference population had a similar percentage (9.6%).11 The rate of voluntary abortion was 14 of 193 (7.3%) patients and 4 of 59 (6.8%) controls, with percentages significantly lower than that of the reference population (13.8%).11 The rate of pregnancy complications in CMT was similar to that of controls and the reference population12,13 except for placenta previa, which occurred in 3 women with CMT (1.6% as compared to 0.4% in the reference population)14 and required cesarean section.
Delivery and newborn conditions
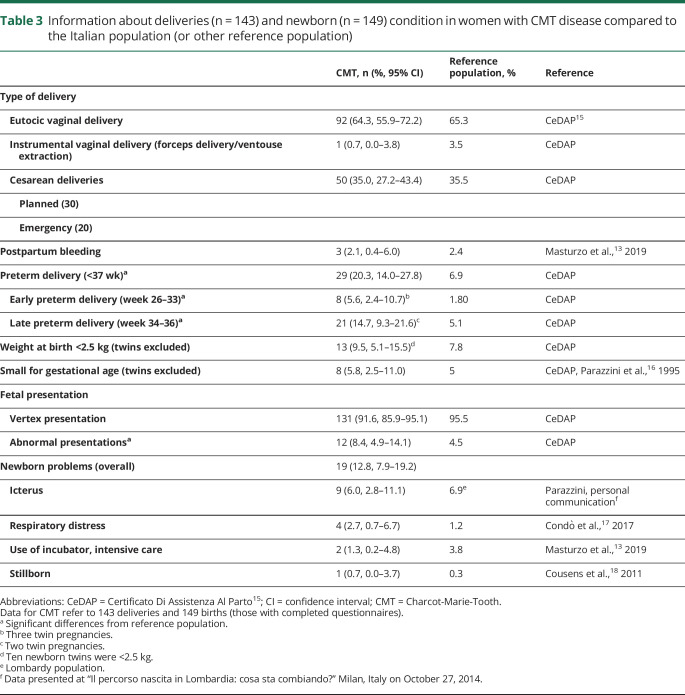
Results are summarized in table 3.
Table 3.
Information about deliveries (n = 143) and newborn (n = 149) condition in women with CMT disease compared to the Italian population (or other reference population)
In summary, type of delivery in women with CMT did not differ from that of the control and reference populations.15 In patients, planned cesarean sections were done in 30 of 143 (21.0%) and eutocic vaginal deliveries in 92 of 143 (64.3%), with induction of labor reported for 16 of them (11.2% of all deliveries) between the 36th and 42nd gestational week. Instrumental vaginal delivery occurred in only 1 case, and emergency cesarean section was needed in 20 of 143 (14.0%) cases. There were only 3 instances of postpartum bleeding in patients with CMT (2.1%), with no difference with respect to the reference population.13
Preterm delivery (before gestational week 37) occurred in 29 of 143 cases (20.3%) for women with CMT and 7 of 43 (16.3%) for healthy controls; the rate in the CMT group is significantly higher compared to the Italian population (6.9%),15 even when twin pregnancies are excluded (24 of 137, 17.5% in CMT). However, it must be said that the difference is due mainly to preterm deliveries occurring in the interval from 34 to 36 gestational weeks. Moreover, for CMT, excluding twins, the rate of small for gestational age newborns below the fifth percentile (8 of 137, 5.8%) and the percentage of newborns <2.5 kg (13 of 137, 9.5%) were comparable to those of the reference population (5% and 7.8%, respectively).15,16 Five of the 6 twin pregnancies in patients with CMT (none in controls) ended with preterm delivery; although the 10 preterm twins all weighed <2.5 kg, none of them was small for gestational age for twins.
Abnormal nonvertex presentations were significantly more frequent in CMT pregnancies (12 of 143, 8.4%) than in the reference population (4.5%)15 and included 9 breech presentations. Three of 12 were twin pregnancies. All but 1 required cesarean section, and none was complicated by postpartum bleeding. For 6 of these pregnancies, the offspring developed CMT but only later in life, ruling out a contribution of CMT in the fetus to the abnormal presentation. Only 1 woman had moderate to severe disease.
Regarding anesthetic management, general anesthesia was performed in 30 of 50 cesarean deliveries and spinal anesthesia in 20 of 50. No complication was reported by patients who underwent general anesthesia, whereas 2 women required intensive care unit admission after spinal anesthesia (1 for urinary retention that lasted 12 hours and 1 for prolonged loss of consciousness, hospitalized for 8 days).
The rate and type of complications in newborns of women with CMT did not differ from controls and the reference populations.13,17,18
Comparison between CMT1A and non-CMT1A
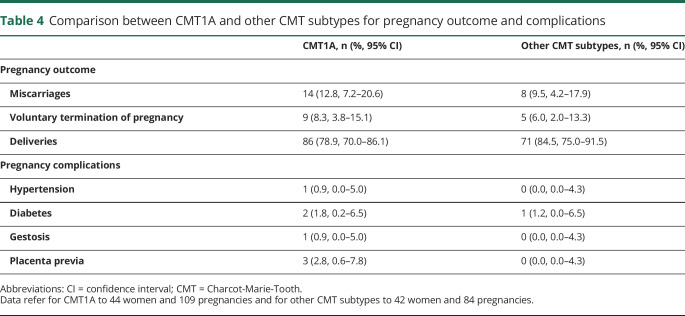
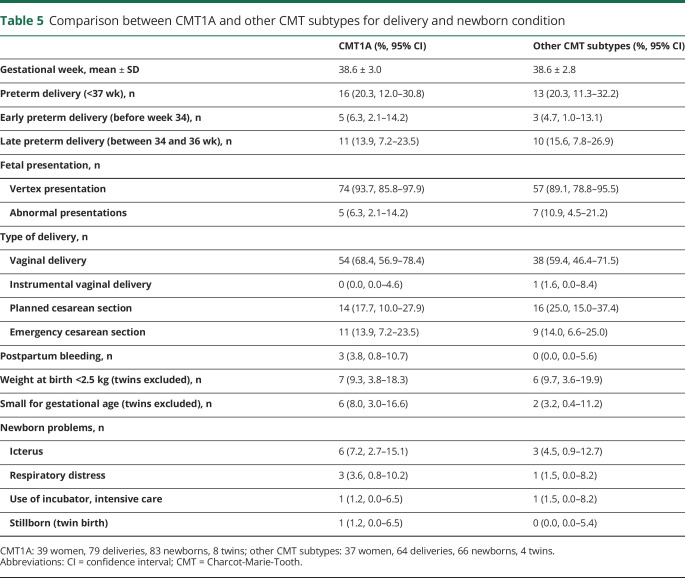
Results are summarized in tables 4 and 5.
Table 4.
Comparison between CMT1A and other CMT subtypes for pregnancy outcome and complications
Table 5.
Comparison between CMT1A and other CMT subtypes for delivery and newborn condition
In brief, we collected data from 44 patients with CMT1A (109 pregnancies) and 42 patients with other forms of CMT (84 pregnancies). The median age at gestation was 27.7 ± 4.9 years (range 17–41 years) in the first group and 29.3 ± 5.5 years (range 18–43 years) in the second. Four patients (9.1%) with CMT1A (5 pregnancies) and 9 (21.4%) (11 pregnancies) women without CMT1A underwent standard prenatal genetic diagnosis for chromosomal abnormalities. Only 5 of them (2 with CMT1A and 3 without CMT1A) knew they were affected by CMT at the time of pregnancy. Of these, only 2 patients affected by CMT1A and CMT2A underwent chorionic villus sampling to search for the PMP22 duplication and a MFN2 mutation, respectively. The analysis turned out to be positive in the former case, but eventually the pregnancy was not terminated, and negative in the latter. No results for pregnancy course, complications, deliveries, and newborns were different between the 2 groups. All 3 instances of placenta previa occurred among patients with CMT1A, but the difference with the non-CMT1A group was not significant.
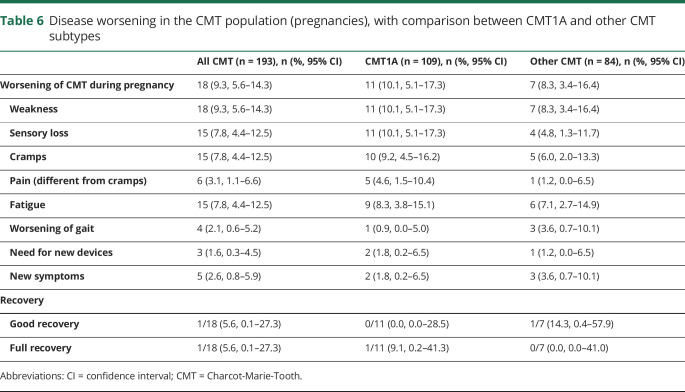
CMT course during pregnancy and puerperium
Table 6 summarizes results regarding the disease course.
Table 6.
Disease worsening in the CMT population (pregnancies), with comparison between CMT1A and other CMT subtypes
CMT disease status worsened during 18 of 193 pregnancies (9.3%) in 14 of 86 patients (16.3%) (8 of 44 with CMT1A, 2 of 6 with CMT2A, 1 of 7 with CMT1B, 1 of 1 with CMT2E, 1 of 1 with CMT2F, and 1 with unknown CMT subtype; mean age at delivery 29.8 ± 4.8 years, range 22–39 years). We observed no significant difference between women who reported CMT worsening and those who did not for age at onset of walking difficulties (22 ± 10 years, range 1–36 years, vs 27.9 ± 15.0 years, range 1–60 years), ability to walk unaided before pregnancy (13 of 14 vs 72 of 72), current disease severity as assessed by CMTES (7.9 ± 4.9, range 1–16, vs 7.5 ± 5.0, range 0–22), and need for an ankle-foot orthotic (4 of 14, 28.6%, vs 24 of 72, 33.3%), although the age at questionnaire completion is lower for the former group (39.6 ± 7.8 years, range 28–56 years, vs 51.3 ± 10.6 years, range 24–73 years).
Ten of 14 women had >1 pregnancy. Recurrence of worsening in subsequent pregnancies was a rare event in that it occurred in 4 women only: 3 patients worsened in 2 pregnancies of 3, and 1 woman reported disease progression in both of her pregnancies. On the other hand, deterioration occurred in only 1 of 2 or 3 pregnancies for 6 patients and in their only pregnancy for 4 women. It occurred during the first pregnancy in 6 cases (which was the only pregnancy in 4 cases) and the second and/or third pregnancy in the remaining 12. Five of these patients had pregnancy complications: placenta previa (n = 2), gestational diabetes (n = 2), or asthma (n = 1).
Worsening or appearance of foot weakness (n = 17), foot sensory loss (n = 15), cramps (n = 15), and fatigue (n = 15) were the most frequent complaints. Ambulation ability remained unchanged in 97.9% of pregnancies, but 3 patients needed new assistive devices (1 shoe insert, 1 walker, 1 assistance with walking with irregular use of wheelchair). No recovery at all from worsening was reported to occur after delivery for 14 of 18 pregnancies, whereas little recovery occurred in 2 cases, good recovery was seen in 1 patient, and only 1 patient recovered completely. For those patients who did not recover from deterioration during pregnancy, the follow-up period ranged from a minimum of 1.8 to a maximum of 18.6 years with a mean of 8.5 years (SD 5.5 years). We found no significant difference between those with and those without CMT1A.
Discussion
This is the largest study ever performed on pregnancy and disease course in patients with CMT.
Our data show that overall pregnancy outcome and deliveries are regular in CMT compared to healthy controls and reference populations, suggesting that the disease does not affect the course of pregnancy. In CMT, miscarriages, emergency cesarean sections, and the main pregnancy complications were not more frequent, with the only exception of placenta previa (1.6% vs 0.4%), which occurred only in 3 women with CMT. Women with CMT had a mean of 1.6 pregnancies, a rate comparable to that of controls and the reference population. Although the difference was not significant, a lower percentage of women with CMT became pregnant compared to controls (66.9% vs 77.4%). This may be due partly to the decision of some of them (n = 17, 12.2%) not to become pregnant because they carried a genetically transmissible disease. On the other hand, we observed a lower frequency of voluntary pregnancy termination with respect to the normal population, and this may be due to a stronger determination to have children for the women with CMT who decided to become pregnant. Although the mean gestational week at delivery did not differ from that of controls, in patients with CMT, we observed a significantly higher rate of preterm deliveries (20.3%). Most of them, however, occurred in the 34- to 36-week interval, with only 8 of 193 deliveries before week 34, and the percentages of newborns with low weight or who were small for gestational age were similar to those of the reference population.
We do not confirm the observation by the Norwegian study3 that reported a higher rate of postpartum bleeding (12%) because it occurred in only 2.1% in our series, thus bringing into question the hypothesis that CMT may cause uterine atony. The authors reported also a high rate of instrumental (forceps and vacuum) deliveries (14.8%),3 which was not the case for patients with CMT in the present research (0.7%). On the other hand, our data are in keeping with the report by the same authors of a higher rate of presentation anomalies (9.3%) in women with CMT. Indeed, we observed 8.4% of abnormal presentations, including 9 of 143 (6.3%) breech presentations. There was no predisposing factor such as more severe CMT disease or occurrence of postpartum bleeding suggesting uterine atony. None of the offspring had early-onset CMT that might have caused reduced fetal movements.
Newborn health did not differ from that of controls and the reference population, and the frequency of complications was similar.
One of the main findings of our study is that the disease course of CMT worsened in a significant percentage of patients (16.3%) and pregnancies (9.3%), with further impairment of strength and sensation; increased occurrence of cramps, pain, and fatigue; or the appearance of new symptoms. Such worsening does not appear to be simply related to common disturbances during pregnancy occurring also in unaffected women such as cramps and fatigue because they reverted or improved after delivery in only a minority of cases. In this respect, our data, although with lower percentages, are in agreement with previous studies that described CMT worsening in 32%6 to 38%4,5 of patients and/or gestations. We do not confirm that deterioration was likely to recur in subsequent pregnancies because only 4 of 14 women had 2 episodes and 3 of them had at least 1 normal pregnancy (the first 1 in 2 cases). Disease severity and age at onset did not appear to be significant risk factors for deterioration. Neuropathy worsening might be expected in CMT1A associated with the PMP22 duplication because progesterone is known to increase PMP22 expression9 and hormonal levels increase markedly during pregnancy. However, we observed CMT worsening in several CMT subtypes, not only in CMT1A, and the deterioration rate was similar in the CMT1A and non-CMT1A series. Pathological mechanisms related to nerve edema have been suggested to be responsible for the exacerbation of neuropathy during pregnancy,19 but the ultimate reasons for such deterioration remain unknown. Notably, a previous study20 reported no difference in disease severity between women who have been pregnant and those who have not and between those reporting worsening during pregnancy (52%)—either temporary or permanent—and those without changes, pointing out that in a percentage of women the deterioration may be either subjective or not clearly detectable on clinical scores.
There are some limitations of our research, and some caution is needed in the interpretation of these findings. It is a retrospective study based mainly on the recollection of patients, and it was impossible to match the collected data with medical records, especially for pregnancies that occurred many years ago. We therefore decided to concentrate analyses on major, easily assessable events. A prospective study would be advisable; however, the collection of the number of pregnancies sufficient to perform statistical analyses requires a long time and a multicenter multinational study. Another drawback is that the pregnancies reported by the patients occurred during a long period, from 1960 to 2015. The data reported for the reference population are related to the last decades in most cases and sometimes only for some Italian regions, which may introduce some biases because data may vary in time and in place. For example, with reference to the lower rate of voluntary abortion reported by the cases, it should be underlined that in Italy voluntary abortion was illegal until 1978. On the other hand, the frequency of preterm births and of nonvertex presentation has been substantially constant during the last 7 decades.15,21 Another minor study limitation is related to the fact that the population of patients who participate in registries is not fully representative of the more general CMT population.
We observed higher rates of abnormal presentations, preterm deliveries, and placenta previa in CMT, but pregnancy outcome and newborn weight and health were similar to those of the reference populations. However, worsening of CMT is not infrequent and does not occurs only in CMT1A. Therefore, our results are overall reassuring with regard to the course and outcome of pregnancy in CMT, but they highlight the need to support patients during and after pregnancy, especially at the end, due to a high rate of preterm deliveries and the occurrence of CMT worsening observed in this series.
Acknowledgements
The authors thank the Associazione del Registro for the creation and maintenance of the Neuromuscular Registry Platform and the Italian CMT Registry with the related questionnaires and for its continuous support; ACMT-Rete for contributing to questionnaire preparation and patient recruitment; all the patients and their families for endless enthusiasm in taking part in the project; and Dr. Luciana Parati for help in study preparation and focus groups. C. Pisciotta., D.C., and D.P. are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD).
Glossary
- CMT
Charcot-Marie-Tooth
Appendix 1. Authors

Appendix 2. Coinvestigators
Footnotes
CME Course: NPub.org/cmelist
Study funding
Funded by Telethon-Italy Foundation grant GUP13006.
Disclosure
G.M. Fabrizi acknowledges donations from Pfizer to support research activities of his Research Unit and financial support from Akcea, Kedrion, Pfizer for participation in national and international meetings and from Ackcea, Alnylam and Pharnext for participation in advisory boards. M. Grandis acknowledges donations from Sanofi Genzyme to support research activities of her research unit and financial support from Alnylam and Sanofi Genzyme for participation in national and international meetings, participation in advisory board from Pfizer, and speaker honorarium from Sanofi Genzyme. A. Mazzeo acknowledges financial support from Pfizer, Alnylam, and Akcea for participation in national and international meetings and participation in advisory board from Pfizer, Alnylam, and Akcea. G. Vita acknowledges donations from Pfizer and PTC to support research activities and participation in advisory board from Pfizer, Alnylam, Akcea, and Pharnext. D. Pareyson acknowledges donations from Pfizer, LAM Therapeutics, and Acceleron to support research activities of his research unit; financial support from Pfizer, Alnylam, and Kedrion for participation in national and international meetings and participation in Advisory Board from Inflectis, Alnylam, and Akcea; and speaker honorarium from Alnylam. C. Pisciotta, D. Calabrese, L. Santoro, I. Tramacere, F. Manganelli, A. Schenone, T. Cavallaro, S.C. Previtali, I. Allegri, L. Padua, C. Pazzaglia, P. Saveri, A. Quattrone, P. Valentino, S. Tozza, L. Gentile, M. Russo, M.C. Trapasso, and F. Parazzini report no disclosure. Go to Neurology.org/N for full disclosures.
References
- 1.Pareyson D, Saveri P, Pisciotta C. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Curr Opin Neurol 2017;30:471–480. [DOI] [PubMed] [Google Scholar]
- 2.Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol 2009;8:654–667. [DOI] [PubMed] [Google Scholar]
- 3.Hoff JM, Gilhus NE, Daltveit AK. Pregnancies and deliveries in patients with Charcot-Marie-Tooth disease. Neurology 2005;64:459–462. [DOI] [PubMed] [Google Scholar]
- 4.Rudnik-Schöneborn S, Röhrig D, Nicholson G, Zerres K. Pregnancy and delivery in Charcot-Marie-Tooth disease type 1. Neurology 1993;43:2011–2016. [DOI] [PubMed] [Google Scholar]
- 5.Rudnik-Schöneborn S, Thiele S, Walter MC, et al. Pregnancy outcome in Charcot-Marie-Tooth disease: results of the CMT-NET cohort study in Germany. Eur J Neurol 2020;27:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awater C, Zerres K, Rudnik-Schoeborn S. Pregnancy course and outcome in women with hereditary neuromuscular disorders: comparison of obstetric risks in 178 patients. Eur J Obstet Gynecol Reprod Biol 2012;162:153–159. [DOI] [PubMed] [Google Scholar]
- 7.Norwood F, Rudnik-Schöneborn S. 179th ENMC international workshop: pregnancy in women with neuromuscular disorders 5-7 November 2010, Naarden, the Netherlands. Neuromuscul Disord 2012;22:183–190. [DOI] [PubMed] [Google Scholar]
- 8.Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth 2009;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melcangi RC, Magnaghi V, Cavarretta I, et al. Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J Neurosci Res 1999;56:349–357. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosini A, Calabrese D, Avato FM, et al. The Italian neuromuscular registry: a coordinated platform where patient organizations and clinicians collaborate for data collection and multiple usage. Orphanet J Rare Dis 2018;13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parazzini F, Cipriani S, Ricci E, et al. I ricoveri ostetrici nella Regione Lombardia nel 2008, parte II. It J Gynecol Obstet 2011;23:48–67. [Google Scholar]
- 12.Gerli S, Favilli A, Franchini D, De Giorgi M, Casucci P, Parazzini F. Is the Robson's classification system burdened by obstetric pathologies, maternal characteristics and assistential levels in comparing hospitals cesarean rates? A regional analysis of class 1 and 3. J Matern Fetal Neonatal Med 2018;31:173–177. [DOI] [PubMed] [Google Scholar]
- 13.Masturzo B, Franzè V, Germano C, et al. Risk of adverse pregnancy outcomes by pre-pregnancy body mass index among Italian population: a retrospective population-based cohort study on 27,807 deliveries. Arch Gynecol Obstet 2019;299:983–991. [DOI] [PubMed] [Google Scholar]
- 14.Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med 2003;13:175–190. [DOI] [PubMed] [Google Scholar]
- 15.Basili F, Di Rosa A, Montorio V, Tamburini C. Certificato di assistenza al parto (CeDAP) Analisi dell’evento nascita-Anno 2013. Available at: www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2431. [Google Scholar]
- 16.Parazzini F, Cortinovis I, Bortolus R, Fedele L, Decarli A. Weight at Accessed September 25, 2020 birth by gestational age in Italy. Hum Reprod 1995;10:1862–1863. [DOI] [PubMed] [Google Scholar]
- 17.Condò V, Cipriani S, Colnaghi M, et al. Neonatal respiratory distress syndrome: are risk factors the same in preterm and term infants? J Matern Fetal Neonatal Med 2017;30:1267–1272. [DOI] [PubMed] [Google Scholar]
- 18.Cousens S, Blencowe H, Stanton C, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet 2011;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 19.Pollock M, Nukada H, Kritchevsky M. Exacerbation of Charcot‐Marie‐Tooth disease in pregnancy. Neurology 1982;32:1311–1314. [DOI] [PubMed] [Google Scholar]
- 20.Swan ER, Fuerst DR, Shy ME. Women and men are equally disabled by Charcot-Marie-Tooth disease type 1A. Neurology 2007;68:873. [DOI] [PubMed] [Google Scholar]
- 21.Parazzini F, Pirotta N, La Vecchia C, Fedele L. Determinants of caesarean section rates in Italy. Br J Obstet Gynaecol 1992;99:203–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available from the corresponding author (D.P.) and will be shared anonymously by request from any qualified investigator.