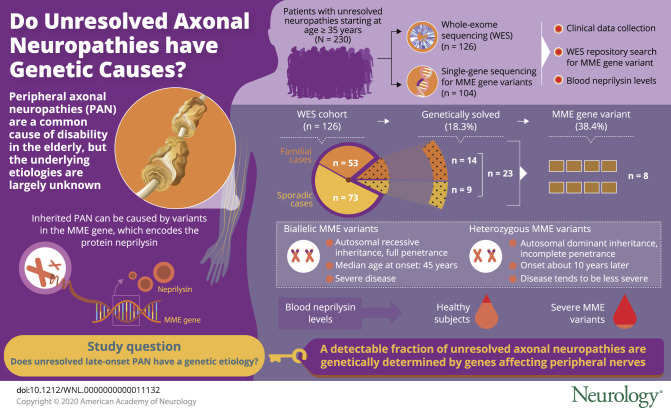
Jan Senderek
Jan Senderek, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,✉,
Petra Lassuthova
Petra Lassuthova, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Dagmara Kabzińska
Dagmara Kabzińska, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Lisa Abreu
Lisa Abreu, MPH
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Jonathan Baets
Jonathan Baets, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Christian Beetz
Christian Beetz, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Geir J Braathen
Geir J Braathen, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
David Brenner
David Brenner, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Joline Dalton
Joline Dalton, MSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Lois Dankwa
Lois Dankwa, MS
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Tine Deconinck
Tine Deconinck, MSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Peter De Jonghe
Peter De Jonghe, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Bianca Dräger
Bianca Dräger, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Katja Eggermann
Katja Eggermann, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Melina Ellis
Melina Ellis, BSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Carina Fischer
Carina Fischer, MSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Tanya Stojkovic
Tanya Stojkovic, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
David N Herrmann
David N Herrmann, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Rita Horvath
Rita Horvath, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Helle Høyer
Helle Høyer, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Stephan Iglseder
Stephan Iglseder, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Marina Kennerson
Marina Kennerson, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Katharina Kinslechner
Katharina Kinslechner, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Jennefer N Kohler
Jennefer N Kohler, MSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,*,
Ingo Kurth
Ingo Kurth, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Nigel G Laing
Nigel G Laing, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Phillipa J Lamont
Phillipa J Lamont, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Löscher Wolfgang N
Löscher Wolfgang N, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Albert Ludolph
Albert Ludolph, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Wilson Marques Jr
Wilson Marques Jr, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Garth Nicholson
Garth Nicholson, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Royston Ong
Royston Ong, BSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Susanne Petri
Susanne Petri, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Gianina Ravenscroft
Gianina Ravenscroft, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Adriana Rebelo
Adriana Rebelo, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Giulia Ricci
Giulia Ricci, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Sabine Rudnik-Schöneborn
Sabine Rudnik-Schöneborn, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Anja Schirmacher
Anja Schirmacher, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Beate Schlotter-Weigel
Beate Schlotter-Weigel, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Ludger Schoels
Ludger Schoels, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Rebecca Schüle
Rebecca Schüle, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Matthis Synofzik
Matthis Synofzik, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Bruno Francou
Bruno Francou, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Tim M Strom
Tim M Strom, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Johannes Wagner
Johannes Wagner, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
David Walk
David Walk, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Julia Wanschitz
Julia Wanschitz, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Daniela Weinmann
Daniela Weinmann, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Jochen Weishaupt
Jochen Weishaupt, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Manuela Wiessner
Manuela Wiessner, MSc
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Reinhard Windhager
Reinhard Windhager, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Peter Young
Peter Young, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Stephan Züchner
Stephan Züchner, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,*,
Stefan Toegel
Stefan Toegel, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Pavel Seeman
Pavel Seeman, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Andrzej Kochański
Andrzej Kochański, MD, PhD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,
Michaela Auer-Grumbach
Michaela Auer-Grumbach, MD
1From the Friedrich-Baur-Institute (J.S., B.S.-W., M.W.), Department of Neurology, LMU Munich, Germany; DNA Laboratory (P.L., P.S.), Department of Pediatric Neurology, 2nd Faculty of Medicine, Charles University in Prague and University Hospital Motol, Czech Republic; Neuromuscular Unit (D.K., A.K.), Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland; Dr. John T. Macdonald Foundation Department of Human Genetics (L.A., A.R., S.Z.), John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, FL; Neurogenetics Group (J.B., T.D., P.D.J.), Center for Molecular Neurology, University of Antwerp; Institute Born-Bunge (J.B., T.D., P.D.J.), University of Antwerp; Neuromuscular Reference Centre (J.B., P.D.J.), Department of Neurology, Antwerp University Hospital, Belgium; Department of Clinical Chemistry and Laboratory Medicine (C.B.), Jena University Hospital; Centogene AG (C.B.), Rostock, Germany; Department of Medical Genetics (G.J.B., H.H.), Telemark Hospital Trust, Skien, Norway; Neurology Department (D.B., A.L., J. Weishaupt), Ulm University, Germany; Department of Neurology (J.D., D. Walk), University of Minnesota, Minneapolis; Department of Neurology (L.D.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia; Department of Sleep Medicine and Neuromuscular Diseases (B.D., A.S., P.Y.), University of Münster; Institute of Human Genetics (K.E., I.K.), Medical Faculty, RWTH Aachen University, Germany; Sydney Medical School (M.E., M.K., G.N.), Concord Hospital, Northcott Neuroscience Laboratory, ANZAC Research Institute, Concord, Australia; Department of Orthopaedics and Trauma Surgery (C.F., K.K., D. Weinmann, R.W., S.T., M.A.-G.), Medical University of Vienna, Austria; AP-HP (T.S.), Institut de Myologie, Centre de référence des maladies neuromusculaires Nord/Est/Ile-de-France, G-H Pitié-Salpêtrière, Paris, France; Department of Neurology (D.N.H.), University of Rochester, NY; Department of Clinical Neurosciences (R.H.), University of Cambridge School of Clinical Medicine, UK; Department of Neurology (S.I.), Konventhospital der Barmherzigen Brüder Linz; Karl Chiari Lab for Orthopaedic Biology (K.K., D. Weinmann, S.T.), Department of Orthopedics and Trauma Surgery, Medical University of Vienna, Austria; Stanford Center for Undiagnosed Diseases (J.N.K.), Stanford, CA; Undiagnosed Diseases Network (UDN) (J.N.K., S.Z.); Centre for Medical Research (N.G.L., R.O., G.Ravenscroft), University of Western Australia, Nedlands; Harry Perkins Institute of Medical Research (N.G.L., R.O., G. Ravenscroft), Nedlands; Neurogenetic Unit (P.J.L.), Royal Perth Hospital, Perth, Australia; Department of Neurology (W.N.L., J. Wanschitz), Medical University of Innsbruck, Austria; Department of Neurosciences and Behavior (W.M.), Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; Department of Neurology (S.P.), Hannover Medical School, Germany; Department of Clinical and Experimental Medicine (G. Ricci), University of Pisa, Italy; Institute of Human Genetics (S.R.-S.), Medical University of Innsbruck, Austria; Department of Neurodegenerative Diseases Hertie–Institute for Clinical Brain Research and Center of Neurology (L.S., R.S., M.S.), University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S., R.S., M.S.), Tübingen, Germany; AP-HP (B.F.), Laboratoire de génétique moléculaire, pharmacogénétique et hormonologie, Hôpital de Bicêtre; Le Kremlin-Bicêtre, France; Institute of Human Genetics (T.M.S.), Helmholtz Zentrum Munich–German Research Center for Environmental Health, Neuherberg; Institute for Human Genetics (T.M.S.), Technical University Munich; and Institut für Klinische Genetik (J. Wagner), Technische Universität Dresden, Medizinische Fakultät Carl Gustav Carus, Germany.
1,✉