Abstract
Objective
To characterize longitudinal MRI and PET abnormalities in autopsy-confirmed Pick disease (PiD) and determine how patterns of neurodegeneration differ with respect to clinical syndrome.
Methods
Seventeen patients with PiD were identified who had antemortem MRI (8 with behavioral variant frontotemporal dementia [bvFTD-PiD], 6 with nonfluent/agrammatic primary progressive aphasia [naPPA-PiD], 1 with semantic primary progressive aphasia, 1 with unclassified primary progressive aphasia, and 1 with corticobasal syndrome). Thirteen patients had serial MRI for a total of 56 MRIs, 7 had [18F]fluorodeoxyglucose PET, 4 had Pittsburgh compound B (PiB) PET, and 1 patient had [18F]flortaucipir PET. Cross-sectional and longitudinal comparisons of gray matter volume and metabolism were performed between bvFTD-PiD, naPPA-PiD, and controls. Cortical PiB summaries were calculated to determine β-amyloid positivity.
Results
The bvFTD-PiD and naPPA-PiD groups showed different foci of volume loss and hypometabolism early in the disease, with bvFTD-PiD involving bilateral prefrontal and anterior temporal cortices and naPPA-PiD involving left inferior frontal gyrus, insula, and orbitofrontal cortex. However, patterns merged over time, with progressive spread into prefrontal and anterior temporal lobe in naPPA-PiD, and eventual involvement of posterior temporal lobe, motor cortex, and parietal lobe in both groups. Rates of frontotemporal atrophy were faster in bvFTD-PiD than naPPA-PiD. One patient was β-amyloid–positive on PET with low Alzheimer neuropathologic changes at autopsy. Flortaucipir PET showed elevated uptake in frontotemporal white matter.
Conclusion
Patterns of atrophy and hypometabolism differ in PiD according to presenting syndrome, although patterns of neurodegeneration appear to converge over time.
Pick disease (PiD) is a neurodegenerative disorder characterized neuropathologically by the presence of 3-repeat tau immunoreactive inclusions in the form of intraneuronal Pick bodies and enlarged neurons (Pick cells).1 PiD most commonly presents with the behavioral variant of frontotemporal dementia (bvFTD), although patients also have been reported to present with language abnormalities and meet criteria for primary progressive aphasia (PPA).2–10 On MRI, patterns of bilateral frontal and anterior temporal volume loss have been observed in patients with PiD and bvFTD5,11–13 and the semantic variant of PPA (svPPA).14 In 2 patients with PiD with the nonfluent/agrammatic variant of PPA (naPPA), asymmetric frontal volume loss was observed.14 However, no studies have directly assessed the degree to which patterns of volume loss and longitudinal progression in PiD differ according to clinical syndrome. Little is also known about the characteristics of PiD on other neuroimaging modalities, such as [18F]fluorodeoxyglucose PET (FDG-PET) or molecular β-amyloid (Aβ) and tau-PET imaging.
We aimed to characterize longitudinal MRI and PET abnormalities in PiD and determine how patterns differ depending on whether the patient presents with bvFTD or naPPA. A better understanding of structural and functional abnormalities, patterns of evolution, and the influence of clinical syndrome will increase understanding of disease progression in this rare pathologic disorder.
Methods
Standard protocol approvals, registrations, and patient consents
The study was approved by the Mayo Clinic institutional review board. All participants consented for enrollment into the study.
Patients
Seventeen patients with PiD were identified from the neuropathology files of the Mayo Clinic, Rochester, MN, who had come to autopsy and had at least one antemortem volumetric MRI scan between April 5, 1999, and August 4, 2017. These patients were all seen by a behavioral neurologist within the Department of Neurology, Mayo Clinic, and had been followed prospectively in either the Neurodegenerative Research Group (PIs Drs. Josephs and Whitwell) or the Mayo Clinic Alzheimer's Disease Research Center (PI Dr. Petersen). The medical records of all cases were reviewed by a behavioral neurologist (K.A.J.) to abstract the clinical diagnosis at presentation. Current clinical criteria for PPA15 and bvFTD16 were applied to the cohort. Of the 17 patients, 8 fulfilled criteria for bvFTD, 6 naPPA, 1 svPPA, and 1 unclassified PPA. One patient met criteria for corticobasal syndrome.17 Of the 6 patients classified as naPPA, 4 received a diagnosis of progressive agrammatic aphasia,18 meaning that they had agrammatic aphasia in the absence of apraxia of speech. The remaining 2 patients with naPPA had a mixture of both agrammatic aphasia and apraxia of speech. Medical records were also reviewed to determine whether patients with naPPA-PiD developed behavioral features and patients with bvFTD-PiD developed language difficulties during follow-up.
Serial MRI data were available for 13 patients with PiD (6 bvFTD-PiD, 5 naPPA-PiD, 1 svPPA, and 1 PPA-U), with a total of 56 MRI scans collected on these 13 patients. FDG-PET data were available for 7 patients (2 bvFTD-PiD, 4 naPPA-PiD, 1 unclassified PPA), with 4 of these having multiple serial FDG (3 naPPA-PiD [1 with 4 yearly serial FDG and 2 with 3 serial FDG] and 1 bvFTD-PiD with 4 FDG spanning 1 year). In addition, 4 patients had undergone Aβ PET (3 naPPA-PiD and 1 bvFTD-PiD), with 1 of these patients with naPPA-PiD also undergoing [18F]flortaucipir PET. Detailed findings from the flortaucipir PET in relation to neuropathology have been published previously.19
Autopsy examination
All patients had undergone standardized neuropathologic evaluations by an experienced neuropathologist (D.W.D. or J.E.P.) in accordance with current diagnostic criteria.20,21 All had routine stains completed including hematoxylin & eosin, glial fibrillary acid protein, and modified Bielschowsky. Immunohistochemistry was performed using a battery of antibodies including markers of glial pathology (glial fibrillary acid protein for astrocytes [clone GA5, 1:1,000; BioGenex, San Ramon, CA]) and markers for microglia (CD68 [clone PG-M1, 1:1,000; DAKO, Carpenteria, CA] or HLA-DR [LN-3, 1:5; ICN, Costa Mesa, CA]). Neuronal pathology was assessed using antibodies to neurofilament protein (NF-L: clone 2F11, 1:75; DAKO; NF-H: clone SMI-31, 1:2,000; Sternberger Monoclonals, Lutherville, MD); ubiquitin (clone Ubi-1 [MAB1510], 1:250; Chemicon, Temecula, CA); α-synuclein LB509 (1:200; Zymed, South San Francisco, CA, or NACP98, polyclonal antibody, 1:2,000; Mayo Clinic Jacksonville, FL), phospho-tau (clone AT8, 1:1,000; Innogenetics, Alpharetta, GA, or CP13: gift from Dr. Peter Davis, Albert Einstein College of Medicine, Bronx, NY) and 3R-tau (RD3, 1:5,000; Millipore, Temecula, CA). Each case was assigned a Braak stage22 using modified Bielschowsky silver stain on the basis of the earliest appearance of neurofibrillary tangles, and the presence of Aβ plaques was assessed using Consortium to Establish a Registry for Alzheimer’s Disease20 and Thal criteria.23 PiD was diagnosed based on the presence of argyrophilic and tau immunoreactive rounded Pick bodies.1
MRI analysis
All patients had undergone a standardized MRI protocol on a GE scanner that included a research quality T1-weighted volumetric MRI. Thirteen patients were scanned on a 1.5T scanner and 4 on a 3T scanner (1.5T = coronal volumetric spoiled gradient recalled echo sequence [slice thickness = 1.6 mm, 24 × 18.5 cm field of view (FOV), minimum full echo time (TE), repetition time (TR) 23 ms, 25° flip angle]; 3T = 3D magnetization-prepared rapid acquisition gradient echo [MPRAGE] [TR/TE/inversion time = 2,300/3/900 ms; flip angle = 8°, 26 cm FOV; 256 × 256 in-plane matrix with phase FOV = 0.94, slice thickness = 1.2 mm]). All longitudinal analyses used serial scans performed at the same field strength within patient. The MRI analysis consisted of both cross-sectional and longitudinal SPM-based group comparisons comparing patients with PiD to controls. The 17 patients with PiD were matched by age, sex, scanner field strength, and scan interval to 45 cognitively unimpaired controls who had been recruited into the Mayo Clinic Study of Aging (PI Dr. Petersen) (median [interquartile range] age 63 years [56–66], scan interval 1.9 [1.3–2.4], 40% female, 69% 1.5T scanner). For the cross-sectional analysis, all MRI scans were normalized to the Mayo Clinic Adult Lifespan Template (MCALT),24 segmented via unified segmentation25 with MCALT priors/settings, and gray matter images were modulated and smoothed at 8 mm full width at half maximum (FWHM). For the longitudinal analysis, we used an in-house developed tensor-based morphometry with symmetric normalization, as previously described.26 Log Jacobian images representing change over time were annualized and smoothed at 8 mm FWHM. Voxel-wise t tests in SPM12 were used to compare baseline images, first available follow-up images, and annualized Jacobians calculated between baseline and first available follow-up between the 17 patients with PiD and controls. Results were assessed after familywise error (FWE) correction for multiple comparisons at p < 0.05. Voxel-wise t tests were also performed comparing the bvFTD-PiD and naPPA-PiD groups to controls and to each other. Age and sex were included in all analyses as covariates, and total intracranial volume and field strength were included in cross-sectional comparisons. Direct comparisons between bvFTD-PiD and naPPA-PiD also included disease duration as a covariate and were assessed using the more lenient false discovery rate (FDR) correction for multiple comparisons at p < 0.05. Region of interest level annualized Jacobians were also calculated for multiple cortical and subcortical regions using the MCALT atlas.
Region-level asymmetry scores were calculated for each participant using the baseline MRI. Regional gray matter volumes were calculated using MCALT and absolute asymmetry scores were calculated as follows: |(left-right) × 2/(left + right)|. Individual-level atrophy maps were created for a few patients who had undergone multiple serial MRI. Regional gray matter volumes were divided by total intracranial volume, and converted into Z scores compared to the 45 cognitively unimpaired controls. Regional Z scores were then displayed on 3D renderings of the brain using MRIcroGL.
PET analysis
All PET scans were acquired using GE PET/CT scanners. For FDG-PET, participants were injected with 18F-FDG of approximately 459 MBq (range 367–576 MBq), and after a 30-minute uptake period an 8-minute 18F-FDG scan was performed. For PiB-PET, participants were injected with ∼628 MBq (range, 385–723 MBq) of PiB and after a 40-minute uptake period a 20-minute PiB scan was obtained.
For flortaucipir-PET, the patient was injected with ∼370 MBq (range 333–407 MBq) of [18F]AV-1451, followed by a 20-minute PET acquisition performed 80 minutes after injection. All 20-minute late-uptake PET scans consisted of four 5-minute dynamic frames, which were averaged. PET sinograms were iteratively reconstructed into a 256 mm FOV (pixel size = 1.0 mm, slice thickness = 3.3 mm). All PET images were coregistered to MPRAGE using 6 degrees of freedom registration in SPM12.
For FDG-PET, all voxels in the MPRAGE space FDG-PET images were divided by median uptake in pons to create standardized uptake value ratio (SUVR) images. SUVR images were normalized to the MCALT and smoothed at 6 mm FWHM. Voxel-wise t tests were performed as explained in the MRI section. These analyses were also repeated using FDG-PET scans that had been corrected for partial volume averaging using a 2-compartment partial volume correction. Group-level assessments of bvFTD-PiD and naPPA-PiD were not performed due to the small number of patients with FDG-PET in each group. Instead, individual-level patterns of hypometabolism were assessed using 3D stereotactic surface projections27 using CortexID (GE Healthcare) whereby activity at each voxel is Z scored to an age-segmented normative database. For PiB-PET, a global PiB SUVR was calculated using the cerebellar crus gray matter as a reference region, with patients classified as Aβ-positive using a cut point of 1.48.28 For flortaucipir-PET, all voxels in the MPRAGE space image were divided by median uptake in cerebellar crus gray matter to create an SUVR image.
Statistical analysis
Demographic features and asymmetry scores that have been converted to absolute scores were compared between bvFTD-PiD and naPPA-PiD using 2-tailed Wilcoxon rank sum tests for continuous variables or Fisher exact test for categorical variables. Region-level MRI-based annualized Jacobians were compared between controls and all PiD cases, bvFTD-PiD, and naPPA-PiD groups, with differences summarized by the area under the receiver operating characteristic curve (AUROC), which can be interpreted as a nonparametric measure of effect size, i.e., group-wise differences, independent of the underlying scale of measurement. The higher the AUROC estimates, the better the discrimination between groups.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
Demographic features
Demographic features of the PiD cohort are shown in the table. The bvFTD-PiD and naPPA-PiD groups did not differ in sex ratio, age at onset, age at death, or age at MRI, although the bvFTD-PiD group had a slightly longer time from onset to first available MRI. Over follow-up, behavioral abnormalities developed in 5 patients with naPPA-PiD (3 marked, 2 mild) and language difficulties developed in 6 patients with bvFTD-PiD (4 marked, 2 mild).
Table.
Demographic, clinical, and imaging variables for the Pick disease (PiD) cohort
MRI analysis
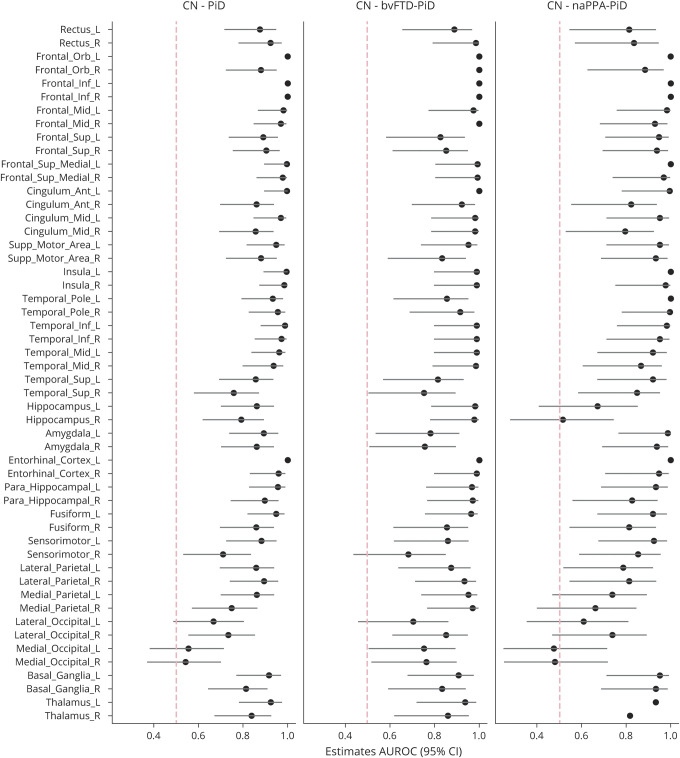
At baseline, patients with PiD showed gray matter volume loss compared to controls throughout bilateral medial and lateral prefrontal cortex and anterior temporal lobes (figure 1), with insula, medial frontal/anterior cingulate, and orbitofrontal regions showing the most loss. Volume loss also was observed in basal ganglia and left supramarginal gyrus. More severe volume loss was observed in these regions at follow-up, with additional progression into posterior inferior and middle temporal lobe (figure 1). In the Jacobian change maps, patients with PiD showed greater atrophy than controls in prefrontal and temporal lobes, as well as insula, cingulate gyrus, left thalamus, and basal ganglia (figure 1). These group maps were unchanged when the corticobasal syndrome, svPPA, and unclassified PPA were removed. In the region of interest (ROI)–level analysis, the ROIs with the highest AUROC effect size comparing patients with PiD and controls were inferior frontal, followed by entorhinal, temporal pole, and insula (figure 2).
Figure 1. Baseline, follow-up, and longitudinal change maps.
Baseline, follow-up, and longitudinal change maps for gray matter volume (A) and FDG-PET hypometabolism (B) in Pick disease (PiD) compared to controls. Gray matter results are shown corrected for multiple comparisons using family-wise error correction at p < 0.05. The top row of the FDG-PET results are shown corrected for multiple comparisons using false discovery rate correction at p < 0.05. The bottom FDG-PET row shows the results after partial volume correction (PVC), uncorrected at p < 0.001. The cross-sectional results are shown on a blue T score scale and the tensor-based morphometry (TBM) with symmetric normalization change maps are shown on a yellow–red scale. Renders were generated using the BrainNet Viewer (nitrc.org/projects/bnv/).
Figure 2. Regional effect size plots for rates of gray matter atrophy in Pick disease (PiD), behavioral variant of frontotemporal dementia (bvFTD)–PiD, and nonfluent/agrammatic variant of primary progressive aphasia (naPPA)–PiD compared to controls (CN).
Effect size and confidence intervals (CIs) are shown for each region. AUROC = area under the receiver operating characteristic curve.
Patients with bvFTD-PiD showed a baseline pattern of gray matter loss that mirrored the findings from the whole PiD group, with striking loss throughout lateral and medial prefrontal cortex and anterior temporal lobes, with additional involvement of insula and basal ganglia, compared to controls (figure 3). At follow-up, volume loss worsened in these regions and progressed into more posterior regions of the temporal lobe, particularly on the right. The Jacobian change maps showed atrophy in frontal pole, anterior and middle cingulate, medial frontal lobe, gyrus rectus, orbitofrontal cortex, inferior and middle frontal gyri, temporal pole, inferior and middle temporal lobes, fusiform, parahippocampal gyrus, right anterior hippocampus, precuneus, right angular and supramarginal gyri, and bilateral basal ganglia. The ROIs with the highest AUROC effect sizes were left and right anterior cingulate, inferior frontal gyrus, and right medial frontal cortex (figure 2).
Figure 3. Baseline, follow-up, and longitudinal change maps for gray matter volume in the behavioral variant of frontotemporal dementia (bvFTD)–Pick disease (PiD) and nonfluent/agrammatic variant of primary progressive aphasia (naPPA)–PiD groups.
(A) Results for each group compared to controls, with results shown after family-wise error correction for multiple comparison at p < 0.05. (B) Results of direct comparisons between the 2 PiD groups, with results shown after false discovery rate correction for multiple comparisons at p < 0.05. The cross-sectional results are shown on a blue T score scale and the tensor-based morphometry (TBM) with symmetric normalization change maps are shown on a yellow–red scale. Renders were generated using the BrainNet Viewer (nitrc.org/projects/bnv/).
Patients with naPPA-PiD showed more focal patterns of gray matter volume loss at baseline predominantly in a bilateral area of loss encompassing insula, inferior frontal gyrus, and orbitofrontal regions, as well as regions of loss in the supplementary motor area and cingulate gyrus, with greater loss observed in the left, compared to controls (figure 3). At follow-up, volume loss slightly worsened in these regions and progressed to involve amygdala. The Jacobian change maps showed atrophy in left entorhinal cortex, middle temporal gyrus, temporal pole, amygdala, left insula, inferior frontal gyrus (worse on the left), and right orbitofrontal cortex. The ROIs with the highest AUROC effect sizes were left and right inferior frontal gyri, left entorhinal cortex, left temporal pole, and left and right insula (figure 2).
Direct comparisons were performed between bvFTD-PiD and naPPA-PiD (FDR p < 0.05). At baseline and follow-up, patients with bvFTD-PiD showed greater volume loss compared to patients with naPPA-PiD predominantly in bilateral prefrontal cortex (including lateral and medial regions), medial and lateral temporal regions, anterior cingulate, rectus gyrus, orbitofrontal cortex, bilateral basal ganglia, and parietal and occipital lobe, with loss most pronounced on the right (figure 2). These differences were more pronounced at follow-up. No regions showed greater loss in naPPA-PiD compared to bvFTD-PiD at either time point. The Jacobian change maps showed greater atrophy in bvFTD-PiD than naPPA-PiD in prefrontal cortex, right middle cingulate, bilateral middle temporal gyri, right parahippocampal gyrus, right angular and supramarginal gyri, and precuneus (figure 2). Conversely, patients with naPPA-PiD showed greater atrophy than patients with bvFTD-PiD in entorhinal cortex, fusiform gyrus, and in small regions in the left posterior middle frontal gyrus and right orbitofrontal gyrus (figure 2).
Using conjunction analysis, overlap was observed between the 2 syndromes at both baseline and follow-up in the bilateral insula, medial prefrontal cortex, anterior cingulate, and orbitofrontal cortex. Overlap in the Jacobian change maps was observed in bilateral inferior frontal gyrus, left insula, and left inferior temporal gyrus.
Asymmetry scores showed frontotemporal asymmetry in bvFTD-PiD and naPPA-PiD, with patients with bvFTD-PiD showing greater absolute asymmetry in orbitofrontal cortex, medial temporal, and temporal pole (table).
Individual-level gray matter Z score maps for a patient with bvFTD-PiD and a patient with naPPA-PiD who each had 5 serial MRIs spanning 4 years are shown in figure 4. At baseline, 5 years after onset, the patient with bvFTD-PiD showed right-sided patterns of volume loss, involving lateral temporal gyri, temporal pole, medial temporal regions, anterior cingulate, medial, lateral, and orbital frontal lobe, gyrus rectus, and insula, with the most severe loss observed in right amygdala, temporal pole, inferior temporal gyrus, temporal pole, and orbitofrontal cortex. Over time, atrophy worsened in the same regions and spread posteriorly to involve caudate, putamen, thalamus, motor cortex, supplementary motor area, inferior parietal lobe, and angular gyrus. At baseline, the patient with naPPA-PiD showed left-sided patterns of volume loss, involving inferior, middle, and medial frontal cortex, orbitofrontal cortex, gyrus rectus, insula, middle cingulate, inferior and middle temporal gyri, temporal pole, amygdala, hippocampus, motor cortex, supramarginal gyrus, and right inferior occipital lobe. The most severe loss at baseline was observed in left inferior frontal cortex. Over time, volume loss worsened in these same regions and spread to involve anterior cingulate, entorhinal cortex, fusiform, parahippocampal gyrus, caudate, putamen, thalamus, sensory cortex, supplementary motor area, inferior parietal lobe, and angular gyrus.
Figure 4. Individual-level MRI gray matter Z score maps for a patient with behavioral variant of frontotemporal dementia (bvFTD)–Pick disease (PiD) and a patient with nonfluent/agrammatic variant of primary progressive aphasia (naPPA)–PiD who each had 5 serial MRIs spanning 4 years.
Each row represents a different MRI date, with the time from onset to MRI shown on each row for each patient. Volume loss is represented as negative Z scores. Only regions with a Z score less than −1.5 are shown.
FDG-PET analysis
The patients with PiD with FDG-PET (n = 7) showed hypometabolism compared to controls in the left insula, inferior frontal gyrus, and supplementary motor area at a FWE-corrected threshold of p < 0.05 (not shown). Using a more lenient correction for multiple comparisons (FDR at p < 0.01) (figure 1), hypometabolism was observed bilaterally throughout medial and lateral frontal lobe, insula, temporal pole, inferior and middle temporal gyri, fusiform, amygdala, entorhinal cortex, caudate, supramarginal gyrus, inferior parietal lobe, and cerebellum. Similar, albeit weaker, patterns of hypometabolism were observed after partial volume correction. The individual FDG-PET CortexID Z score images are shown in figure 5. The 2 patients with bvFTD-PiD both showed striking prefrontal hypometabolism, although one case is left and the other right lateralized. Both cases also showed some involvement of the anterior and inferior temporal lobe, basal ganglia, and supramarginal gyrus. Subtle progression in hypometabolism was observed over 1 year in the patient with bvFTD-PiD with serial scans, with progression particularly notable in posterior temporal lobe and supramarginal gyrus. The 4 patients with naPPA-PiD all showed a focus of hypometabolism in the posterior inferior frontal cortex, with 2 cases showing left lateralized patterns, 1 bilateral and 1 right lateralized. The supplementary motor area was also involved in all 4 naPPA-PiD cases, yet was spared in the bvFTD-PiD cases. Serial FDG-PET scans were obtained in 3 patients with naPPA-PiD (figures 5 and 6). Progression in hypometabolism was observed in all 3 patients in the frontal cortex, particularly Broca area, with additional areas of progression within temporal and parietal regions.
Figure 5. Individual baseline FDG-PET CortexID Z score maps.
Clinical diagnosis, age, and time from onset to FDG-PET are shown for each patient. For patients with serial FDG, time from onset to each FDG-PET is shown. bvFTD = behavioral variant of frontotemporal dementia; naPPA = nonfluent/agrammatic variant of primary progressive aphasia.
Figure 6. Molecular PET findings.
Top row shows the Pittsburgh compound B (PiB) PET standard uptake value ratio (SUVR) images for 5 patients with Pick disease (PiD), with the global PiB SUVR shown for each. Asterisks highlight the 2 cases that had evidence of Aβ plaques at autopsy. Bottom panel shows the flortaucipir PET SUVR image (left) and longitudinal MRI (middle) and FDG-PET (right) Z score maps for 1 patient with nonfluent/agrammatic variant of primary progressive aphasia–PiD. For the Z score maps, each row represents a different scan date, with the time from onset to scan shown on each row. The MRI and FDG-PET images were performed within 1 day of each other at every visit. Volume loss and hypometabolism are represented as negative Z scores. Only regions with a Z score less than −1.5 are shown on the MRI maps. The flortaucipir PET was performed at the time of the last MRI/FDG-PET visit.
PiB and flortaucipir PET analysis
Of the 5 patients with PiB-PET, only 1 patient with naPPA-PiD was Aβ+ (SUVR = 1.66), with Aβ uptake observed in frontal and parietal lobes (figure 6). Sparse to moderate non-neuritic Aβ senile plaques were observed in the cortex in this patient on autopsy (Thal phase 1), with a Braak neurofibrillary tangle stage of I. Of the 4 PET Aβ- cases, 3 showed no Aβ plaques on autopsy (Thal phase 0), and 1 showed sparse neuritic and moderate diffuse Aβ plaques in the cortex (Thal phase 1). Flortaucipir PET in 1 patient with naPPA-PiD showed mild flortaucipir uptake in white matter of the frontal and anterior temporal lobes, greater on the right, and also in basal ganglia, thalamus, and midbrain (figure 6). Consistent with flortaucipir PET, volume loss and hypometabolism were more severe in the right hemisphere, involving inferior frontal cortex, orbitofrontal cortex, supplementary motor area, insula, temporal pole, lateral temporal gyri, and amygdala at baseline, spreading to involve entorhinal, fusiform, gyrus rectus, putamen, thalamus, sensorimotor cortex, and supramarginal gyrus (figure 6).
Discussion
We performed a cross-sectional and longitudinal assessment of MRI and FDG-PET in a relatively large cohort of patients with autopsy-confirmed PiD. We showed that PiD is associated with striking volume loss and hypometabolism in the prefrontal cortex and anterior temporal lobes, with progression in these regions over time and spreading into more posterior regions of the brain. Regional patterns at baseline differed according to the presenting syndrome, with patients with bvFTD-PiD showing typical frontotemporal abnormalities, and patients with naPPA-PiD showing patterns more restricted to the frontal lobe. However, with disease progression, patterns of neurodegeneration somewhat converge.
The cross-sectional patterns of bilateral frontotemporal volume loss and hypometabolism identified in the bvFTD-PiD group were consistent with previous MRI findings in patients with bvFTD-PiD.11–13 We found that the bilateral insula, medial frontal lobe/anterior cingulate, and orbitofrontal cortex were the most severely affected structures in the group maps. However, loss did occur in regions outside of the frontotemporal regions to involve the basal ganglia and supramarginal gyrus, as others have also found.5,11–13 Regional patterns of volume loss and hypometabolism at baseline were somewhat different in the naPPA-PiD group. This group showed a relative sparing of the temporal lobe, with abnormalities observed predominantly in the insula, inferior frontal gyrus, and supplementary motor area. This pattern is very similar to that reported previously in 2 cases of naPPA-PiD.14 These regions are typically abnormal in patients with the clinical syndrome of naPPA,2,29,30 with MRI and FDG-PET abnormalities in the inferior frontal gyrus shown to correlate with agrammatism and abnormalities in the supplementary motor area shown to correlate with apraxia of speech.31 The bvFTD-PiD group showed more striking involvement of the most anterior portions of the frontal lobes and the temporal lobes compared to the naPPA-PiD group. Patterns of loss were asymmetric at the group level in naPPA-PiD, with greater involvement of the left hemisphere, and more bilateral at the group level in bvFTD-PiD. However, at the patient level, asymmetry was observed in both groups, with bvFTD-PiD even showing greater asymmetry in some regions. Within each cohort, patients were observed with both left-sided and right-sided patterns. One case of naPPA-PiD with right-sided patterns of atrophy, i.e. crossed aphasia, has previously been reported.14
Patterns of progression also differed according to clinical syndrome at the group level, with bvFTD-PiD showing volume loss spreading posteriorly in the right temporal lobe and into the parietal lobe, while naPPA-PiD showed spread anteriorly within the frontal lobe, both laterally and medially, and spread into the left temporal lobe, including the entorhinal cortex and lateral temporal cortices. The bvFTD-PiD group showed faster rates in frontotemporal regions, with the exception of the entorhinal cortex, compared to naPPA-PiD, suggesting more aggressive disease progression. We did not observe any involvement of the parietal lobe in the group maps in naPPA-PiD, although there was evidence of spread of atrophy into the supramarginal gyrus, angular gyrus, and inferior parietal lobe in individual patients both on MRI and FDG-PET; similar patterns were observed at the individual level in patients with bvFTD-PiD. There was also evidence for spread into the motor cortex in both bvFTD-PiD and naPPA-PiD in individual patients. Therefore, despite different early patterns of loss, which reflect clinical syndrome, it appears as though the disease spreads into similar regions, albeit in a different order, in patients with both clinical syndromes, and hence they may end up looking more similar late in the disease. This was evident in the patients who had multiple serial MRI; the patients with bvFTD-PiD and naPPA-PiD ultimately showed similar patterns of loss affecting medial and lateral temporal and frontal lobes, motor cortex, and inferior parietal regions. Time from onset to baseline scan was slightly shorter in the patients with naPPA-PiD, so they were likely earlier in their disease course, which may explain the milder patterns of baseline volume loss in this group. There was evidence that the clinical syndromes also converge to some degree over time, with behavioral abnormalities commonly developing in the patients with naPPA-PiD and language difficulties commonly developing in the patients with bvFTD-PiD.
The indirect progression of PiD has been assessed pathologically, with one study finding that tau deposition starts in frontotemporal limbic cortices (anterior cingulate, insula, amygdala; phase I limbic) and neocortical regions (middle frontal, orbitofrontal cortex, angular gyrus, temporal cortex; phase I cortical) before spreading into basal ganglia (phase II), motor cortex (phase III), and finally visual cortex and cerebellum (phase IV).10 Longitudinal imaging was available in a handful of patients in that study and they also found early volume loss in anterior cingulate, insula, orbitofrontal cortex, middle frontal gyrus, amygdala, and striatum, with spread over time into lateral temporal lobe and motor cortex.10 Our findings were largely consistent with the pathologic staging scheme. We did observe early volume loss in the insula, cingulate, and orbitofrontal cortex in both bvFTD-PiD and naPPA-PiD, and evidence of early involvement of the lateral temporal lobe and amygdala in individual patients from both groups. Involvement of some temporal lobe structures, including the entorhinal cortex, parahippocampal gyrus, and fusiform gyrus, differed across groups, with evidence for earlier involvement in bvFTD-PiD. We also observed progressive involvement of the basal ganglia and motor cortex, although this seemed to occur at a similar time to involvement of the parietal lobe (angular gyrus, supramarginal, and inferior parietal) in the individual cases. Involvement of the basal ganglia was more prominent in bvFTD-PiD compared to naPPA-PiD. We did not find any evidence that the cases had yet spread to involve visual cortex or cerebellum on MRI, although we did observe some mild cerebellar hypometabolism on FDG-PET.
Despite the different clinical presentations, a set of core regions, including the inferior frontal gyrus, insula, orbitofrontal cortex, and medial frontal regions (including anterior cingulate), were atrophic in both bvFTD-PiD and naPPA-PiD. The presence of atrophy in these regions could, therefore, be a clue to the presence of underlying PiD. Indeed, these regions have come up as associated with PiD in studies that have compared different pathologies within patients with bvFTD. For example, compared to bvFTD with corticobasal degeneration pathology, bvFTD-PiD showed greater involvement of the anterior medial frontal lobe in one study,12 and greater involvement of insula and inferior frontal gyrus in another.11 The finding of dorsolateral and medial frontal volume loss was also unique to bvFTD-PiD compared to both bvFTD with underlying FTLD-ubiquitinated and bvFTD with mutations in the microtubule-associated protein tau (MAPT) gene.13 It is less clear whether these regions would be useful to help predict pathology within naPPA, with one study failing to find differences between naPPA cases with different pathologies, although the number of participants was small.14
Of the 5 patients with PiD who had undergone PiB-PET, only 1 (20%) showed evidence of Aβ deposition. Indeed, this patient did have sparse to moderate diffuse plaques in the cortex on autopsy. However, the patient only received a Thal phase of 1 and a Braak stage of I, and hence the autopsy findings would meet National Institute on Aging–Alzheimer’s Association criteria for low AD neuropathologic change.21 The PiB-PET scan failed to identify Aβ deposition in the one other case that showed sparse neuritic plaques and moderate diffuse plaques in the cortex; this case also met criteria for low AD neuropathologic change.21 Previous studies have shown that PiB-PET can detect diffuse Aβ plaques,32 that the global PiB SUVR correlates well with Thal Aβ phase,23,33,34,35 and that PiB-PET can start to detect Aβ from a Thal phase of 1 and upwards,32 although PiB-PET often cannot detect sparse to moderate Aβ plaques.36 It is unclear why the PET scan only identified 1 of these 2 cases, although it is possible that differences in plaque burden could have influenced the PET findings. In both cases the PiB-PET scan was performed approximately 3 years before death.
One of our patients underwent a flortaucipir PET scan and showed evidence for mild uptake in frontotemporal regions. This patient did not have any AD neuropathologic changes at autopsy. We have previously reported this patient and showed that regional flortaucipir uptake correlated well with volume loss, hypometabolism, and 3R tau burden at autopsy.19 However, autoradiographic studies show no to minimal binding of flortaucipir to non 3R/4R tauopathies, such as PiD,19,37–39 and hence it is unclear whether the flortaucipir findings truly reflect underlying 3R tau burden. An interesting observation from this patient is that uptake was predominantly observed in the white matter. While pathology is found in the white matter at autopsy in PiD, predominant flortaucipir white matter uptake has been observed in patients who have 4R tau40 and non-tau disorders,41 suggesting a possible off-target binding site in the white matter.
A limitation of the study was that we did not have enough patients with svPPA or corticobasal syndrome with neuroimaging in order to determine patterns of atrophy or hypometabolism in these Pick syndromes. This may limit the generalizability of our conclusions to other clinical syndromes that result from PiD. The number of patients with PET scans was also relatively small. This is, however, expected since this cohort has been collected over the last 21 years.
This study provided a comprehensive neuroimaging assessment of patients with pathologically confirmed PiD. Our findings help to better characterize how the disease progresses through the brain in PiD and demonstrated how neurodegeneration in PiD differs according to presenting clinical syndrome. This knowledge not only sheds light on the biology and disease process in PiD but improves the antemortem characterization of PiD.
Acknowledgment
The authors thank AVID Radiopharmaceuticals for provision of AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
Glossary
- Aβ
β-amyloid
- AUROC
area under the receiver operating characteristic curve
- bvFTD
behavioral variant of frontotemporal dementia
- FDG-PET
[18F]fluorodeoxyglucose PET
- FDR
false discovery rate
- FOV
field of view
- FWE
familywise error
- FWHM
full width at half maximum
- MCALT
Mayo Clinic Adult Lifespan Template
- MCSA
Mayo Clinic Study of Aging
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
- naPPA
nonfluent/agrammatic variant of primary progressive aphasia
- PiD
Pick disease
- PPA
primary progressive aphasia
- ROI
region of interest
- SUVR
standardized uptake value ratio
- svPPA
semantic variant of primary progressive aphasia
- TE
echo time
- TI
inversion time
- TR
repetition time
Appendix. Authors

Study funding
The study was funded by NIH grants R01-DC010367, R01-DC12519, R21-NS094684, R01-DC14942, P50-AG16574, P30-AG062677, U01-AG006786, and R01-AG11378.
Disclosure
J.L. Whitwell receives funding from the NIH. N. Tosakulwong reports no disclosures. C.C. Schwarz receives funding from the NIH. M.L. Senjem and A.J. Spychalla report no disclosures. J.R. Duffy receives funding from the NIH. J. Graff-Radford receives funding from the NIH. M.M. Machulda receives funding from the NIH. B.F. Boeve serves on the scientific advisory board of the Tau Consortium; receives publishing royalties for Behavioral Neurology of Dementia (Cambridge University Press); and receives grant support from Biogen, Alector, Mangurian Foundation, Little Family Foundation, Turner Family, and the NIH. D.S. Knopman serves on the scientific board of Consultant Bluefield project (frontotemporal dementia), Lundbeck Pharmaceuticals, and DIAN study DSMB; receives research support from A4 clinical trial partially funded by Lilly (site PI), Biogen Alzheimer Study, and the NIH. R.C. Petersen serves as a consultant to Roche Incorporated, Merck, Genentech (DSMB), Biogen, GE Healthcare (presentation), and Eisai, Inc.; receives royalties for Mild Cognitive Impairment (Oxford University Press); and receives research support from the NIH. V.J. Lowe serves on the scientific advisory board of Piramal Imaging, Merck Research, and Bayer Schering Pharma; and receives research support from GE Health Care, AVID Radiopharmaceuticals, Siemens Molecular Imaging, MN Partnership for Biotechnology and Medical Genomics, Elsie and Marvin Dekelboum Family Foundation, Liston Family Foundation, and the NIH. C.R. Jack receives research support from The Alexander Family Professorship for Alzheimer's Disease Research, Mayo Clinic, and the NIH. D.W. Dickson receives funding from the NIH. J.E. Parisi reports no disclosures. K.A. Josephs receives funding from the NIH. Go to Neurology.org/N for full disclosures.
References
- 1.Dickson DW. Neuropathology of Pick's disease. Neurology 2001;56:S16–S20. [DOI] [PubMed] [Google Scholar]
- 2.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005;128:1996–2005. [DOI] [PubMed] [Google Scholar]
- 4.Piguet O, Halliday GM, Reid WG, et al. Clinical phenotypes in autopsy-confirmed Pick disease. Neurology 2011;76:253–259. [DOI] [PubMed] [Google Scholar]
- 5.Rohrer JD, Lashley T, Schott JM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 2011;134:2565–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs KA, Hodges JR, Snowden J, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 2011;122:137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006;66:41–48. [DOI] [PubMed] [Google Scholar]
- 8.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol 2007;114:31–38. [DOI] [PubMed] [Google Scholar]
- 9.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004;56:399–406. [DOI] [PubMed] [Google Scholar]
- 10.Irwin DJ, Brettschneider J, McMillan CT, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 2016;79:272–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin KP, Mayo MC, Seeley WW, et al. Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick's disease. J Mol Neurosci 2011;45:594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitwell JL, Jack CR Jr, Parisi JE, et al. Imaging signatures of molecular pathology in behavioral variant frontotemporal dementia. J Mol Neurosci 2011;45:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol 2005;62:1402–1408. [DOI] [PubMed] [Google Scholar]
- 14.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 2017;81:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tetzloff KA, Duffy JR, Clark HM, et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain 2019;142:2466–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utianski RL, Schwarz CG, Murray ME, et al. In vivo imaging and autoradiography in a case of autopsy-confirmed Pick disease. Neurol Clin Pract Epub 2019 Oct 23. [DOI] [PMC free article] [PubMed]
- 20.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 21.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 23.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz CG, Gunter JL, Ward CP, et al. The Mayo Clinic Adult lifespan template: better quantification across the lifespan. Alzheimers Demen 2017;13:792. [Google Scholar]
- 25.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology 2014;82:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995;36:1238. [PubMed] [Google Scholar]
- 28.Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid-beta and tau: relationship with established neurocognitive clinical syndromes. Brain 2019;142:3230–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josephs KA, Duffy JR, Fossett TR, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol 2010;67:596–605. [DOI] [PubMed] [Google Scholar]
- 31.Whitwell JL, Duffy JR, Strand EA, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang 2013;125:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe VJ, Lundt ES, Albertson SM, et al. Neuroimaging correlates with neuropathologic schemes in neurodegenerative disease. Alzheimers Dement 2019;15:927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantarci K, Lowe VJ, Chen Q, et al. beta-Amyloid PET and neuropathology in dementia with Lewy bodies. Neurology 2020;94:e282–e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Joie R, Ayakta N, Seeley WW, et al. Multisite study of the relationships between antemortem [(11)C]PIB-PET Centiloid values and postmortem measures of Alzheimer's disease neuropathology. Alzheimers Dement 2019;15:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray ME, Lowe VJ, Graff-Radford NR, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain 2015;138:1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghirelli A, Tosakulwong N, Weigand SD, et al. Sensitivity-Specificity of Tau and Amyloid β Positron Emission Tomography in Frontotemporal Lobar Degeneration. Ann Neurol EPub 2020 August 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [F]AV-1451 binding to postmortem tissue in Alzheimer's disease, primary tauopathies, and other dementias. Alzheimers Dement 2016;12:1116–1124. [DOI] [PubMed] [Google Scholar]
- 40.Josephs KA, Martin PR, Botha H, et al. [(18) F]AV-1451 tau-PET and primary progressive aphasia. Ann Neurol 2018;83:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitwell JL, Martin PR, Duffy JR, et al. The influence of beta-amyloid on [(18)F]AV-1451 in semantic variant of primary progressive aphasia. Neurology 2019;92:e710–e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.