Abstract
Objective
To examine the use of benzodiazepines and the association between low benzodiazepine dose, breakthrough seizures, and respiratory support in patients with status epilepticus.
Methods
In this cross-sectional analysis of adult patients with status epilepticus treated by an emergency medical services agency from 2013 to 2018, the primary outcome was treatment with a second benzodiazepine dose, an indicator for breakthrough seizure. The secondary outcome was receiving respiratory support. Midazolam was the only benzodiazepine administered.
Results
Among 2,494 patients with status epilepticus, mean age was 54.0 years and 1,146 (46%) were female. There were 1,537 patients given midazolam at any dose, yielding an administration rate of 62%. No patients received a dose and route consistent with national guidelines. Rescue therapy with a second midazolam dose was required in 282 (18%) patients. Higher midazolam doses were associated with lower odds of rescue therapy (odds ratio [OR], 0.8; 95% confidence interval [CI], 0.7–0.9) and were not associated with increased respiratory support. If anything, higher doses of midazolam were associated with decreased need for respiratory support after adjustment (OR, 0.9; 95% CI, 0.8–1.0).
Conclusions
An overwhelming majority of patients with status epilepticus did not receive evidence-based benzodiazepine treatment. Higher midazolam doses were associated with reduced use of rescue therapy and there was no evidence of respiratory harm, suggesting that benzodiazepines are withheld without clinical benefit.
Classification of evidence
This study provides Class III evidence that for patients with status epilepticus, higher doses of midazolam led to a reduced use of rescue therapy without an increased need for ventilatory support.
Status epilepticus is a neurologic emergency affecting approximately 160,000 people in the United States each year.1–3 To limit status epilepticus–related morbidity and mortality, guidelines recommend emergent treatment with intramuscular midazolam, IV lorazepam, or IV diazepam based on the results of 2 high-quality randomized controlled trials.4–7 For out-of-hospital status epilepticus, immediate benzodiazepine administration is provided by emergency medical services (EMS) providers who administer first responder care as employees of a given EMS agency. EMS agencies provide oversight and training to their provider team in order to ensure the management of out-of-hospital medical emergencies is standardized and high quality. Nonetheless, studies demonstrate that out-of-hospital status epilepticus is frequently undertreated, as EMS providers may not administer benzodiazepines or may administer them at doses lower than recommended.8–12
Undertreatment may occur because EMS providers believe that recommended doses of benzodiazepines will cause respiratory depression or that lower dosages and alternative routes of administration will be equally effective for status epilepticus, but there is limited evidence to support either of these assumptions. Different benzodiazepine medications and routes of administration have differential success in terminating status epilepticus.6,7 Treatment with a lower benzodiazepine dose has been shown to increase the likelihood of recurrent seizures.13 Although high benzodiazepine doses carry a theoretical risk of respiratory depression, trial data using small sample sizes suggest that the risk of respiratory depression may actually be higher for those who are treated with lower benzodiazepine doses, perhaps due to continued ictal activity.7 However, the exact relationship between rates of seizure termination as well as rates of respiratory depression and benzodiazepine dose is incompletely characterized.
Given the morbidity and potential costs associated with undertreated status epilepticus, inadequate benzodiazepine dosing could have large consequences. We used EMS medical record data to study the rate of evidence-based benzodiazepine use and the association between benzodiazepine dose and clinical outcomes in order to provide insight about the scope and implications of this gap in care.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review board of the University of California, San Francisco.
Classification of evidence
The primary research question was to examine whether higher doses of midazolam would reduce the rate of recurrent seizure and respiratory depression among patients with status epilepticus. This study provides Class III evidence that for patients with status epilepticus, higher doses of midazolam led to a reduced use of rescue therapy without an increased need for ventilatory support.
Study design and setting
This is an analysis of EMS medical records of adult patients evaluated for out-of-hospital status epilepticus from January 2013 to January 2018. The EMS agency provides EMS services for an urban/suburban county with 11 cities and a population of 1.6 million. Medical records are entered electronically and include primary and secondary diagnostic impressions, which providers select from a predetermined list. We examined the association between midazolam dose and 2 clinical outcomes: recurrent seizure and respiratory depression.
Selection of participants
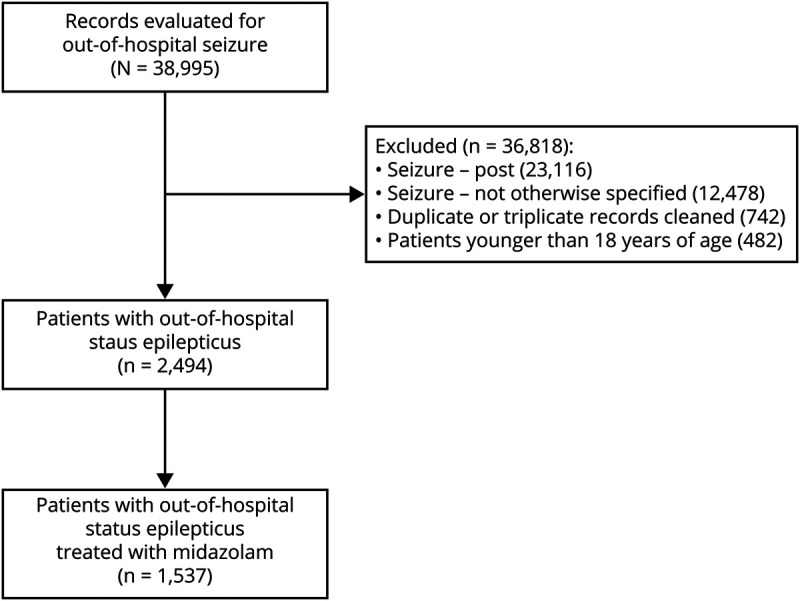
Patients with seizure were identified as any patient with a primary or secondary diagnostic impression indicating 1 of 3 seizure options: seizure-active, seizure-post, and seizure–not otherwise specified (NOS). The EMS agency protocol defines active seizures as 3 or more seizures within a 5-minute period or any seizure lasting more than 5 minutes, which is similar to guideline-based definitions of status epilepticus as 5 minutes of continuous seizure activity or 2 or more seizures without recovery of consciousness.4,14 Using temporal criteria of 5 minutes instead of 30 minutes to define status epilepticus has been widely adopted to encourage earlier treatment. We have chosen to employ this more practical definition and use the term status epilepticus to refer to patients with a primary or secondary impression of active seizures. Patients younger than 18 years were excluded (figure 1). We used these data to examine the use of benzodiazepines among patients with status epilepticus and examine the association between benzodiazepine dose and 2 clinical outcomes: the need for rescue therapy and the need for respiratory support.
Figure 1. Data available on adult patients evaluated by an emergency medical services agency for out-of-hospital status epilepticus (2013–2018).
Exposure
The primary exposure of interest was the initial midazolam dose administered by an EMS provider. Midazolam is the only benzodiazepine available for providers to administer at this EMS agency. Providers have the option of administering midazolam as an intramuscular, intranasal, IV, or intraosseous medication. Agency protocol recommends either a single dose of 0.1 mg/kg (up to a maximum dose of 6 mg) for intramuscular, IV, or intraosseous administration or a single dose of 5 mg for intranasal administration. This differs from national guidelines, which state that first-line evidence-based treatment with midazolam requires administering the medication intramuscularly as a single 10-mg dose, and does not recommend the intranasal, IV, or intraosseous route of administration. Agency protocol also does not recommend treatment with IV lorazepam or IV diazepam, which are alternative first-line treatment options included in national guidelines.
Measurements and outcomes
Rates of midazolam use were calculated as (1) the proportion of patients with status epilepticus who received any midazolam dose and (2) the proportion of patients with status epilepticus who received a midazolam dose of 5 mg or higher among those who received any midazolam dose, which was the median midazolam dose used during the study period and differs from the guidelines recommending 10 mg as a single dose.
The primary outcome was the need for rescue therapy, defined as treatment with a second benzodiazepine dose, which was used as an indicator for ongoing seizure activity requiring treatment; this has previously been used as a marker of status epilepticus treatment failure.7,8 The data did not include detailed information about clinically evident seizures, level of consciousness, or electrographic seizures. The secondary outcome was the need for respiratory support defined by the use of ventilatory assistance (endotracheal intubation, supraglottic airway, continuous positive airway pressure device, bag valve mask), an oropharyngeal airway, or a nasopharyngeal airway.
Patient age, sex, and race were extracted from the patient record along with patient comorbidities, allergies, vital sign data, and Glasgow Coma Scale (GCS) score. Race was narrowed to 3 options: white, black, other/unknown. Patient comorbidities were recorded when available and we included 2 relevant comorbidities: alcohol or drug use (patient-reported or suspected by the provider) and epilepsy. We used the initial vital sign values recorded because these were likely most relevant to medication decisions. We also abstracted an EMS provider's experience treating patients with seizure by calculating their case volume over the study period. Provider case volume was defined as the number of records for out-of-hospital seizure associated with a given provider's name. Every encounter has one provider who submits the documentation and multiple EMS providers who are part of the treating team. Provider case volume was equal to the number of cases for out-of-hospital seizure that a given provider was the documenting member of the team, as this was the information available in the medical record.
A subset of patients had duplicate records for the same patient encounter, which occurred when separate evaluation and transport teams were dispatched to a patient and each team documented their encounter separately. We identified duplicate records by matching patient name and encounter date. There were 742 encounters with either duplicate or triplicate records. To accurately record midazolam administration and consolidate information into a single record, we developed an algorithm for abstracting data based on subanalysis of these files and included these cleaned records in the main dataset (data available from Dryad: “Method to condense duplicate emergency medical services records”).
Statistical analysis
We examined demographic data and clinical characteristics using Pearson χ2 tests to compare categorical variables and Student t tests to compare continuous variables. To compare GCS and EMS provider case volume between groups, we used Wilcoxon rank-sum tests because these variables were not normally distributed.
For the patients with status epilepticus who received midazolam, we examined the association between midazolam dose and the need for rescue therapy and the need for respiratory support by univariable and multivariable logistic regression. We treated midazolam dose as a continuous variable in our primary analysis. We included patient and provider characteristics as covariates. Our multivariable analysis was a mixed effects linear model, which included a random agency-level intercept to address potential clustering by ambulance provider. There were missing baseline data for a small percentage of patients who had received midazolam (systolic blood pressure missing in 75 [4.9%], diastolic blood pressure missing in 89 [5.8%], oxygen saturation missing in 91 [5.9%], pulse missing in 2 [0.1%], age in 1 [0.1%]). We used multiple imputation to handle these missing observations in the primary analysis.
In addition to the primary analysis, we treated midazolam dose as a binary variable (receiving the median dose of 5 mg or higher vs not) to determine whether our findings were robust to this dose threshold and to ease interpretability. We also explored the relationship between midazolam administration and outcomes further by incorporating route of administration and the interaction between midazolam dose and route in the model. We used average marginal effects to better characterize the association between midazolam dose, midazolam route, and our outcome variables. We further evaluated respiratory outcomes by examining the association between midazolam dose and the need for ventilatory assistance (as opposed to all forms of respiratory support). All tests were 2-sided and all reported odds ratios (ORs) are from the adjusted models unless otherwise specified. Statistical significance was declared based on p < 0.05. Statistical analyses were performed using Stata (version 15.1, StataCorp, College Station, TX).
Data availability
Anonymized data related to the current article are available and will be shared by request from any qualified investigator. Persons interested in obtaining access to the data should contact the corresponding author (E.L.G.).
Results
We identified 38,995 encounters for out-of-hospital seizure. There were 3,401 (8.7%) patients with status epilepticus, 23,116 (59.3%) patients whose seizures had terminated, and 12,478 (32.0%) patients whose seizures were NOS. There were 2,494 unique cases of status epilepticus (figure 1). Patients with status epilepticus had a mean age of 54 years and there were 1,146 (46.0%) female patients.
Clinical characteristics and benzodiazepine use
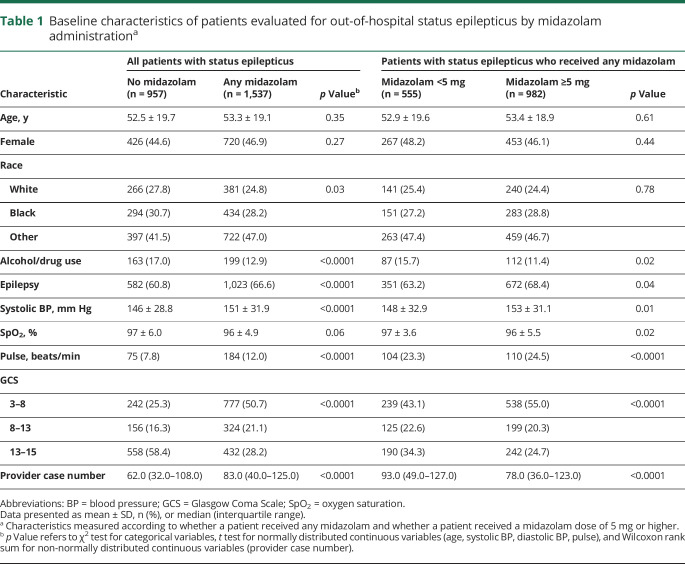
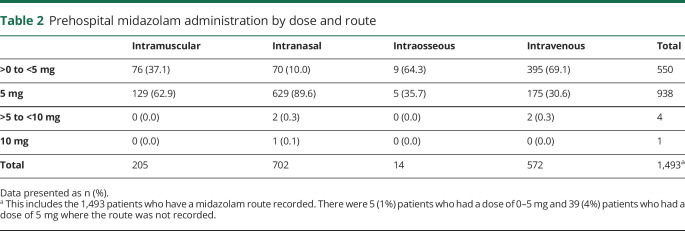
Among those with status epilepticus, 1,537 patients were given midazolam at any dose, yielding a benzodiazepine administration rate of 61.6% for status epilepticus. There were 982 patients who received a dose of midazolam that was 5 mg or higher, which comprised 39.4% of all patients with status epilepticus and 63.9% of patients with status epilepticus who received midazolam at any dose (table 1). Zero percent received the guideline-based dose of 10 mg. Patients received midazolam as an intranasal (47.0%) and IV (38.3%) injection more commonly than intramuscular (13.7%) and intraosseous (1%) injection. Those who received intranasal and intramuscular midazolam were most commonly given a dose of 5 mg (89.6% and 62.9%, respectively) and those who received IV midazolam were most commonly given a dose less than 5 mg (69.1%). Of the 943 patients who received a dose of midazolam that was 5 mg or higher, 938 (99.5%) received a dose of 5 mg and only 5 patients (0.5%) received a dose greater than 5 mg (table 2).
Table 1.
Baseline characteristics of patients evaluated for out-of-hospital status epilepticus by midazolam administrationa
Table 2.
Prehospital midazolam administration by dose and route
In univariable and multiple logistic regression analysis, patients with epilepsy (OR, 1.4; 95% confidence interval [CI], 1.2–1.7) and with a lower GCS score (GCS 8–12: OR, 0.7; 95% CI, 0.5–0.9; GCS 13–15: OR, 0.3; 95% CI, 0.2–0.3) were more likely to receive midazolam. Providers who administered midazolam had a median of 83 (interquartile range [IQR] 40–125) seizure encounters during the study period as compared to 62 (IQR 32–108) seizure encounters among providers who did not administer midazolam to patients with status epilepticus (OR, 1.0; 95% CI, 1.0–1.0), while those with more seizure encounters during the study period were less likely to give a dose of 5 mg or higher (OR, 0.7; 95% CI, 0.6–0.9) (table 1). The multivariable model had moderate discrimination (c statistic 0.61).
Primary outcome: rescue therapy
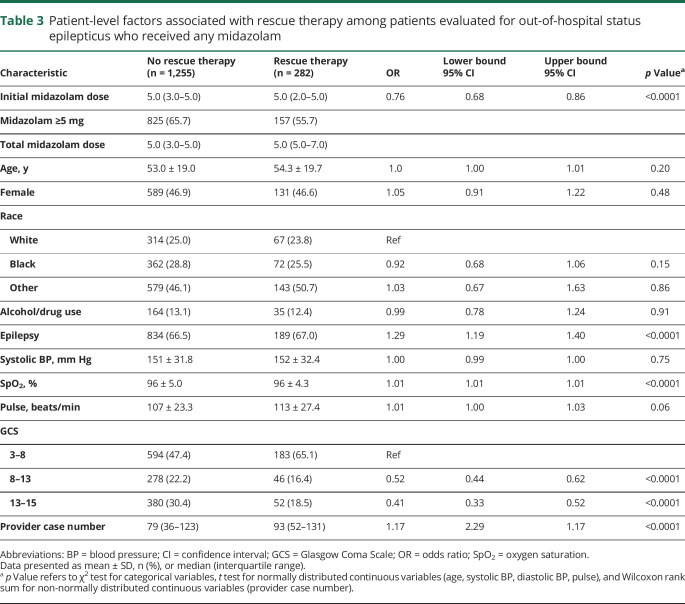
Rescue therapy with a second dose of midazolam was required in 282 of the 1,537 patients who received any dose of midazolam (18.3%). Those who received rescue therapy were less likely to have received a midazolam dose of 5 mg of higher, were more frequently tachycardic, had a lower GCS, and were evaluated by providers who had seen a higher number of patients with total seizure and status epilepticus during the study period (table 3).
Table 3.
Patient-level factors associated with rescue therapy among patients evaluated for out-of-hospital status epilepticus who received any midazolam
Patients who received a higher dose of midazolam were less likely to require rescue therapy (unadjusted OR, 0.8; 95% CI, 0.7–0.9). In our mixed effects model examining the relationship between the initial dose of midazolam and need for rescue therapy, every additional milligram of midazolam was associated with lower odds of rescue therapy (OR, 0.8; 95% CI, 0.7–0.9), and the probability of requiring rescue therapy was considerably lower among those who received 10 mg as compared to 1 mg of midazolam (4.5% vs 32.3%, respectively, using average marginal effects). Receiving a midazolam dose of 5 mg of higher was also associated with lower odds of receiving rescue therapy after adjustment (OR, 0.6; 95% CI, 0.4–0.9) (table 3).
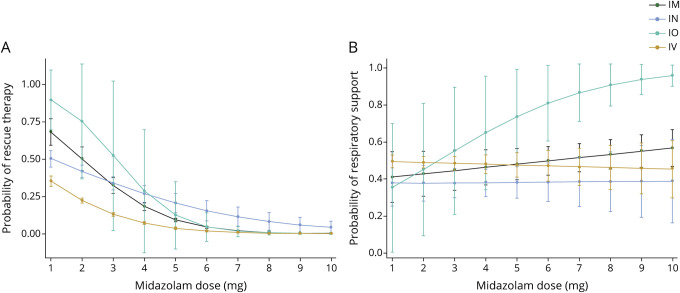
We used a marginal effects analysis to examine the relationship between route of administration, dose, and likelihood of requiring rescue therapy. All routes of administration demonstrated the same association between increasing dose and decreased likelihood of rescue therapy; however, the change in likelihood of rescue therapy between high and low doses was higher for intraosseous and intramuscular injections than intranasal and IV injections (figure 2).
Figure 2. Association between midazolam dose and rescue therapy or respiratory support.
(A) Association between midazolam dose and rescue therapy. (B) Association between midazolam dose and respiratory support. Number of observations for each dose and route available from Dryad (appendix “Detailed emergency medical services midazolam administration by dose and route”).23 Five patients received a midazolam dose greater than 5 mg (3 intravenous, 2 intranasal, 0 intramuscular, 0 intraosseous).
Secondary outcome: respiratory support
Of the 1,537 patients who received any dose of midazolam, there were 678 patients (44.1%) who required any form of respiratory support and 517 patients (33.6%) who required ventilatory support. Those who required respiratory support were more frequently male, were more likely to have comorbid alcohol and drug use, were more likely to be tachycardic, had lower oxygen saturation, had a lower GCS, and were evaluated by providers who had seen a higher number of total patients with seizure and status epilepticus during the study period (table 4). Receiving a higher dose of midazolam was not associated with an increased need for respiratory support (unadjusted OR, 1.0; 95% CI, 0.9–1.0). After adjusting for potential confounders in our mixed effects models, receiving a higher dose of midazolam was associated with a decreased need for respiratory support (OR, 0.9; 95% CI, 0.8–1.0). Receiving a midazolam dose of 5 mg or higher was associated with lower odds of respiratory support (OR, 0.8; 95% CI, 0.7–1.0). The interaction between route of administration, dose, and respiratory support did not reach statistical significance for any route of other than intraosseous injection, which 14 patients received (figure 2).
Table 4.
Patient-level factors associated with the need for respiratory support among patients evaluated for out-of-hospital status epilepticus who received any midazolam
Discussion
In a single-site study of adult patients with out-of-hospital status, midazolam was administered in 62% of cases and there were no patients who received midazolam at a dose and route consistent with national guidelines. Higher midazolam doses were associated with reduced use of rescue therapy and there was no evidence of harm. If anything, higher doses were associated with a lower need for respiratory support, perhaps due to successfully treated ictal activity. These results suggest that a higher initial midazolam dose, even if the dose is below that recommended in national guidelines, is associated with increased rates of successful seizure termination.
The rate of midazolam administration was similar to benzodiazepine administration rates reported in past studies, though these earlier studies were restricted to small cohorts of pediatric patients, adult populations outside of the United States, and preliminary analyses.8,9,15 In the 2 cohort studies, the low rate of benzodiazepine administration was attributed to ineffective identification of status epilepticus by prehospital providers.9,15 However, we found low rates of benzodiazepine use among patients who EMS providers had identified as having active seizures, suggesting that the findings may represent a failure to deliver appropriate treatment in spite of appropriately diagnosing status epilepticus. There are multiple possible contributors to this non–evidence-based care. A study examining barriers to the appropriate care of pediatric patients with seizure identified inadequate clinical training, concerns about respiratory depression, and systems barriers to the administration of controlled substances as consistent hurdles for a large number of providers.16 These same obstacles likely impede adult status epilepticus management in addition to discomfort with the diagnosis and management of neurologic emergencies among some providers. The severity of the problem and potential educational remedies deserve further study. For instance, we were not able to capture patients whose diagnosis of status epilepticus was missed in the prehospital setting, which, if substantial, may have lowered the rates of benzodiazepine administration further. Conversely, there may be patients who received an impression of active seizures but did not meet criteria for status epilepticus according to national guidelines. If these patients did not have guideline-concordant status epilepticus and did not receive midazolam, then removing them from the study cohort would raise the observed proportion of patients with status epilepticus who received midazolam. Yet, even among the patients who were treated with midazolam, nearly 40% were given a dose less than 5 mg, demonstrating that those who were treated for status epilepticus were often not treated appropriately.
To our knowledge, previous studies have not closely characterized the dose-dependent relationship between benzodiazepine administration and seizure termination. Earlier studies have provided strong evidence that benzodiazepines are effective in the treatment of status epilepticus and many of these studies have provided high-quality comparisons of the safety and efficacy of different benzodiazepine drug types and routes.7,8,17–20 However, there are limited data demonstrating how seizure termination rates change with low vs high dosing of a single medication type. Our finding that treatment with a higher midazolam dose leads to decreased need for rescue therapy therefore extends the literature and supports the guideline recommendation for higher midazolam dosing. Furthermore, whereas the perceived risk of respiratory depression may contribute to the current practice of underdosing benzodiazepines, we found evidence that higher midazolam doses are, if anything, associated with lower need for respiratory support, although the majority of the midazolam doses used in this study were lower than guideline recommendations.
In addition, to our knowledge, the majority of studies comparing the efficacy of different benzodiazepine routes have been performed in children and only compared 2 combinations of dose and route.21 We were able to examine the interaction between midazolam dose and midazolam route and found the greatest difference in rates of rescue therapy at lower midazolam doses. There were also minimal differences in rates of respiratory support across different routes of administration. These findings suggest that different routes of administration may be safe and effective when used at the higher, guideline-concordant doses.
There are limitations to this study. First, incomplete and inaccurate prehospital medical records could lead to misclassification of our study population. Providers may have used the diagnostic impression of active seizures for patients who did not meet criteria for status epilepticus and may have missed status epilepticus for other patients. This would limit the specificity and sensitivity of our definition. Thus, the rate of benzodiazepine administration that we report applies to patients identified as having status epilepticus by prehospital providers in this EMS agency but does not necessarily correspond to the rate of benzodiazepine administration among patients with true status epilepticus nationally. We also restricted our main analysis to patients with a diagnostic impression of active seizures who had received midazolam at any dose in order to increase the specificity of our definition and ensure our outcome was sensible. Second, patients cared for by the same EMS provider will be correlated but we did not include EMS provider as a random effect because there are multiple providers responding to every EMS call, and we were unable to determine whether the EMS provider providing documentation was the same provider making the clinical decision about how to treat the patient. Third, we were unable to confirm whether lack of rescue therapy truly represented lack of seizure recurrence. EMS providers may have withheld rescue therapy from patients who continued to have seizures, and we did not have information about seizure recurrence in the emergency department or other hospital outcomes such as intensive care unit admission and in-hospital mortality. Fourth, this study used a nonrandomized design to examine the relationship between midazolam administration and outcomes, which can lead to unrecognized differences between the patients who receive low as compared to high doses of midazolam. We found differences in comorbidities and vital sign values between the 2 populations, which were included as potential confounders; however, there are potential sources of residual confounding such as seizure duration and etiology. Nonetheless, higher midazolam doses were given to patients with more severe vital sign abnormalities, who may be at higher risk for recurrent seizure. In this circumstance, confounding would bias our results away from the protective effect of midazolam that we found. Finally, we only included patients from a single EMS agency. This inherently limits the generalizability of our findings to other EMS agencies; however, the findings support previous findings in other populations and we would expect the effect of benzodiazepine dose and route on seizure termination to be consistent across populations.22
Midazolam was either not administered or administered at a dose lower than the guidelines recommend in the majority of patients with status epilepticus evaluated by an EMS agency. Undertreatment with midazolam was associated with increased need for rescue therapy and did not affect rates of respiratory complications. This finding identifies an important gap in the treatment of status epilepticus and carries important clinical implications if EMS agencies nationally parallel this single-site experience. The next challenge will be evaluating the true scope of the problem and identifying effective interventions to correct it.
Glossary
- CI
confidence interval
- EMS
emergency medical services
- GCS
Glasgow Coma Scale
- IQR
interquartile range
- NOS
not otherwise specified
- OR
odds ratio
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study funding
Research reported in this publication was supported by the American Academy of Neurology under the Practice Based Training Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Academy of Neurology.
Disclosure
E.L. Guterman receives funding from the National Institute of Neurologic Disorders and Stroke (1K23NS116128-01) and the National Institute on Aging (5R01AG056715), American Academy of Neurology, as well as consulting fees from Marinus Pharmaceuticals, Inc. J.K. Sanford reports no disclosures. J.P. Betjemann receives consulting fees from Marinus Pharmaceuticals, Inc. and personal compensation from JAMA Neurology. L. Zhang receives funding from the National Cancer Institute (1U54CA242646-01). J.F. Burke receives funding from the National Institute on Minority Health and Health Disparities (5R01MD008879-05) and the National Institute on Aging (5R01AG059733-02). D.H. Lowenstein receives funding from the National Institute of Neurologic Disorders and Stroke (U01NS088034), the Office of the Director, NIH (U54 OD020351), and the Human Epilepsy Project. S.A. Josephson received personal compensation from JAMA Neurology and Continuum Audio, which are unrelated to the submitted work. K.A. Sporer reports no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46:1029–1035. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein DH, Alldredge B. Status epilepticus. N Engl J Med 1998;338:970–976. [DOI] [PubMed] [Google Scholar]
- 3.Betjemann JP, Josephson SA, Lowenstein DH, Burke JF. Trends in status epilepticus-related hospitalizations and mortality: redefined in US practice over time. JAMA Neurol 2015;72:650–655. [DOI] [PubMed] [Google Scholar]
- 4.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brophy GM, Bell R, Claassen J, et al. ; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 6.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631–637. [DOI] [PubMed] [Google Scholar]
- 7.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvis J, Barton D, Sager L, Nudell N. EMS Compass Benchmarks Using a National EMS Dataset: Status Epilepticus and Hypoglycemia Performance Measures. NAEMSP Annual Meeting Abstract; 2018. [Google Scholar]
- 9.Semmlack S, Yeginsoy D, Spiegel R, et al. Emergency response to out-of-hospital status epilepticus: a 10-year observational cohort study. Neurology 2017;89:376–384. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez Fernández I, Abend NS, Agadi S, et al. ; Pediatric Status Epilepticus Research Group (pSERG). Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology 2015;84:2304–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sairanen JJ, Kantanen AM, Hyppölä HT, Kälviäinen RK. Treatment delay in status epilepticus: more effective prehospital symptom recognition warranted. Scand J Trauma Resusc Emerg Med 2019;27:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sathe AG, Tillman H, Coles LD, et al. Underdosing of benzodiazepines in patients with status epilepticus enrolled in established status epilepticus treatment trial. Acad Emerg Med 2019;26:940‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter Healthcare Company. Ativan (lorazepam) [package insert]. US Food and Drug Administration website. Available at: accessdata.fda.gov/drugsatfda_docs/label/2006/018140s028lbl.pdf. Accessed May 30, 2020. [Google Scholar]
- 14.Alameda Emergency Medical Services Agency, Alameda County Field Assessment and Treatment Protocols. Available at: ems.acgov.org/ClinicalProcedures/FieldTreatmtProtocols.page? Accessed September 14, 2020. [Google Scholar]
- 15.Sánchez Fernández I, Gaínza-Lein M, Abend NS, et al. ; Pediatric Status Epilepticus Research Group (pSERG). Factors associated with treatment delays in pediatric refractory convulsive status epilepticus. Neurology 2018;90:e1692–e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey JM, Studnek JR, Browne LR, et al. Paramedic-identified enablers of and barriers to pediatric seizure management: a multicenter, qualitative study. Prehosp Emerg Care 2019;23:870–881. [DOI] [PubMed] [Google Scholar]
- 17.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. N Engl J Med 1998;339:792–798. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav 2004;5:253–255. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet 2005;366:205–210. [DOI] [PubMed] [Google Scholar]
- 20.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ 2000;321:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mula M. New non-intravenous routes for benzodiazepines in epilepsy: a clinician perspective. CNS Drugs 2017;31:11–17. [DOI] [PubMed] [Google Scholar]
- 22.Betjemann JP, Josephson SA, Lowenstein DH, Guterman EL. Emergency medical services protocols for generalized convulsive status epilepticus. JAMA 2019;321:1216–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guterman E. Detailed Emergency Medical Services Midazolam Administration by Dose and Route. UC San Francisco, Dataset; 2020. doi: 10.7272/Q6NS0S3R. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data related to the current article are available and will be shared by request from any qualified investigator. Persons interested in obtaining access to the data should contact the corresponding author (E.L.G.).