We report here the observation of a 60-year-old male jeweller who was suffering from severe asthma. Asthma onset was reported by age 30. The patient also reported comorbid severe chronic rhinosinusitis with nasal polyposis since adolescence. Aspirin and nonsteroidal anti-inflammatory drug intolerance was considered to worsen the patient's asthma symptoms, since he had experienced one episode of emergency room attendance shortly after aspirin ingestion. Episodes of generalised chronic urticaria led to genuine anaphylactic reactions that were treated with epinephrine twice in the past, but fortunately without the need for orotracheal intubation. No trigger for these episodes could be identified despite appropriate provocation tests.
Short abstract
In this case report, relapse of urticaria after a switch from oma- to mepolizumab successfully led to combination of biologics https://bit.ly/2GykNtI
To the Editor:
We report here the observation of a 60-year-old male jeweller who was suffering from severe asthma. Asthma onset was reported by age 30. The patient also reported comorbid severe chronic rhinosinusitis with nasal polyposis since adolescence. Aspirin and nonsteroidal anti-inflammatory drug intolerance was considered to worsen the patient's asthma symptoms, since he had experienced one episode of emergency room attendance shortly after aspirin ingestion. Episodes of generalised chronic urticaria led to genuine anaphylactic reactions that were treated with epinephrine twice in the past, but fortunately without the need for orotracheal intubation. No trigger for these episodes could be identified despite appropriate provocation tests.
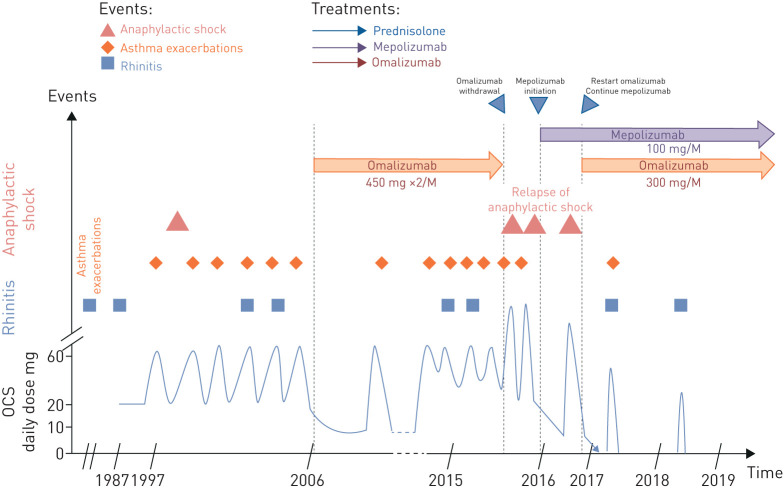
Recombinant anti-immunoglobulin E antibody (omalizumab) was initiated in 2006 and continued until 2015 because the patient's asthma was uncontrolled despite being managed according to Global Initiative for Asthma (GINA) guidelines: he presented with at least five exacerbations the year before receiving high doses of systemic corticosteroids, and he was eligible for the drug as he had known perennial sensitisations (house dust mite, cypress pollens, etc.) and an elevated serum total IgE level (739 kU·L−1). Oral corticosteroid (OCS) maintenance was established at 20 mg·day−1.
During the first years of treatment with omalizumab the number of exacerbations was reduced and the control of asthma was improved.
Progressive tapering of a maintenance dose of OCS could be achieved down to 10 mg·day−1, but complete weaning was unsuccessful due to asthma relapse.
After the good initial response to omalizumab, exacerbation outbreak and deterioration in the control of the asthma forced us to consider alternative therapies (figure 1). The eosinophil blood count at that time was 1640 per mm3, without grounds for a diagnosis of either ANCA-associated vasculitis or bronchopulmonary aspergillosis based on dedicated examinations including a chest computed tomography scan and autoimmunity assessment. The inhaled treatment observance was good and included maintenance and reliever therapy with a fixed combination of inhaled corticosteroids (ICS) (1500 μg beclometasone dipropionate equivalent) – long-acting β agonist (LABA) and a long-acting muscarinic antagonist (LAMA). Forced expiratory volume in 1 s (FEV1) was 67% of predicted value (June 2015).
FIGURE 1.
At a glance: clinical and therapeutic vignette.
The patient was given the opportunity to benefit from more immunotherapy in a clinical trial with anti-IL5R monoclonal antibody (i.e. the ZONDA trial at that time), which suggested the suspension of omalizumab then a washout time of at least 4 months.
At 2 months and also 3 months after omalizumab withdrawal, the patient presented at the emergency department with anaphylactic shock that required use of parenteral steroids at 2 mg·kg−1 for 3 consecutive days. No convincing triggering factor could be identified. On the other hand, the patient's asthma remained relatively unaffected by the withdrawal of omalizumab in terms of control of symptoms, exacerbation rates and lung function.
Considering the risk to vital functions related to these anaphylactic shocks, we introduced another biological, an anti-IL-5 monoclonal antibody (mepolizumab) as part of the registered temporary authorisation utilisation before ZONDA inclusion criteria could be completed, in December 2015. After five injections of this monoclonal antibody, and the daily dose of OCS progressively tapered as the level of asthma control was continuously improving (of note, FEV1 was then at 73% of predicted value), he continued to suffer from episodes of giant urticaria despite a regularly observed treatment with fexofenadine hydrochloride with another emergency admission for angioedema requiring a novel burst of OCS.
After a multidisciplinary concertation meeting, we decided to introduce omalizumab as a concomitant biological treatment at a dose regimen of 300 mg every 4 weeks as indicated for chronic idiopathic urticaria because of the absence of anaphylactic shock during previous treatment with omalizumab.
Since that time, he has received a monthly injection of both mepolizumab and omalizumab on the same day in different shoulders.
Nowadays, this patient is totally weaned from oral glucocorticoids, with controlled asthma and stable lung function with FEV1 at 2.71 L (86% of predicted value) for a forced vital capacity of 4.11 L (103% of predicted value). He no longer complains of skin itching or other anaphylactic manifestations. He has not been hospitalised or admitted to an emergency department. He no longer uses his reliever therapy, has stopped his LAMA therapy and has reduced his daily dose of ICS to 1000 µg·day−1. He has had only one mild exacerbation (probable viral trigger) during the past 3 years of follow-up, which was treated with OCS for 5 days at 0.5 mg·kg−1. The troublesome symptoms of rhinosinusitis are still present.
The use of monoclonal antibodies in asthma is based on the understanding of T2 pathophysiological mechanisms. Currently, the choice between those directed against IL-5 or IgE is relatively insoluble in patients who are eligible for both, which was and still is the case for this patient [1]. Combining biologicals for treating patients with partially responding severe asthma is an attractive option, but to date it has not been used to its full potential because of concerns related to costs, as indicated by the provocative title of this article. Interestingly, this patient presents a relatively clear-cut symptomatology with no overlapping mechanisms despite being placed under the T2 umbrella; he seems to have IgE-dependent urticaria and anaphylactic manifestations considering the good response to omalizumab, especially since these manifestations relapsed during this drug's withdrawal. On the other hand, he appears to have IL5-driven asthma, as there is a connection between the elevated eosinophilic blood count, cortico-dependency, respiratory symptoms and the rhinosinusitis manifestations, and the beautiful response to mepolizumab.
We acknowledge that these elements are mostly clinical and therefore subject to discussion, in particular in terms of the symptoms’ subjectivity, their relatively low specificity, the very long disease evolution and some reported difficulty in establishing a clear clinical distinction between the two. The risk to vital functions and the unacceptable side-effects of systemic corticosteroids have prompted us to propose this exceptional management, but has raised an issue not tackled until today. This patient's profile therefore is in line with approval of both biologicals, because omalizumab is also approved for use in chronic urticaria when resistant to conventional treatments [2]. In addition, the switch of this dual-therapy to dupilumab, an anti-IL4/IL13 monoclonal antibody, could reasonably be considered as evidence of benefits are clear for both severe asthma and chronic urticaria [3]. The quite high blood eosinophil count recorded before initiating mepolizumab (1640 per mm3) might be the only limit as there are no data in this range, patients with counts higher than 1500 per mm3 having been excluded from the randomised controlled trial [4, 5].
Footnotes
The patient gave oral and written consent for this clinical communication.
Conflict of interest: M. Volpato has nothing to disclose.
Conflict of interest: S. Nowak has nothing to disclose.
Conflict of interest: J.L. Bourrain has nothing to disclose.
Conflict of interest: P. Demoly reports grants from Stallergène Greer, ALK, AstraZeneca, Bausch & Lomb and Thermo Fisher Scientific, and personal fees from Sanofi, outside the submitted work.
Conflict of interest: E. Ahmed has nothing to disclose.
Conflict of interest: A. Bourdin reports grants, personal fees, nonfinancial support and other from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Novartis; personal fees and nonfinancial support from Teva; personal fees, nonfinancial support and other from Regeneron and Chiesi Pharmaceuticals; grants, personal fees, nonfinancial support and other from Actelion; personal fees from Gilead; nonfinancial support and other from Roche; and other from Nuvaira, all outside the submitted work.
Conflict of interest: J. Charriot has nothing to disclose.
References
- 1.Bousquet J, Brusselle G, Buhl R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J 2017; 50: 1701782. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018; 73: 1393–1414. [DOI] [PubMed] [Google Scholar]
- 3.Lee JK, Simpson RS. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract 2019; 7: 1659–1661. [DOI] [PubMed] [Google Scholar]
- 4.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. [DOI] [PubMed] [Google Scholar]
- 5.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. [DOI] [PubMed] [Google Scholar]